PbSe nanocrystal growth as nanocubes and nanorods on peptide nanotubes via different directed-assembly pathways
Menglu
Shi
,
Wei
Su
and
Hiroshi
Matsui
*
Department of Chemistry and Biochemistry, City University of New York – Hunter College, New York, NY 10065, USA. E-mail: hmatsui@hunter.cuny.edu; Tel: 212-650-3918; Fax: 212-772-5332
First published on 13th September 2010
Abstract
Pb-binding TAR-1 peptides (Ile-Ser-Leu-Leu-His-Ser-Thr) were covalently conjugated on a bolaamphiphile peptide nanotube substrate and the precursors of PbSe were incubated at room temperature. This resulted in the growth of highly crystalline PbSe nanocubes on this biomimetic cylindrical substrate. The growth mechanism to generate nanocubes occurs via the directed self-assembly of nanoparticles and then nanoparticle fusion. The peptide conformation and the cylindrical peptide nanotube substrate play important roles in the mesoscopic crystallization of PbSe nanocubes. Changing the buffer for the peptide immobilization process from 2-(N-morpholino)ethanesulfonic acid to phosphate induces a transformation in the nanocrystal shape from nanocube to nanorods. The conformational change of the TAR-1 peptide on the nanotubes due to the change in the buffer seems to be responsible for aggregating intermediate nanoparticles in different directions for the directed fusion and mesoscopic crystallization of PbSe into the different shapes.
As an important semiconductor with a narrow bandgap (0.27 eV at 300 K), nanosized lead selenide (PbSe) is of scientific interest due to its great potential in the applications of optics,1–3 the IR spectra regime, thermoelectric devices, biological labels and solar cells.4–6 Currently the synthesis of morphology-controlled or shape-controlled PbSe nanocrystals requires heating of the reaction mixture to a high temperature. While Pb ions and Se ions have no problem reacting at any temperature, the low solubility of PbSe, Ksp = 1.0 × 10−37, makes the synthesis difficult at room temperature and under an ambient conditions the reaction tends to form shapeless particles. Meanwhile in nature, biomineralization enables manipulation and crystallization of crystals into complex shapes in vitro via the transport of intermediates, controlled by the topology and the chemical functionality of biological substrates.7–11 Biomineralizing organisms such as starfish and marine sponges have evolved the capability of synthesizing inorganic structures in mild conditions with an outstanding degree of complexity on a nano- and microscale.12,13 By mimicking natural systems, target crystals can be mineralized on functional flat substrates where peptides, which have a high affinity to specific precursor ions and catalyze the reaction, are patterned.14 This approach enables the growth of targeted inorganic crystals, however the resulting morphology is difficult to control on these 2D surfaces. The shape of the substrate plays a significant role in controlling the size and the shape of nanocrystals, as observed in nature. For example, when the mineralizing peptides for Au were immobilized on a cylindrical peptide nanotube surface and Au was mineralized onto this surface, the resulting size of the nanocrystals was very sensitive to the pH of the growth solution15 while the same degree of control was not achieved by the same peptide monolayer on flat Au substrates.14 This comparison indicates that non-flat substrates have certain advantages in the morphology control of mineralization. This is not surprising since the natural mineralization template is rarely flat. This flexibility of morphology in peptide-assisted mineralization could be due to the ease of the conformational change of peptides on cylindrical surfaces.16
Here we report the growth of PbSe with Pb-binding peptides patterned on cylindrical peptide nanotube surfaces (Fig. 1). Since the peptide that can bind Pb ions with a high affinity can overcome the low solubility problem by concentrating ions on the peptide surface, this system is expected to grow PbSe at room temperature.
 | ||
| Fig. 1 Schematic diagram of PbSe nanoparticle growth on the peptide nanotubes. The TAR-1 peptide (ISLLHST), immobilized on the template nanotubes, coordinates Pb ions and forms PbSe nanocrystals after they react with Se ions on the cylindrical nanotube substrate. | ||
The template nanotubes used for the PbSe mineralization were self-assembled from bis(N-α-amido-glycylglycine)-1,7-heptane dicarboxylate monomers. Details of the monomer synthesis and the method for the cylindrical self-assembly are described elsewhere.17,18 The peptide TAR-1, whose sequence is Ile-Ser-Leu-Leu-His-Ser-Thr, was found to have a high affinity and specificity towards coordination of Pb ions via charge interaction.19,20 After amine groups of the TAR-1 peptide were covalently bound to the carbonyl groups of the nanotubes via the NHS/EDAC reaction in a 2-(N-morpholino)ethanesulfonic acid (MES) buffer (0.1 M, pH 5.6), Pb ions were incubated with the TAR-1 peptide-conjugated nanotube and subsequently Se ions were reacted with these Pb ions. This final step of Se incubation was carried out in deionized water in order to reduce the production of Pb(OH)x which competes with PbSe generation. During 1 h of incubation, the majority of the coating consisted of small spherical nanoparticles with an average diameter of 8 nm (Fig. 2a). Also observed after this short incubation time were some cubic nanocrystals of larger sizes.
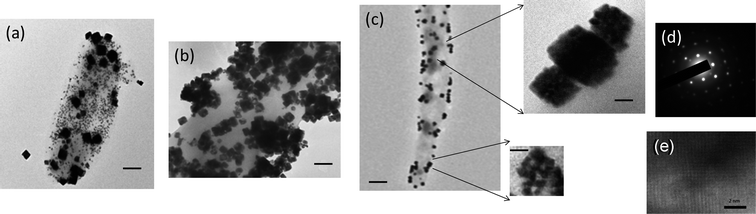 | ||
| Fig. 2 TEM images of (a) the TAR-1 peptide-conjugated nanotube after incubating with PbSe precursors for 1 h, scale bar = 50 nm, (b) 12 h, scale bar = 40 nm and (c) 20 h, scale bar = 210 nm right: a magnified TEM image, scale bar = 30 nm. (d) Electron diffraction pattern of the TAR-1 peptide-conjugated nanotube after incubating with PbSe precursors for 20 h. (e) HR-TEM image of a PbSe nanocube in (c). | ||
When the precursor incubation was extended to 12 h, the number of PbSe nanocubes, with an average size of 55 nm, increased while the small spherical nanoparticles were observed at a smaller population (Fig. 2b). After 20 h of the reaction, the spherical nanoparticles were completely transformed into cubic nanocrystals on the nanotube surface (Fig. 2c, left). These results for different incubation times reveal the growth steps of PbSe nanocubes; that the spherical nanoparticles are grown first, and then these nanoparticles fuse into larger cubic nanocrystals. The electron diffraction pattern of the PbSe nanocube on the nanotube in Fig. 2d shows the (200), (220), (222), (400), (420), and (422) planes of a face-centered-cubic crystal of PbSe. A high-resolution TEM image of the resulting nanocube (Fig. 2e) resolves the lattice fringes of (200) and (220) faces and it shows a single crystalline nature. We hypothesize that the subsequent crystallographic fusion of the high-energy crystal faces induces crystallization of the self-assembled nanoparticles. This hypothesis for the nonclassical crystallization process is supported by magnified TEM images of some of the PbSe nanocubes that do not complete the mesoscopic fusion process in Fig. 2c (right). These images show the particle domains in the cubic structure. This type of mesoscopic transformation has been reported for the growth of various inorganic systems.11 In the case of the orientated attachment of PbSe nanoparticles, the formation of 1D PbSe nanowires has been reported with high-temperature synthesis in organic solvents,21 however previously 2D PbSe mesoscopic crystallization has not been achieved at room temperature in aqueous solution. It should be noted that in the crystallization there is another possible growth mechanism in which spherical and cubic nanoparticles co-develop with different kinetics without the fusion of small particle domains. However, all of our observations point the other way. For example, the number of spherical nanoparticles decreases as the number of nanocubes increases (Fig. 2a and b), and the resulting nanocubes consist of a spherical particle domain as a resulting of fusing (Fig. 2c). If both shapes of nanoparticles grow at different rates, the number of spherical nanoparticles should not diminish as the reaction proceeds. Therefore, it is more likely that the mesoscopic transformation takes place in the crystallization of PbSe nanocubes.
In order to confirm the role of the TAR-1 peptide on the nanotube for the growth of PbSe nanocubes, two control experiments were performed. When the neat nanotubes without the TAR-1 peptides were incubated with Pb(NO)2 and then with Na2SeSO3 solution, no coating was observed around the nanotube (Fig. 3a). In another control experiment, when the TAR-1 peptides and the precursors were incubated without the nanotubes, random aggregations of nanoparticles were seen in the TEM image (Fig. 3b). It should be noted that the aggregate in Fig. 3b does not have a defined electron diffraction pattern and therefore it is amorphous. This observation indicates that the TAR-1 peptides immobilized on the nanotube surface concentrate precursors more effectively for nucleation, and the peptide-functionalized nanotubes play a crucial role in the crystallization of PbSe nanocubes. While the TAR-1 peptides concentrate Pb ions on the nanotube surface for mineralization, the nanotubes provide adequate platforms for the directed self-assembly of nanoparticles and their fusion into PbSe nanocube crystals rather than random particle aggregates.
 | ||
| Fig. 3 TEM images of (a) a neat nanotube template without TAR-1 peptides after incubating with PbSe precursors for 20 h, scale bar = 200 nm. (b) Only TAR-1 peptides without the nanotube substrate after incubating with PbSe precursors for 20 h, scale bar = 100 nm. | ||
The conformation of the mineralizing peptides on the nanotube substrate plays an important role in determining the shape and the size of nanocrystals.16 Buffers are known to influence the conformation and aggregation states of peptides and proteins,22 and we examined this buffer effect by changing to a phosphate buffer with the same concentration and pH as with the previous MES buffer. Since water is the best solvent to grow PbSe nanocrystals, we only changed the buffer at the first step of the peptide incubation. When the immobilization step of TAR-1 peptide on the nanotubes was carried out in phosphate buffer (0.1 M, pH 5.6) and the resulting peptide nanotubes were incubated with the PbSe precursors, PbSe nanorods were grown on the peptide nanotube surfaces (Fig. 4a). The electron diffraction pattern of the PbSe nanorods on the nanotubes in Fig. 4b shows (111), (200), (220), (222), (400), (420), and (422) planes for a face-centered-cubic crystal of PbSe. This shape transformation from nanocube to nanorods indicates that the peptide conformation is indeed important in determining the crystal shape of PbSe. At the early stage of PbSe growth in this condition, cubic nanoparticles were seen to undergo directed-assembly in one-dimension to grow nanorods (Fig. 4c). Phosphate buffer has been shown to induce aggregation of proteins,22 and the conformation of TAR-1 peptide in phosphate buffer is expected to be significantly different compared to different buffers under strong peptide–peptide interactions. It is possible that the directed-fusion of nanoparticles for mesoscopic crystallization is influenced by the conformation of TAR-1 peptides in the phosphate buffer. Previously the adsorption/desorption properties of small molecules on TiO2 surfaces were seen to become more sensitive to the crystalline structure in phosphate rather than MES buffers.23 In this case the assembly of PbSe nanoparticles could be more directional along the crystalline faces to form nanorods on the peptide nanotube. It should be emphasized that the buffer effect was only examined in the peptide incubation step to understand the influence of the peptide conformation on the crystal shapes; buffers were only added in the peptide immobilization step and then rinsed out with deionized water before the precursors were added. Therefore, the possibility of the direct influence of buffers on the crystal growth is eliminated.
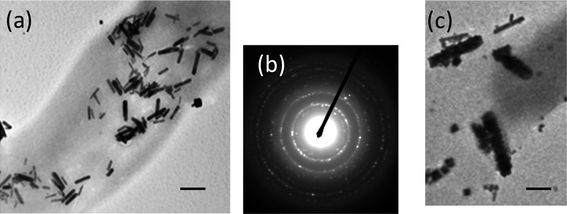 | ||
| Fig. 4 TEM images of (a) the TAR-1 peptide-conjugated nanotube after incubating with PbSe precursors for 20 h when the TAR-1 peptide immobilization on the nanotubes took place in phosphate buffer instead of MES buffer, scale bar = 100 nm. (b) An electron diffraction pattern of (a). (c) The TAR-1 peptide-conjugated nanotube after incubating with PbSe precursors for 1 h when the TAR-1 peptide was immobilized in phosphate buffer, scale bar = 30 nm. | ||
In conclusion, PbSe nanocubes were successfully grown on the peptide nanotubes at room temperature, which were conjugated with Pb-binding TAR-1 peptides. The growth mechanism follows the directed self-assembly of nanoparticles and the nanoparticle fusion to generate nanocubes. The peptide conformation and the cylindrical peptide nanotube substrate play important roles in the mesoscopic crystallization of PbSe nanocubes. Changing the buffer for the peptide immobilization process from MES to phosphate induces a nanocrystal shape transformation from nanocube to nanorods, and a conformation change of TAR-1 peptide on the nanotube via the buffer change seems to be responsible for aggregating intermediate nanoparticles in different directions for the directed fusion and mesoscopic crystallization of PbSe in different shapes.
Experimental section
Bis(N-α-amido-glycylglycine)-1,7-heptane dicarboxylate monomers were synthesized and assembled into peptide nanotubes as reported previously.17,18 After these peptide nanotubes were centrifuged and rinsed with deionized water, they were used as substrates for the immobilization of Pb mineralizing TAR-1 peptides and for the subsequent PbSe nanocrystal growth. For the covalent immobilization of TAR-1 peptide, the rinsed nanotubes were incubated in the N-hydroxysuccinamide and 1-ethly-3-(3-dimethlyaminopropyl) carbodiimide (NHS/EDAC) solution (NHS (100 mM) and EDAC (100 mM) in MES buffer (0.1 M, pH 5.6)) for 2 days, and then the nanotubes were mixed with TAR-1 peptides (4 mM) for 2 days. After removing the unreacted TAR-1 peptides by centrifuging and rinsing with deionized water, (PbNO3)2 (0.5 mM) was injected and incubated for 4 days and then the excess lead ions were removed by centrifuging and rinsing. Then, Na2SeSO3 (2 mM) was mixed with the resulting nanotube solution to complete the PbSe nanocrystal growth. The incubation time of Na2SeSO3 varied from 1 h to 20 h to monitor the growth mechanism. Finally, the PbSe nanocrystal-coated nanotubes were centrifuged and rinsed with deionized water before microscopic characterization.Acknowledgements
This research was supported by the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under Award No. DE-FG-02-01ER45935. Hunter College infrastructure is supported by the National Institutes of Health, the RCMI program (G12-RR003037-245476). M. L. thanks Dr Jorge Morales and Areti Tsiola for their assistance with TEM instrumentation.References
- M. Law, J. M. Luther, O. Song, B. K. Hughes, C. L. Perkins and A. J. Nozik, J. Am. Chem. Soc., 2008, 130, 5974 CrossRef CAS.
- W. G. Lu, P. X. Gao, J. W. Bin, Z. L. Wang and J. Y. Fang, J. Am. Chem. Soc., 2004, 126, 14816 CrossRef CAS.
- H. Du, C. L. Chen, R. Krishnan, T. D. Krauss, J. M. Harbold, F. W. Wise, M. G. Thomas and J. Silcox, Nano Lett., 2002, 2, 1321 CrossRef CAS.
- J. J. Choi, Y. F. Lim, M. B. Santiago-Berrios, M. Oh, B. R. Hyun, L. F. Sung, A. C. Bartnik, A. Goedhart, G. G. Malliaras, H. D. Abruna, F. W. Wise and T. Hanrath, Nano Lett., 2009, 9, 3749 CrossRef CAS.
- J. M. Pietryga, R. D. Schaller, D. Werder, M. H. Stewart, V. I. Klimov and J. A. Hollingsworth, J. Am. Chem. Soc., 2004, 126, 11752 CrossRef CAS.
- Q. Y. Lin, M. Smeller, C. L. Heideman, P. Zschack, M. Koyano, M. D. Anderson, R. Kykyneshi, D. A. Keszler, I. M. Anderson and D. C. Johnson, Chem. Mater., 2010, 22, 1002 CrossRef CAS.
- J. Aizenberg, D. A. Muller, J. L. Grazul and D. R. Hamann, Science, 2003, 299, 1205 CrossRef CAS.
- J. Aizenberg, G. Lambert, S. Weiner and L. Addadi, J. Am. Chem. Soc., 2002, 124, 32 CrossRef CAS.
- T. Tsuji, K. Onuma, A. Yamamoto, M. Iijima and K. Shiba, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 16866 CrossRef CAS.
- B. D. Reiss, C. B. Mao, D. J. Solis, K. S. Ryan, T. Thomson and A. M. Belcher, Nano Lett., 2004, 4, 1127 CrossRef CAS.
- F. C. Meldrum and H. Colfen, Chem. Rev., 2008, 108, 4332 CrossRef CAS.
- J. Aizenberg, J. C. Weaver, M. S. Thanawala, V. C. Sundar, D. E. Morse and P. Fratzl, Science, 2005, 309, 275 CrossRef CAS.
- J. C. Weaver, J. Aizenberg, G. E. Fantner, D. Kisailus, A. Woesz, P. Allen, K. Fields, M. J. Porter, F. W. Zok, P. K. Hansma, P. Fratzl and D. E. Morse, J. Struct. Biol., 2007, 158, 93 CrossRef CAS.
- N. Nuraje, S. Mohammed, L. L. Yang and H. Matsui, Angew. Chem., Int. Ed., 2009, 48, 2546 CrossRef CAS.
- R. Djalali, Y.-F. Chen and H. Matsui, J. Am. Chem. Soc., 2003, 125, 5873 CrossRef CAS.
- I. A. Banerjee, L. T. Yu and H. Matsui, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 14678 CrossRef CAS.
- H. Matsui and B. Gologan, J. Phys. Chem. B, 2000, 104, 3383 CrossRef CAS.
- M. Kogiso, S. Ohnishi, K. Yase, M. Masuda and T. Shimizu, Langmuir, 1998, 14, 4978 CrossRef CAS.
- B. D. Reiss, G. R. Bai, O. Auciello, L. E. Ocola and M. A. Firestone, Appl. Phys. Lett., 2006, 88, 083903 CrossRef.
- R. L. Willett, K. W. Baldwin, K. W. West and L. N. Pfeiffer, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 7817 CrossRef CAS.
- K. S. Cho, D. V. Talapin, W. Gaschler and C. B. Murray, J. Am. Chem. Soc., 2005, 127, 7140 CrossRef CAS.
- D. Kameoka, E. Masuzaki, T. Ueda and T. Imoto, J. Biochem., 2007, 142, 383 CrossRef CAS.
- M. E. Barbour, D. J. O'Sullivan and D. C. Jagger, Colloids Surf., A, 2007, 307, 116 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
