Formation of cobalt-Prussian Blue nanoparticles in a biopolymer matrix†
Andrew M.
Collins
,
Stephen
Mann
and
Simon R.
Hall
*
Centre for Organized Matter Chemistry, School of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS, UK. E-mail: simon.hall@bristol.ac.uk; Tel: +44(0)1173316797
First published on 28th September 2010
Abstract
The molecular magnet material, cobalt-Prussian Blue, has been synthesized using chitosan as a morphological structure-directing agent. The strong chelating ability of the biopolymer ensures that the inorganic phase remains nanoparticulate. SQUID magnetometry reveals that the nanoparticles exhibit a sharp ferrimagnetic to paramagnetic transition at 16 K.
The synthesis of functional nanoparticles is to a large extent dependant on the reagents and protocols employed as their progenitors. Nanoparticles can be synthesized and organized in situ using top-down moulding methods1 or created using bottom-up biomimetic principles.2 As an example of the latter, chitosan is a good choice of material for nanoparticle synthesis, due to its low toxicity and high affinity for transition and post-transition metal ions in solution.3 This strong chelating ability has enabled chitosan to be used for the morphological control of a range of inorganic materials.4 For example, nanoparticles of iron oxide,5 gold6 and more complex materials such as high-temperature cuprate superconductors7 have been formed in the presence of chitosan by a variety of synthetic strategies. In the case of nanoparticulate gold formation in chitosan, the composite had excellent antioxidant properties, and was approximately 80 times more effective in the neutralisation of hydroxyl radicals than ascorbic acid.6
Previous work has shown that it is possible to synthesize molecular magnetic materials in chitosan,8 although in that study, the chitosan matrix was removed to leave a colloidal final product. In this paper we demonstrate that chitosan can be used to prepare discrete functional nanoparticles of a molecular magnetic material using a facile and rapid synthetic method that confers increased resistance to chemical degradation of the chitosan. By way of example, we use the Prussian Blue complex, cobalt hexacyanoferrate, (KxCo4II/III[FeIII/II(CN)6]y·nH2O), which is of particular interest due to its optically induced magnetic properties. Morphological control of this material at the nanometre length scale should facilitate the integration of molecular magnet materials as functional components in the next generation of advanced materials for applications in energy generation, computing, data storage, biosensing and optical film devices.
In a typical synthesis, an aqueous solution of Co2+ (0.1–0.5 M) was added to a 1 wt% acetic acid solution of chitosan (0.3–1 wt%) to produce a pink clear solution consisting of [Co(H2O)6]2+ ions and cationic biopolymer molecules The solution was then placed onto a glass slide and dried at 50 °C to give a cast film that turned blue due to dehydration of the Co2+ ions and complexation with the chitosan amide and hydroxyl groups, as confirmed by UV–visible spectroscopy. Upon exposure to potassium hexacyanoferrate solution, the blue films were transformed into red-brown composites containing dispersed cobalt hexacyanoferrate nanoparticles.
The nanoparticle-containing chitosan films were studied by transmission electron microscopy (TEM) and UV–visible spectroscopy. TEM samples were prepared by prolonged sonication in acetic acid followed by dispersion on the solution onto copper grids. Temperature dependent magnetisation curves were obtained on intact films using a Quantum Design Magnetic Property Measurement System (MPMS) SQUID (Superconducting Quantum Interference Device) magnetometer in the temperature range 4–40 K and under an applied magnetic field of 5 kOe. Control samples of chitosan were prepared by casting chitosan–acetic acid solutions onto glass slides followed by addition of 1 M NaOH. Samples of cobalt hexacyanoferrate were prepared in the absence of chitosan by adding 10 mL of a 0.1 M CoCl2·6H2O (Aldrich) solution in distilled water (pH 5.5) to 10 mL of a 0.1 M K3FeIII(CN)6 (Aldrich) solution (pH 6.3) to produce a red-brown precipitate.
Solid-state UV–visible spectroscopy of the composite films were consistent with the optical properties of a cobalt-based hexacyanoferrate, showing two broad bands centred at 388 nm and 519 nm indicative of a {CoIII[(t42g)]FeII[(t62g)]} → {CoII[(t52g)(e2g)FeIII[(t32g)(e2g)]}* electronic transition (Fig. 1).
 | ||
| Fig. 1 UV–visible spectra of a chitosan-cobalt hexacyanoferrate composite film. | ||
From FTIR measurements, the characteristic CoIII–CN–FeII cyanide-stretching mode could be seen at 2117 cm−1, confirming that cobalt hexacyanoferrate was formed in the film (Fig. 2).
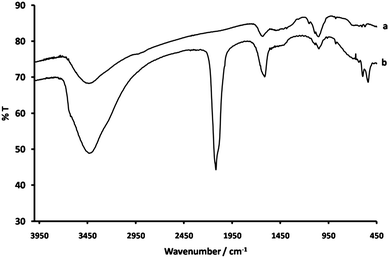 | ||
| Fig. 2 FTIR spectra of (a) a control film of chitosan prepared by NaOH treatment and (b) a chitosan-cobalt hexacyanoferrate composite film. | ||
A definite shift for the amide group vibrational mode was observed from 1637 cm−1 in the control sample to 1610 cm−1 in the composite. In addition, a shift in the C–O/chitosan stretching mode from 1049 cm−1 to 1054 cm−1 was seen. This suggests a mechanism of formation in which the chitosan polysaccharide ring is bonded to the cobalt hexacyanoferrate by amide linkages to the nanoparticle surface (Fig. 3).
 | ||
| Fig. 3 Schematic representation of chitosan-cobalt hexacyanoferrate chelation. | ||
TEM was used to characterise cobalt hexacyanoferrate nanoparticles prepared in the absence or presence of chitosan cast films. Size distribution analysis of control samples of cobalt hexacyanoferrate prepared free in solution revealed a wide size distribution with a mean nanoparticle size of 34 nm (σ = 14). This contrasted sharply with the cobalt hexacyanoferrate composite films prepared from a 0.3 wt%, chitosan solution, which revealed a high number of evenly distributed discrete spherical particles, 5.5 nm in mean size (σ = 1.4), and consisting of cobalt and iron (energy-dispersive X-ray (EDX) analysis) (Fig. 4).
![(a) TEM micrograph of a chitosan-cobalt hexacyanoferrate composite film (0.3 wt% chitosan solution, [CoCl2] 0.1 M casting solution) partially dissolved in acetic acid for analysis. The homogeneous dispersion of the cobalt hexacyanoferrate nanoparticles delineates the edge of the less-electron-dense chitosan film, which can be distinguished against the carbon background. Inset (b) shows EDX spectrum of the composite film and (c) particle size distribution. Scale bar in (a) is 200 nm.](/image/article/2010/NR/c0nr00382d/c0nr00382d-f4.gif) | ||
| Fig. 4 (a) TEM micrograph of a chitosan-cobalt hexacyanoferrate composite film (0.3 wt% chitosan solution, [CoCl2] 0.1 M casting solution) partially dissolved in acetic acid for analysis. The homogeneous dispersion of the cobalt hexacyanoferrate nanoparticles delineates the edge of the less-electron-dense chitosan film, which can be distinguished against the carbon background. Inset (b) shows EDX spectrum of the composite film and (c) particle size distribution. Scale bar in (a) is 200 nm. | ||
Significantly, the nanoparticles were spatially isolated within the biopolymer matrix, suggesting that nucleation in the film in the presence of a [Fe(CN)6]3− diffusion gradient was confined to localized regions, and that aggregation of the clusters was constrained by the cocooning effect of the surrounding chitosan matrix.
Samples prepared using twice the chitosan concentraion (0.6 wt%) also contained discrete cobalt hexacyanoferrate nanoparticles but with a decreased mean size of 2.8 nm (σ = 0.6). This was attributed to the reduction in mass transport of [Fe(CN)6]3− ions and higher localised concentrations of complexed Co2+ for increased concentrations of the biopolymer, both of which would increase the nucleation density in the chitosan matrix. Interestingly, when the chitosan concentration was increased to 1 wt% no discrete nanoparticles were observed and a continuous composite film of cobalt hexacyanoferrate and chitosan was formed (see Fig. S1 of the ESI†). Moreover, the mean sizes of the cobalt hexacyanoferrate nanoparticles formed in chitosan cast films were independent of changes in CoII concentration up to a concentration of 0.5 M. In all cases, Gaussian particle size distributions were observed, indicating unrestricted crystal growth within the chitosan matrix rather than a mechanism of physically limited crystal growth in a biopolymer network cavity, which would produce Poisson distributions skewed towards larger particles.
SQUID magnetometry measurements of the composite films showed a sharper transition towards the paramagnetic region upon heating than observed for the control sample of cobalt hxacyanoferrate (Fig. 5). We attribute this to the relatively uniform particle size, which has a direct correlation to the number of magnetic domains in each particle and the respective energy barriers associated with magnetic domain transitions. The nanoparticles behave as single magnetic domains and due to the narrow distribution of energy barriers in the chitoson-mediated samples a rapid transition from ferrimagnetism to paramagnetism is observed at 16 K. In contrast, a smooth exponential decay curve was observed for the control sample due to a wider polydispersity. A magnetic anomaly appears at around 10 K in both the control and composite materials which we were unable to resolve. It is likely that this is due to an impurity phase, although more work will be required in order to identify this, as XRD does not reveal any crystalline potential candidates (see Fig. S2 of the ESI†).
 | ||
| Fig. 5 Graph showing the magnetic susceptibility of control cobalt hexacyanoferrate (◆) and cobalt hexacyanoferrate prepared using chitosan (□). | ||
Finally, we observed that optimal preparations of the composite films produced with 0.3 wt% chitosan and a [Co2+] greater than 0.1 M showed increased resistance to dissolution in a 10% solution of acetic acid compared to chitosan alone. Whilst the control chitosan film dissolved in a few seconds, the composite film took hours to days to dissolve completely. The rate of chitosan dissolution is determined principally by the level of protonation at the amide group, implying that blockage of these sites by binding of the chitosan to the cobalt hexacyanoferrate nanoparticles stabilised the matrix in acidic solutions. Although the transition band of cobalt hexacyanoferrate was observed to disappear in both the control and film samples by UV–visible spectroscopy after dissolution, TEM analysis of these solutions showed that nanoparticles were still present (Fig. 6). These results indicate that it is possible to produce a template-free colloidal solution containing cobalt hexacyanoferrate nanoparticles via our synthetic approach. Indeed, it was possible to raise the pH of the colloidal solution from 2.2 to 8.0, where it was stable for over 24 h after which flocculation occurred.
 | ||
| Fig. 6 TEM image showing cobalt hexacyanoferrate nanoparticles after dissolution of the chitosan templating film. Scale bar is 200 nm. | ||
In summary nanoparticles of cobalt hexacyanoferrate molecule-based magnets have been produced in a biological matrix, by a diffusion-limited process. The films have enhanced resistance to acid digestion. Moreover, the characteristics of the inorganic component demonstrated a sharper magnetic response to temperature. This methodology may be applied to a range of functional inorganic materials and could herald new opportunities in sensing, optical and medical films and delivery systems. In addition, as many organisms use chitosan-based materials as morphologically complex structural scaffolds, it may be possible to prepare cobalt hexacyanoferrate materials which mimic these structures in three dimensions. Mineralisation of chitosan-based constructs such as cuttlefish bone matrix for example, would give unique functional materials with periodic porosity and low overall weight.9,10
Acknowledgements
The authors thank the Royal Society (SRH, University Research Fellowship) for financial support, and Adam Robinson for SQUID magnetometry.References
- P. D. Yang, T. Deng, D. Y. Zhao, P. Y. Feng, D. Pine, B. F. Chmelka, G. M. Whitesides and G. D. Stucky, Science, 1998, 282, 2244 CrossRef CAS.
- S. Mann, Nature, 1993, 365, 499 CrossRef CAS.
- K. Okuyama, K. Noguchi, T. Miyazawa, T. Yui and K. Ogawa, Macromolecules, 1997, 30, 5849 CrossRef CAS.
- C. Muzzarelli and R. A. A. Muzzarelli, J. Inorg. Biochem., 2002, 92, 89 CrossRef CAS.
- A. Kaushik, R. Khan, P. R. Solanki, P. Pandey, J. Alam, S. Ahmad and B. D. Malhotra, Biosens. Bioelectron., 2008, 24, 676 CrossRef.
- K. Esumi, N. Takei and T. Yoshimura, Colloids Surf., B, 2003, 32, 117 CrossRef CAS.
- S. R. Hall, Adv. Mater., 2006, 18, 487 CrossRef CAS.
- Y. Guari, J. Larionova, K. Molvinger, B. Folch and C. Guérin, Chem. Commun., 2006, 2613 RSC.
- W. Ogasawara, W. Shenton, S. A. Davis and S. Mann, Chem. Mater., 2000, 12, 2835 CrossRef CAS.
- E. Culverwell, S. C. Wimbush and S. R. Hall, Chem. Commun., 2008, 1055 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: XRD pattern obtained from a cast film of chitosan containing cobalt hexacyanoferrate nanoparticles and a TEM micrograph depicting a film of chitosan and cobalt hexacyanoferrate prepared from a 1 wt% chitosan casting solution. See DOI: 10.1039/c0nr00382d |
| This journal is © The Royal Society of Chemistry 2010 |
