Stabilization of amorphous calcium carbonate by controlling its particle size†
Fabio
Nudelman
,
Ela
Sonmezler
,
Paul H. H.
Bomans
,
Gijsbertus
de With
and
Nico A. J. M.
Sommerdijk
*
Laboratory of Materials and Interface Chemistry and Soft Matter CryoTEM Unit, Eindhoven University of Technology, P. O. Box 513, 5600 MB, Eindhoven, The Netherlands. E-mail: n.sommerdijk@tue.nl; Fax: +31 40 245 1036
First published on 13th September 2010
Abstract
Amorphous calcium carbonate (ACC) nanoparticles of different size are prepared using a flow system. Post-synthesis stabilization with a layer of poly[(α,β)-DL-aspartic acid] leads to stabilization of the ACC, but only for particles <100 nm. Larger and uncoated particles readily convert into the crystalline forms of CaCO3. This shows that ACC is intrinsically stable below 100 nm.
The use of a transient amorphous phase as an intermediary in the formation of crystalline biogenic minerals is a common strategy in biomineralization.1 The most well known examples are amorphous calcium phosphate as a precursor to apatite in bone2 and amorphous calcium carbonate (ACC), which transforms either in calcite3,4 or aragonite.5 Although the use of amorphous precursor phases is not a requirement for the formation of complex crystalline morphologies,6 it does allow their formation at higher supersaturations and hence at higher mineralization rates compared to ion-by-ion nucleation and growth.7,8 Moreover, the volume of saturated solution necessary for the formation of a biomineral would be very large, and would represent a logistic problem for the organism.9
Employing an amorphous phase as a precursor to crystalline materials requires its transient stabilization until it is delivered to the mineralization front. Crystallization then is triggered either by a nucleation site or by fusion with a growing crystal.9 The mechanisms of stabilization often employed by organisms involve the use of additives such as Mg ions10 or specialized macromolecules,11 which may be incorporated into the mineral where they inhibit crystallization.
Several synthetic routes have been used to produce ACC in vitro either through the use of additives12 or low temperatures.8 In addition, ACC has also been shown to form as an intermediary to vaterite and calcite in template-directed mineralization.12–15 Intriguingly, the transformation of ACC into vaterite only occurs in particles above 100 nm in size, suggesting that the amorphous phase is intrinsically stable below a certain critical size.16
Here, we investigate the particle size dependency on the development of crystallinity in ACC. Such a study demands the development of a system where calcium carbonate particles can be formed with controllable sizes. We designed a microfluidics-based system17 where solutions of 10 mM of CaCl2 and 10 mM of Na2CO3 were pumped with constant flow rates and mixed through a T-junction.‡ Calcium carbonate formation was dependent on the diffusion of the ions at the interface between the two solutions after mixing, and by controlling the length of the exit tube from the T-junction, we could precisely control the reaction time and hence the particle size. The experiment was done at 4 °C in order to slow down the rate of mineral formation and transiently stabilize the ACC.8 Poly[(α,β)-DL-aspartic acid] (pAsp) was introduced through a second T-junction to cover the surface of the newly formed ACC particles and therefore inhibit their further growth and coalescence. This particular polymer was selected for our study since it binds well to calcium carbonate. Samples were plunge-frozen in liquid ethane for investigation by cryoTEM (see the ESI†), either immediately after the reaction or after being stored for 48 h at 4 °C.
In order to characterize our mineralization system, samples were collected at different time points without any additives. At 5 and 10 s of reaction, we observed particles with similar size distributions, ranging from 10 to 25 nm (Fig. 1a, b). Longer reaction times yielded particles significantly bigger, in the range of 250 to 300 nm after 20 s and between 250 and 350 nm after 40 s (Fig. 1c–d). LD-SAED showed that all the particles formed at all reaction time points were amorphous (Fig. 1a–d, insets). CryoEDX analysis confirmed that the particles formed after 20 and 40 s of reaction were indeed composed of CaCO3 and therefore consisted of ACC (see the ESI†). CryoEDX could not be performed on the particles formed after 5 and 10 s, as they immediately disintegrated under the electron beam during spectrum acquisition. Thus, in order to verify whether these particles were indeed CaCO3 or ice crystals, we analyzed their electron diffraction pattern, using radial integration and comparing the positions of the first and second diffraction rings in comparison to the amorphous ice layer.15
 | ||
| Fig. 1 CryoTEM images of calcium carbonate particles formed for 5 s (a, white circles); 10 s (b, white circles); 20 s (c) and 40 s (d). Insets: LD-SAED patterns showing that the particles are amorphous. (e) Radial integration of the diffraction patterns at the different time points, in comparison with vitrified pure water. Vertical lines mark the position of the diffraction peaks of the ACC particles. | ||
As shown in Fig. 1e and in the ESI,† the second diffraction peak of the ACC particles formed in 20 and 40 s was shifted in comparison with amorphous ice (X1 = 0.385 ± 0.02 nm for 20 s and X1 = 0.384 ± 0.02 nm for 40 s, as opposed to X1 = 0.376 ± 0.01 nm for the ice layer). This shift is caused by the amorphous calcium carbonate, as previously described.15 These values closely matched the position of the diffraction rings of the particles formed at 5 s of reaction (X1 = 0.385 ± 0.03 nm), clearly indicating that all particles consisted of ACC. In addition, it must also be noted that if all these particles were in fact ice, spots in the LD-SAED corresponding to hexagonal ice would have been observed. Although we did not perform LD-SAED on the particles formed after 10 s reaction, we can assume that they are also amorphous since only ACC was found, even at longer reaction times. When the reaction was allowed to proceed for 1 h at 4 °C, the ACC particles developed into rhombohedral calcite crystals 2 μm in size (see the ESI†).
Poly[(α,β)-DL-aspartic acid] at 0.6 mg ml−1 was introduced after 10 s of CaCl2 and Na2CO3 reaction, and samples were collected and vitrified immediately (t = 10 s), or stored for 48 h at 4 °C and then vitrified for cryoTEM analysis (Fig. 2a–b). At t = 10 s, we observed ACC particles with a size distribution of 20 to 60 nm. After 48 h, the average particle size was measured as 50 nm, with a few particles measuring 100 nm, indicating very slow particle growth after encapsulation with pAsp. Importantly, after 48 h the particles that were smaller than 100 nm still remained amorphous (Fig. 2b). Further evidence that these particles indeed consisted of ACC was obtained from the radial integration of the LD-SAED as described above (Fig. 2c). The positions of the first and second diffraction rings matched those of the ACC particles formed at 20 and 40 s, without additives (X1 = 0.384 ± 0.03 nm, ESI†). The fact that the diffraction peaks of the background were also shifted in relation to that of pure water (X1 = 0.382 ± 0.04 nm, ESI†) indicates the presence of pre-nucleation clusters in solution, as described.15,18 On the other hand, particles that were 100 nm in size started to develop crystallinity, exhibiting the (100) plane of vaterite at the d-spacing of 0.357 nm (Fig. 3).
 | ||
| Fig. 2 CryoTEM images of calcium carbonate particles formed for 10 s and encapsulated with 0.6 mg ml−1 of pAsp. (a) Particles immediately after mixing with pAsp (t = 0 h). (b) Particles after 48 h. Inset: LD-SAED pattern showing that the particles are still amorphous. (c) Radial integration of the diffraction pattern of the particles in (b), in comparison with the amorphous ice layer at the background and with vitrified pure water. Vertical lines mark the position of the diffraction peaks of the ACC particles. | ||
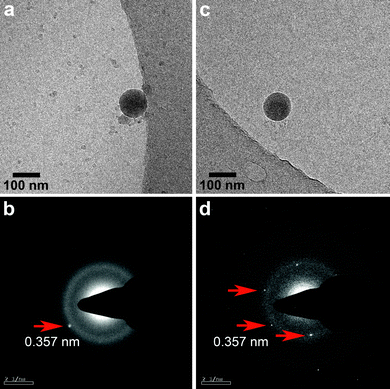 | ||
| Fig. 3 CryoTEM images of calcium carbonate particles 100 nm in size formed after 10 s and encapsulated with 0.6 mg ml−1 of pAsp. (b) LD-SAED pattern of the particle in (a). The appearance of a diffraction spot at a d-spacing corresponding to that of vaterite shows that crystallinity started to develop. (d) LD-SAED pattern of particle in (c) shows that the particle has crystallized into vaterite, as given by the characteristic d-spacing of 0.357 nm. | ||
Our results clearly show that the transformation of an amorphous phase into a crystalline one in calcium carbonate is dependent on the size of the particle. Our data conforms with computer simulations which predicted a minimum threshold size for the transition from an amorphous to a crystalline state, where sufficient low bulk energy can be accommodated to offset the entropic penalty.19 According to our observations, the minimum size for crystallinity to start to develop in the present system is ∼100 nm. Possibly, this critical size is system-specific and may vary according to the experimental conditions. Nevertheless, these results are consistent with previous reports that also described the crystallization of ACC only in particles in the range of 70 to 100 nm,14,15 suggesting that 100 nm is the critical upper limit for the stability of the amorphous phase. These conclusions are further corroborated by observations that while 70–100 nm ACC particles promptly crystallized once exposed to the electron beam, the smaller ones remained amorphous.14
It is interesting to note that although vaterite has a hexagonal lattice, the particles still remained spherical. These results suggest that the transition from ACC to vaterite occurred through a solid-state transformation and not through a dissolution–reprecipitation mechanism. This crystallization mechanism is likely to be caused by the coverage of the particle with pAsp, and is comparable to other in vitro systems, where a template was used to direct the formation of calcium carbonate,8,13–15 and to biological systems.4 It is also noteworthy that after 48 h only vaterite was observed. Whether this material will further transform into calcite or not still needs to be investigated.
To date, several methods have been used to produce ACC in vitro. However, long-term stabilization of ACC could not be achieved unless inhibitory agents occluded inside the mineral phase, such as Mg ions,10 acidic polymers and other (macro)molecules12,20 extracted from biogenic CaCO3, were used. Here, using pAsp to inhibit particle growth, we report the stabilization of ACC for 48 h by keeping the particles below the threshold in which ACC is no longer stable. It is likely that under these conditions the amorphous phase can be stabilized for longer periods as long as they remain below 100 nm.
It is conceivable that this mechanism is also employed by nature to stabilize ACC. In vivo, the presence of granules 50–100 nm in size being transported to the mineralization front has been reported.21 These granules were proposed to be composed of ACC, and although it is likely that they contained acidic proteins inhibiting the crystallization, it is also likely that restricting particle size is an additional mechanism to ensure that the amorphous phase remains stable until its delivery to the mineralization site.
Acknowledgements
We thank the Netherlands Science Foundation, NWO, for financial support.References
- S. Weiner, I. Sagi and L. Addadi, Science, 2005, 309, 1027–1028 CrossRef CAS.
- J. Mahamid, A. Sharir, L. Addadi and S. Weiner, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 12748–12753 CrossRef CAS.
- R. Dillaman, S. Hequembourg and M. Gay, J. Morphol., 2005, 263, 356–374 CrossRef.
- Y. Politi, T. Arad, E. Klein, S. Weiner and L. Addadi, Science, 2004, 306, 1161–1164 CrossRef CAS.
- J. C. Marxen, W. Becker, D. Finke, B. Hasse and M. Epple, J. Molluscan Stud., 2003, 69, 113–121 CrossRef.
- R. J. Park and F. C. Meldrum, Adv. Mater., 2002, 14, 1167–1169 CrossRef CAS.
- J. Aizenberg, D. A. Muller, J. L. Grazul and D. R. Hamann, Science, 2003, 299, 1205–1208 CrossRef CAS; S. Raz, P. C. Hamilton, F. H. Wilt, S. Weiner and L. Addadi, Adv. Funct. Mater., 2003, 13, 480–486 CrossRef CAS.
- E. Loste, R. J. Park, J. Warren and F. C. Meldrum, Adv. Funct. Mater., 2004, 14, 1211–1220 CrossRef CAS.
- L. Addadi, D. Joester, F. Nudelman and S. Weiner, Chem.–Eur. J., 2006, 12, 980–987 CrossRef CAS.
- S. Raz, S. Weiner and L. Addadi, Adv. Mater., 2000, 12, 38 CrossRef CAS; M. M. Reddy and G. H. Nancollas, J. Cryst. Growth, 1976, 35, 33–38 CrossRef CAS.
- L. B. Gower, Chem. Rev., 2008, 108, 4551–4627 CrossRef CAS; F. C. Meldrum and H. Colfen, Chem. Rev., 2008, 108, 4332–4432 CrossRef CAS; N. A. J. M. Sommerdijk and G. de With, Chem. Rev., 2008, 108, 4499–4550 CrossRef CAS.
- J. J. J. M. Donners, B. R. Heywood, E. W. Meijer, R. J. M. Nolte, C. Roman, A. P. H. J. Schenning and N. A. J. M. Sommerdijk, Chem. Commun., 2000, 1937–1938 RSC; J. J. J. M. Donners, B. R. Heywood, E. W. Meijer, R. J. M. Nolte and N. A. J. M. Sommerdijk, Chem.–Eur. J., 2002, 8, 2561–2567 CrossRef CAS; L. B. Gower and D. J. Odom, J. Cryst. Growth, 2000, 210, 719–734 CrossRef CAS; T. Kato, Adv. Mater., 2000, 12, 1543–1546 CrossRef CAS; Y. Politi, J. Mahamid, H. Goldberg, S. Weiner and L. Addadi, CrystEngComm, 2007, 9, 1171–1177 RSC; D. C. Popescu, E. N. M. van Leeuwen, N. A. A. Rossi, S. J. Holder, J. A. Jansen and N. A. J. M. Sommerdijk, Angew. Chem., Int. Ed., 2006, 45, 1762–1767 CrossRef CAS; A. W. Xu, Q. Yu, W. F. Dong, M. Antonietti and H. Colfen, Adv. Mater., 2005, 17, 2217–2221 CrossRef CAS.
- T. Y. J. Han and J. Aizenberg, Chem. Mater., 2008, 20, 1064–1068 CrossRef CAS; J. R. I. Lee, T. Y. J. Han, T. M. Willey, D. Wang, R. W. Meulenberg, J. Nilsson, P. M. Dove, L. J. Terminello, T. van Buuren and J. J. De Yoreo, J. Am. Chem. Soc., 2007, 129, 10370–10381 CrossRef CAS.
- B. P. Pichon, P. H. H. Bomans, P. M. Frederik and N. A. J. M. Sommerdijk, J. Am. Chem. Soc., 2008, 130, 4034–4040 CrossRef CAS.
- E. M. Pouget, P. H. H. Bomans, J. A. C. M. Goos, P. M. Frederik, G. de With and N.A.J.M. Sommerdijk, Science, 2009, 323, 1455–1458 CrossRef CAS.
- A. Navrotsky, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 12096–12101 CrossRef CAS.
- Microfluidics systems have also been applied for cryoTEM analysis ( J. Lee, A. K. Jha, A. Bose and A. Tripathi, Langmuir, 2008, 24, 12738–12741 Search PubMed ); and for monitoring biomimetic mineralization ( H. B. Yin, B. Z. Ji, P. S. Dobson, K. Mosbahi, A. Glidle, N. Gadegaard, A. Freer, J. M. Cooper and M. Cusack, Anal. Chem., 2009, 81, 473–478 CrossRef CAS ).
- D. Gebauer, A. Volkel and H. Colfen, Science, 2008, 322, 1819–1822 CrossRef CAS.
- D. Quigley and P. M. Rodger, J. Chem. Phys., 2008, 128, 221101–221104 CrossRef CAS.
- A. Ulčinas, M. F. Butler, M. Heppenstall-Butler, S. Singleton and M. J. Miles, J. Cryst. Growth, 2007, 307, 378–385 CrossRef CAS.
- J. M. Neff, Tissue Cell, 1972, 4, 311 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental setup, light microscopy of calcium carbonate formed without additives, radial integration of low-dose selected-area electron diffraction and cryoEDX analysis of calcium carbonate particles. See DOI: 10.1039/c0nr00432d |
‡ All chemicals were of analytical grade and used without further purification. Calcium chloride dehydrate (CaCl2·2H2O, Sigma Aldrich) and sodium carbonate (Na2CO3 Merck) were prepared at concentrations of 10 mM in MilliQ water, filtered using sintered glass filters and stored at 4 °C. Encapsulation of ACC particles (see below) was carried out with 0.6 mg ml−1 of poly[(α,β)-DL-aspartic acid] sodium salt (pAsp, Sigma Aldrich, Mw = 2000–11![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000), freshly prepared in MilliQ water and cooled to 4 °C before each experiment. 000), freshly prepared in MilliQ water and cooled to 4 °C before each experiment.Our experimental setup consisted of using syringe pump-to-pump solutions of 10 mM of CaCl2 and 10 mM of Na2CO3 continuously, with a flow rate of 1.5 ml min−1 through a T-mixer and then through an exit tube (see the ESI†). The reaction time, corresponding to the length of the exit tube, varied from 10 to 40 s. Poly[(α,β)-DL-aspartic acid] was added to the mineralization solution through an additional T-junction connected to the setup at a reaction time of 10 s, with a flow rate of 3 ml·min−1. The reaction time after the addition of the polymer was kept constant at 10 s, after which samples were collected at the end of the tube and characterized by cryoTEM immediately or after storage for 48 h at 4 °C. Throughout the whole experiment, the temperature of the solution was kept at 4 °C in order to slow down the rate of formation of calcium carbonate and temporarily stabilize the amorphous phase. The cryoTEM experiments were performed on a FEI Tecnai 20 (type Sphera) TEM or on the TU/e CryoTitan (FEI). Low-dose selected-area electron diffraction (LD-SAED) and energy-dispersive X-ray analysis (EDX) were performed on a Tecnai 20, equipped with a LaB6 filament operating at 200 kV and a Gatan cryoholder operating at ca. −170 °C was used. The images were recorded using a 1k × 1k Gatan CCD camera and cryoEDX spectra was recorded on an EDAX detector in cryoSTEM mode spot size 3. The TU/e CryoTitan was equipped with a field-emission gun (FEG) operating at 300 kV and a post-column Gatan Energy Filter (GIF). Images were recorded using a post-GIF 2k × 2k Gatan CCD camera. The sample vitrification procedure was carried out using an automated vitrification robot (FEI Vitrobot™ Mark III). CryoTEM grids, R2/2 Quantifoil Jena grids, were purchased from Quantifoil Micro Tools GmbH. Prior to the vitrification procedure the grids were surface plasma treated using a Cressington 208 carbon coater. Samples were collected from the mineralization solution and applied to the cryoTEM grids inside the vitrobot chamber, at 100% humidity and 4 °C. |
| This journal is © The Royal Society of Chemistry 2010 |
