“Nanoarmoured” droplets of different shapes formed by interfacial self-assembly and crosslinking of metal nanoparticles†
Bartlomiej
Kowalczyk
,
István
Lagzi
and
Bartosz A.
Grzybowski
*
Department of Chemistry and Department of Chemical and Biological Engineering, Northwestern University, 2145 Sheridan Road, Evanston, IL, USA. E-mail: grzybor@northwestern.edu; Fax: (+1) 847 491 3728; Tel: (+1) 847 491 3024
First published on 11th October 2010
Abstract
Films comprising metal nanoparticles are assembled on the surfaces of liquid droplets of different shapes and macroscopic dimensions. These films are reinforced by dithiol crosslinks and are mechanically rugged yet permeable to the diffusion of small molecules.
Interest in coatings and films comprising nanoparticles (NPs) of various types has been fuelled by the singular optical,1,2 biological,3 magnetic,4,5 and electronic6–8 properties of these nanostructured materials and as well as by their potential applications in sensors,9–11 catalysis,12 displays,13 corrosion protection,14,15 antireflective films,16–18 bacteriostatic surfaces,19,20 and supports for bioresistant SAMs.21 Recently, several groups have investigated the possibility of creating NP films, in which strong interactions between the particles would render these films freestanding without the need for encapsulation in supporting matrices.22 For example, Lin et al. reported23 the synthesis of monolayer films in which CdSe particles were connected by covalent crosslinks and were sturdy enough to fold like sheets of paper. Mueggenburg et al. prepared monolayer superlattices of AuNPs, in which the particles were held together by the intercalation of the stabilizing monolayers of long-chain alkane thiols; these films were freestanding over small (250 nm) circular holes.24 Our own group developed a method25 in which large, centimeter-sized nanoparticle mono- or multilayers are first crosslinked on a solid substrate and then lifted-off by chemical underetching; the freestanding films thus produced can be subsequently transferred or micropatterned onto other surfaces. Notwithstanding their ingenuity, the above approaches are largely restricted to planar interfaces such that forming freestanding NP films over curved manifolds remains challenging. In particular, it would be desirable to form resilient NP films over liquid droplets—such nanoparticulate “armouring” could alter the mechanical properties of the droplets (as in the so-called colloid-decorated “armoured bubbles”26) and, by virtue of the nanoscale porosity of the films, could constitute membrane-like coating with interesting transport/permeability characteristics. The “armoured” droplets studied to date have been prepared either from colloidal particles held by large van der Waals (vdW) attractions27 or with the help of macromolecular/polymeric conjugates28 or even gels.29 Another approach has been to use segmented nanorods which assemble in a kinetically controlled fashion on the liquid/air interface upon drying.30,31 On the other hand, there are scarcely any examples of macroscopic droplets reinforced by sturdy nanoparticle films without the help of large stabilizers. One difficulty here is that unlike nanorods, spherical nanoparticles lack inherent “surfactant-like” symmetry that would promote their dense, side-by-side assembly on the interface.32,33,34 It appears that with small nanoparticles, the non-covalent interactions need to be enhanced by covalent NP crosslinking performed in such a way that the NPs do not precipitate but rather form a continuous film over the droplet's surface. Here, we describe a straightforward method that achieves such an interfacial assembly by combining the tendency of appropriately functionalized AuNPs to localize onto the water/toluene (or water/toluene-dichloromethane) interface with a crosslinking reaction taking place therein. With appropriately adjusted NP and crosslinker concentrations, the particles form continuous films over droplets several millimeters in diameter. These “armoured” droplets do not coalesce with one another but, at the same time, allow osmotic and diffusive transport through the NP film. Coupling of interfacial crosslinking reaction with the assembly of nanoscopic components can be performed at deformed liquid/liquid interfaces suggesting that such interfaces can be used as general-purpose templates for the assembly of NP films of different topographies.
Our experiments were based on gold nanoparticles AuNPs (5.5 ± 0.7 nm in diameter) functionalized with self-assembled monolayers (SAMs35) of 2,6-difluoro p-mercaptophenol (DFMP) prepared according to the published procedure.36,37 These nanoparticles were suspended in water at pH 10 (by the addition of NMe4OH) such that the DFMP ligands were mostly deprotonated. The key property of the DFMP-decorated particles is that while they are readily soluble in water, they also localize onto and spread over the water/toluene (or water/toluene-dichloromethane) interface. We have previously argued36 that these properties reflect a delicate balance between the electrostatic repulsions stablizing the NPs in an aqueous phase and partly hydrophobic character of the particles due to the fluorine groups. Additionally, once close to one another at the interface, the NPs can interact by hydrogen bonds between the DFMP ligands and by relatively large vdW attractions between particles' metal cores (for details, see ref. 37 and 38).
In a typical experiment (Fig. 1), the concentration of DFMP particles was 36 mM (in terms of metal atoms). Droplets of this solution (∼10–100 μL, diameters ∼1–3 mm) were placed onto a hydrophobic glass surface silanized with trichloro(1H,1H,2H,2H-perfluorooctyl)silane and immersed in either toluene or toluene-dichloromethane mixture. Under pure toluene, the heavier aqueous droplets were not spherical but rather “compressed” into ellipsoids. When the densities of the aqueous and organic phases were matched (e.g., with the use of a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v toluene-dichloromethane, density 0.982 g/cm3), the droplets were approximately spherical and had the contact angle with the glass surface >160 degrees. These droplets appeared dark red (Fig. 1a) reflecting the surface plasmon resonance, SPR, of the free AuNPs at ∼520 nm. Free NPs were present both at the interface and in the droplet's bulk (as verified by experiments in which droplets were frozen and then ruptured and examined under the optical microscope). When, however, hexanedithiol crosslinker; typical concentration, 10 μM) was added to the organic phase and the NPs at the droplet's surface were crosslinked,21,25 the appearance of the droplets gradually changed until after ca. 12 h they developed a golden hue (Fig. 1c) characteristic of AuNP multilayers.21 When these droplets were examined (again, by freezing, rupturing, and microscope imaging), the aqueous solution they contained was colorless. UV-Vis spectrum of this solution showed no characteristic AuNP SPR peak indicating that all available NPs were incorporated into the metallic film whose thickness was ∼400 nm (or 60 NP layers). We note that the key parameter controlling the formation of the film was the concentration of the dithiol crosslinkers. When this concentration was below ∼5 μM, a continuous film visible to a naked eye was not formed. Conversely, for concentrations higher than ∼25μM, the NPs aggregated rapidly and instead of coating the droplet uniformly over its entire surface, formed a large clump at one location of the interface; this clump then slid from the droplet's wall and sedimented at its bottom as a black precipitate.
1 v/v toluene-dichloromethane, density 0.982 g/cm3), the droplets were approximately spherical and had the contact angle with the glass surface >160 degrees. These droplets appeared dark red (Fig. 1a) reflecting the surface plasmon resonance, SPR, of the free AuNPs at ∼520 nm. Free NPs were present both at the interface and in the droplet's bulk (as verified by experiments in which droplets were frozen and then ruptured and examined under the optical microscope). When, however, hexanedithiol crosslinker; typical concentration, 10 μM) was added to the organic phase and the NPs at the droplet's surface were crosslinked,21,25 the appearance of the droplets gradually changed until after ca. 12 h they developed a golden hue (Fig. 1c) characteristic of AuNP multilayers.21 When these droplets were examined (again, by freezing, rupturing, and microscope imaging), the aqueous solution they contained was colorless. UV-Vis spectrum of this solution showed no characteristic AuNP SPR peak indicating that all available NPs were incorporated into the metallic film whose thickness was ∼400 nm (or 60 NP layers). We note that the key parameter controlling the formation of the film was the concentration of the dithiol crosslinkers. When this concentration was below ∼5 μM, a continuous film visible to a naked eye was not formed. Conversely, for concentrations higher than ∼25μM, the NPs aggregated rapidly and instead of coating the droplet uniformly over its entire surface, formed a large clump at one location of the interface; this clump then slid from the droplet's wall and sedimented at its bottom as a black precipitate.
 | ||
| Fig. 1 Preparation and appearance of NP-armoured droplets. (a) Droplet containing basic solution of AuNPs functionalized with DFMP ligands is placed onto silanized glass immersed in an organic solvent. The optical image in the bottom row shows that this droplet appears dark red due to the SPR of the free NPs. (b) When 10 μM HS-(CH2)6-SH, insoluble in water, is added to the organic phase, it crosslinks the NPs at the interface. (c) As more and more NPs reach the interface and are crosslinked therein, the nanoparticulate armour develops a characteristic metallic gloss. | ||
Also, NPs functionalized with other thiols did not produce uniform films. For example, while the NPs coated with either positively charged N,N,N-trimethyl(11-mercaptoundecyl)-ammonium chloride (TMA) or negatively charged 11-mercaptoundecanoic acid (MUA) were soluble in water, they did not assemble on the interface between aqueous and organic phases (likely, because van der Waals attractions between the particles' metal cores were weakened by the relatively long thiols). In addition, these NPs were hard to crosslink with dithiols, even ones longer than C6, reflecting the fact that TMA or MUA SAMs were more stable and less prone to thiol-dithiol exchange than the “thinner” monolayers of DFMP ligands.39
The droplets' “armoured” appelation derives from their mechanical robustness. This is vividly illustrated in Fig. 2b which shows two droplets brought into contact—as seen, the nanoparticle armour prevents the droplets from coalescing. We emphasize that this effect is due to NP crosslinking—when the droplets are not fully crosslinked/armoured, the fragile films break and coalescence does occur readily. Also, the rightmost image in Fig. 2 shows that the armour behaves akin to a thin metallic film and as a result of mechanical deformation develops pronounced wrinkles.
 | ||
| Fig. 2 Colliding and “interacting” droplets. Images in (a)–(c) illustrate the case when armoured droplets are brought into contact but do not coalesce (a) As-obtained droplet with smooth film on the surface. (b) Two droplets pushed into contact. (c) One of the droplets after contact – the droplet maintains its integrity but the AuNP film on its surface is wrinkled. Image in (d) illustrates the situation when an armoured droplet is brought into contact with a droplet of pure water. (e) Because of the difference in salt concentrations, water is “sucked” into the armoured droplet which increases its volume. (f) Upon this increase, the droplet initially appears intact but ultimately the nanoparticulate armour cracks/breaks. The process of water uptake is illustrated vividly in Movie 1 in the Supporting Information.† | ||
Although mechanically sturdy, the nanoparticulate armour behaves as a permeable membrane. To show this, we performed two experiments. In the first experiment (Fig. 2d–f), a droplet of pure water was placed next to an armoured drop. Because salt concentrations in the two droplets were different, the gradient of osmotic pressure caused the flow of pure water into the armoured droplet until the NP film finally gave away and cracked. In the second experiment, we demonstrated that the NP film was permeable to solutes contained in the organic phase. Specifically, when bromothymol blue (a dye soluble in both organic and aqueous phases) was dissolved in the organic phase around an armoured droplet, it slowly diffused into this droplet and colored it blue. This is illustrated in Fig. 3b,c, where the dye-infused droplet was frozen (to remove intact from the organic phase) but then, upon thawing, ruptured spilling a blue solution.
 | ||
| Fig. 3 Diffusive transport through the droplet's nanoparticulate armour. (a) Scheme illustrating diffusion of the bromothymol blue dye from the organic into the aqueous phase. (b) Optical image of an armoured droplet initially immersed in toluene containing bromothymol blue and then frozen at −78 °C (in dry ice) (c) After ∼3–5 min ice melts, and the NP film on the thawing droplet disintegrates. Water that spills is colored blue confirming that the dye migrated into the droplet through the NP armour. (d) Control experiment aimed to rule out transport through micro- or even macroscopic cracks in the NP film. Here, the droplet was first placed into the red-colored solution of toluene-dichloromethane containing ∼4.5 nm AuNPs functionalized with HS-(CH2)11-EG3. Even after prolonged soaking, the liquid contained in the droplet is clear (no SPR peak in UV-Vis spectrum) indicating that no AuEG3 nanoparticles migrated from the organic into the aqueous phase. | ||
The above result, however, is by itself not conclusive. One can argue that the dye is not transported into the droplet through the nanoscopic spaces between the NPs forming the film, but rather through microscopic or even macroscopic cracks in this film that are not visible during visual examination of the droplets. To rule out this possibility, we used larger, colored (red) “tracers” in the form of ∼4.5 nm gold nanoparticles functionalized with alkane thiols terminated in triethylene glycol units (HS-(CH2)11-EG3). These NPs were chosen because they were soluble in both aqueous and organic phases (with the preference for the former) and could withstand the basic conditions inside of the droplet (unlike, for example, fluorescently tagged proteins that denatured). Most importantly, these particles were small enough to migrate readily through any putative microscopic cracks yet were too large to diffuse through the nanoscopic voids of the densely-packed NP films coating the droplet (diffusion coefficient40D < 10−20 m2/s). The result of this experiment was that even after prolonged soaking (days), the solution inside of the droplet contained no NPs and appeared clear (Fig. 3d). From this experiment we conclude that the NP film is continuous over the droplet's surface and the only mode of transport is by the diffusion through the interparticle spaces in this membrane-like film.
Of course, the formation of the NP films is not restricted to spherical droplets and can take place over liquid–liquid interfaces of different shapes. To show this, we prepared pyramid-shaped droplets by constraining their bottom portions in polymeric, polygonal molds. Specifically, aliquots of aqueous solution of AuDFMP NPs were placed into differently shaped holes cut into a layer of poly (dimethyl siloxane), PDMS, and immersed in toluene-dichloromethane mixture containing dithiol crosslinker. The droplets thus prepared were “armoured” over their entire surfaces giving, for example, triangular (Fig. 4a) or square (Fig. 4b) “pyramids”. The mechanical properties and permeability of such droplets were identical to those of their spherical counterparts.
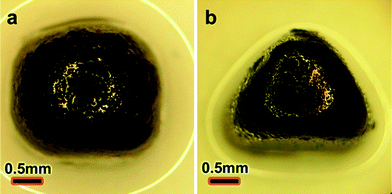 | ||
| Fig. 4 Optical images of armoured droplets of non-spherical shapes prepared by constraining the droplets' bases in PDMS molds. | ||
In summary, we described a straightforward method for the formation of continuous nanoparticle films over three dimensional liquid–liquid interfaces including spherical droplets as well as other, “deformed” surfaces. We believe that the most exciting feature of this system is that the films combine mechanical ruggedness (sufficient to prevent coalescence of water droplets) with permittivity to small molecule transport. Since the diffusivity of larger entities (macromolecules and/or nanoparticles) is limited, the “armoured” droplets could act as size-selective membranes and be used in dialysis-related applications. One of the drawbacks of the present system, however, is that the high-pH environment inside of the droplets is not conducive to biomolecules (especially proteins), and other types of NP functionalizations should be sought to achieve armour formation under more benign (less basic) conditions.
Notes and references
- X. Xu, T. H. Gibbons and M. B. Cortie, Gold Bulletin, 2006, 39, 156–165 CAS.
- J. Q. Liu, B. Cankurtaran, L. Wieczorek, M. J. Ford and M. Cortie, Adv. Funct. Mater., 2006, 16, 1457–1461 CrossRef CAS.
- M. Es-Souni and H. Fischer-Brandies, Adv. Funct. Mater., 2008, 18, 3179–3188 CrossRef CAS.
- T. Thurn-Albrecht, J. Schotter, C. A. Kastle, N. Emley, T. Shibauchi, L. Krusin-Elbaum, K. Guarini, C. T. Black, M. T. Tuominen and T. P. Russell, Science, 2000, 290, 2126–2129 CrossRef CAS.
- V. F. Puntes, K. M. Krishnan and A. P. Alivisatos, Science, 2001, 291, 2115–2117 CrossRef CAS.
- M. Brust, D. Bethell, C. J. Kiely and D. J. Schiffrin, Langmuir, 1998, 14, 5425–5429 CrossRef CAS.
- M. Brust, D. Bethell, D. J. Schiffrin and C. J. Kiely, Adv. Mater., 1995, 7, 795 CAS.
- H. Nakanishi, K. J. M. Bishop, B. Kowalczyk, A. Nitzan, E. A. Weiss, K. V. Tretiakov, M. M. Apodaca, R. Klajn, J. F. Stoddart and B. A. Grzybowski, Nature, 2009, 460, 371–375 CrossRef CAS.
- L. Han, D. R. Daniel, M. M. Maye and C. J. Zhong, Anal. Chem., 2001, 73, 4441–4449 CrossRef CAS.
- F. P. Zamborini, M. C. Leopold, J. F. Hicks, P. J. Kulesza, M. A. Malik and R. W. Murray, J. Am. Chem. Soc., 2002, 124, 8958–8964 CrossRef CAS.
- Y. Joseph, I. Besnard, M. Rosenberger, B. Guse, H. G. Nothofer, J. M. Wessels, U. Wild, A. Knop-Gericke, D. S. Su, R. Schlogl, A. Yasuda and T. Vossmeyer, J. Phys. Chem. B, 2003, 107, 7406–7413 CrossRef CAS.
- J. Luo, V. W. Jones, M. M. Maye, L. Han, N. N. Kariuki and C. J. Zhong, J. Am. Chem. Soc., 2002, 124, 13988–13989 CrossRef CAS.
- E. P. K. Currie and M. Tilley, J. Soc. Inf. Disp., 2005, 13, 773–780 CrossRef CAS.
- K. C. Chang, H. F. Lin, C. Y. Lin, T. H. Kuo, H. H. Huang, S. C. Hsu, J. M. Yeh, J. C. Yang and Y. H. Yu, J. Nanosci. Nanotechnol., 2008, 8, 3040–3049 CrossRef CAS.
- G. X. Shen, Y. C. Chen and C. J. Lin, Thin Solid Films, 2005, 489, 130–136 CrossRef CAS.
- X. T. Zhang, O. Sato, M. Taguchi, Y. Einaga, T. Murakami and A. Fujishima, Chem. Mater., 2005, 17, 696–700 CrossRef CAS.
- H. Hattori, Adv. Mater., 2001, 13, 51 CrossRef CAS.
- B. G. Prevo, Y. Hwang and O. D. Velev, Chem. Mater., 2005, 17, 3642–3651 CrossRef CAS.
- S. K. Smoukov, K. J. M. Bishop, B. Kowalczyk, A. M. Kalsin and B. A. Grzybowski, J. Am. Chem. Soc., 2007, 129, 15623–15630 CrossRef CAS.
- G. F. Fu, P. S. Vary and C. T. Lin, J. Phys. Chem. B, 2005, 109, 8889–8898 CrossRef CAS.
- B. Kowalczyk, M. Byrska, G. Mahmud, S. Huda, K. Kandere-Grzybowska and B. A. Grzybowski, Langmuir, 2009, 25, 1905–1907 CrossRef CAS.
- C. Y. Jiang and V. V. Tsukruk, Adv. Mater., 2006, 18, 829–840 CrossRef CAS.
- Y. Lin, H. Skaff, A. Boker, A. D. Dinsmore, T. Emrick and T. P. Russell, J. Am. Chem. Soc., 2003, 125, 12690–12691 CrossRef CAS.
- K. E. Mueggenburg, X. M. Lin, R. H. Goldsmith and H. M. Jaeger, Nat. Mater., 2007, 6, 656–660 CrossRef CAS.
- B. Kowalczyk, M. M. Apodaca, H. Nakanishi, S. K. Smoukov and B. A. Grzybowski, Small, 2009, 5, 1970–1973 CrossRef CAS.
- A. B. Subramaniam, M. Abkarian, L. Mahadevan and H. A. Stone, Nature, 2005, 438, 930–930 CrossRef.
- A. D. Dinsmore, M. F. Hsu, M. G. Nikolaides, M. Marquez, A. R. Bausch and D. A. Weitz, Science, 2002, 298, 1006–1009 CrossRef CAS.
- B. Samanta, X. C. Yang, Y. Ofir, M. H. Park, D. Patra, S. S. Agasti, O. R. Miranda, Z. H. Mo and V. M. Rotello, Angew. Chem., Int. Ed., 2009, 48, 5341–5344 CrossRef CAS.
- H. W. Duan, D. Y. Wang, N. S. Sobal, M. Giersig, D. G. Kurth and H. Mohwald, Nano Lett., 2005, 5, 949–952 CrossRef CAS.
- J. W. Ciszek, L. Huang, S. Tsonchev, Y. H. Wang, K. R. Shull, M. A. Ratner, G. C. Schatz and C. A. Mirkin, ACS Nano, 4, pp. 259–266 Search PubMed.
- J. W. Ciszek, L. Huang, Y. Wang and C. A. Mirkin, Small, 2008, 4, 206–210 CrossRef CAS.
- H. W. Duan, D. Y. Wang, D. G. Kurth and H. Mohwald, Angew. Chem., Int. Ed., 2004, 43, 5639–5642 CrossRef CAS.
- D. Y. Wang, H. W. Duan and H. Mohwald, Soft Matter, 2005, 1, 412–416 RSC.
- K. J. M. Bishop, C. E. Wilmer, S. Soh and B. A. Grzybowski, Small, 2009, 5, 1600–1630 CrossRef CAS.
- D. Witt, R. Klajn, P. Barski and B. A. Grzybowski, Curr. Org. Chem., 2004, 8, 1763–1797 CrossRef CAS.
- B. Kowalczyk, M. M. Apodaca, S. Soh and B. A. Grzybowski, Langmuir, 2009, 25, 12855–12859 CrossRef CAS.
- D. W. Wang, B. Kowalczyk, I. Lagzi and B. A. Grzybowski, J. Phys. Chem. Lett., 1, 1459–1462 Search PubMed.
- K. J. M. Bishop, B. Kowalczyk and B. A. Grzybowski, J. Phys. Chem. B, 2009, 113, 1413–1417 CrossRef CAS.
- C. J. Campbell, S. Soh and B. A. Grzybowski, Langmuir, 2008, 24, 11600–11604 CrossRef CAS.
- S. Soh, M. Byrska, K. Kandere-Grzybowska and B. A. Grzybowski, Angew. Chem., Int. Ed., 2010, 49, 4170–4198 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Movie 1 illustrating water uptake experiments (Fig. 2d–f). Movie 2 illustrating thawing and disintegration of a droplet infused with bromothymol blue. Experimental details. See DOI: 10.1039/c0nr00381f |
| This journal is © The Royal Society of Chemistry 2010 |
