Recent advancements in boron nitride nanotubes
Jiesheng
Wang
,
Chee Huei
Lee
and
Yoke Khin
Yap
*
Department of Physics, Michigan Technological University, 118 Fisher Hall, 1400 Townsend Drive, Houghton, MI 49931, U.S.A. E-mail: ykyap@mtu.edu
First published on 14th September 2010
Abstract
This article provides a concise review of the recent research advancements in boron nitride nanotubes (BNNTs) with a comprehensive list of references. As the motivation of the field, we first summarize some of the attractive properties and potential applications of BNNTs. Then, latest discoveries on the properties, applications, and synthesis of BNNTs are discussed. In particular, we focus on low-temperature and patterned growth, and mass production of BNNTs, since these are the major challenges that have hindered investigation of the properties and application of BNNTs for the past decade. Finally, perspectives of future research on BNNTs are discussed.
 Jiesheng Wang | Dr Jiesheng Wang is a postdoctoral research scientist at Michigan Technological University (MTU). He received his BS degree in physics (1999) from Ji Lin University (China) and his PhD degree in physics (2007) from MTU. Dr Wang demonstrated the first success in patterned growth of boron nitride nanotubes (BNNTs) in 2005 based on a phase-selective approach. He has continued research interest on the growth, characterization, and application of BNNTs and related nanostructures. |
 Chee Huei Lee | Mr Chee Huei Lee is a PhD candidate at Michigan Technological University. He received his BS degree from the University Technology of Malaysia in 2002 and the MS degree from the University of Malaya (Malaysia) in 2004. Mr Lee demonstrated controlled growth of boron nitride nanotubes (BNNTs) by simple catalytic chemical vapor deposition (CCVD) by the growth vapor trapping approach. He has also discovered the superhydrophobicity of BNNT films. He is currently exploring novel properties and applications of BNNTs. |
 Yoke Khin Yap | Professor Yoke Khin Yap received his PhD in 1999 (Osaka University, Japan) while being sponsored as a “Monbusho” scholar. He was a fellow of the Japan Society for the Promotion of Science before becoming an Assistant Professor at Michigan Technological University (MTU) in 2002. Professor Yap received the National Science Foundation CAREER Award in 2005. He was the first elected Chair of the user group of the Center for Nanophase Materials Sciences (CNMS) at Oak Ridge National Laboratory (ORNL) in 2008. Professor Yap received his BSc (1992) and MSc (1994) degrees from the University of Malaya (Malaysia). |
1. Introduction
The unique structural properties of carbon nanotubes (CNTs) have stimulated tremendous interest in the extraordinary properties of nanotubular structures.1 Boron nitride nanotubes (BNNTs) are structurally similar to CNTs and consist of co-axial hexagonal boron nitride (h-BN) networks.2,3 As shown in Fig. 1, the possible chiral vectors (n, m) for single walled BNNTs on a h-BN sheet can be assigned identically, as for single walled CNTs.4 For example, the appearance of single-walled CNT (10, 0) and BNNT (10, 0) are shown in Fig. 2. As is shown, both CNTs and BNNTs have seamless structures, without dangling bonds on their surfaces. In fact, they are the strongest light-weight nanomaterials on Earth, with a Young's modulus of ∼1.2 TPa.5,6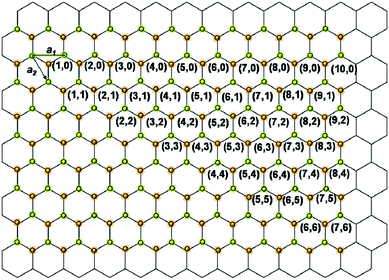 | ||
| Fig. 1 Vectors (n, m) for single-walled BNNT on a h-BN sheet. | ||
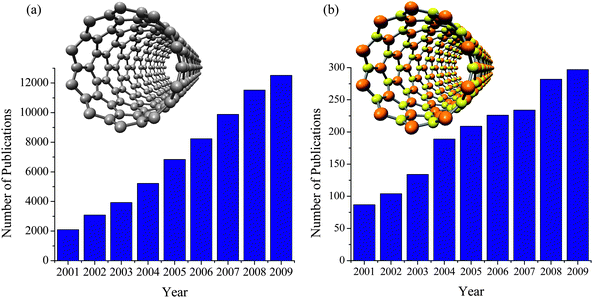 | ||
| Fig. 2 The number of publications on (a) carbon nanotubes, and (b) boron nitride nanotubes (based on the ISI Web of Knowledge). The models of single-walled carbon nanotube (10, 0) and boron nitride nanotube (10, 0) are shown as the insets in (a) and (b), respectively. (c) The vectors (m, n) of single walled BNNTs on a h-BN sheet. | ||
Despite this extraordinary property, research activities on CNTs are much more popular than that of BNNTs. As shown in Fig. 2(a) and 2(b), the number of papers published on the subject of CNTs and BNNTs have both been increasing since 2001. However, the numbers of publications on CNTs in 2001 and 2009 are 24- and 42- times larger than those of BNNTs, respectively. Among the major reasons for this situation is that the syntheses of BNNTs are much tougher than that of CNTs. As discussed in a recent review, the growth of BNNTs usually requires excessively high growth temperatures (>1300 °C), involving special instrumentation or dangerous chemicals.7 This has been a major issue for more than a decade, and has limited the availability of BNNT samples for experimental study of their properties and applications.
It is apparent that a breakthrough in the synthesis of BNNTs would potentially enhance the research activities of BNNTs. Effective growth techniques of BNNTs with common instrumentation that is available in research laboratories are highly desired. In view of this, we focus on summarizing recent research advancements in the synthesis of BNNTs. As discussed later in this article, low-temperature and patterned growth of BNNTs were demonstrated by plasma-enhanced pulsed-laser deposition (PE-PLD)8 and plasma-enhanced chemical vapor deposition (PE-CVD).9–11 Later, high-yield synthesis of BNNTs was recently reported by using pressurized vapor/condenser method (PVC).12 Finally, a simple growth technique of BNNTs with high structural properties and chemical purity was demonstrated by catalytic chemical vapor deposition (CCVD),13 much like the growth of CNTs.14 We hope to highlight some of these growth techniques so as to stimulate more attention and research efforts on the growth of BNNTs and the investigation on their novel properties and applications.
In the coming sections, we will first review the properties and potential applications of BNNTs, followed by the historical evolution on the synthesis of BNNTs. Recent advancements in the growth of BNNTs by PE-CVD, PVC and CCVD will then be highlighted. Readers are encouraged to refer to more comprehensive review articles on BNNTs.7,15–17
2. Properties and potential applications of BNNTs
As mentioned, BNNTs have an exceptional stiffness, with a Young's modulus of ∼1.2 TPa.6,18 Therefore, they are promising for reinforcement of polymeric composites and ceramics.19 These will lead to applications in lighter, faster and affordable transportation, as well as lightweight armors. However, the other physical and chemical properties of BNNTs are different from those of CNTs, making BNNTs complementary nanomaterials for the applications of CNTs at the cutting edge of nanotechnology.The unique nature of BNNTs is associated with the nature of their partial ionic bonds. For example, BNNTs are preferable to CNTs for hydrogen storage because ionic B–N bonds induce a dipole moment between hydrogen and the nanotubes for stronger binding.20 Experimentally, multi-walled and bamboo-like BNNTs can take up 1.8 wt% and 2.6 wt% of hydrogen, respectively, at ∼10 MPa at room temperature.21 The storage rates can even be higher (4.2 wt%) at ∼10 MPa for collapsed BNNTs.22 These are at least comparable to the reported capacity for multi-walled CNTs.23
Because of this ionic bonding nature, BNNTs are wide band gap materials like other nitride materials. BNNTs possess electronic properties that are not sensitive to the change of their diameter, chirality and number of tubular walls.2,3 In addition, zig-zag single-walled BNNTs are expected to have a direct band gap while armchair single-walled BNNTs will have an indirect band gap.2 An earlier study by tunneling spectroscopy estimated that BNNTs will have band gap in the range of ∼4.5–4.9 eV.8,24 In fact, BNNTs are a good electrical insulator at room temperature.13 They have been experimentally utilized to shield many kinds of nanowires, such as non-metal SiC nanowires25 and Co and Ni nanowires.26 More interestingly, the band gap can be theoretically engineered by carbon substitution,27 radial deformation28 or applying transverse electric fields.29–31 Doping of BNNTs by fluorine, for example, can cause their resistivity to reduce to 0.2–0.6 Ω cm.32 However, such an approach produced defective BNNTs.
The magnetic properties of BNNTs are attractive. As indicated by theoretical calculations, spontaneous magnetization for BNNTs can be induced by carbon-doping (i.e., substituting either B or N atoms in BNNTs).33 Moreover, those properties can be tuned by chemisorption treatment. For example, fluorine adsorption on B atoms in BNNTs can induce strong magnetism and the magnetic moment increases with increasing F coverage.34 Experimentally, magnetic behavior of BNNTs could be introduced by functionalization with Fe3O4 nanoparticles35 or due to the presence of Fe catalyst particles.36 In addition, BNNTs were also excellent piezoelectric systems.37 A recent calculation showed that an axial deformation of up to 1% can be induced by an electric field with a strength of 10 V nm−1 due to both the converse piezoelectric and electrostatic effects.38 This is about nine times larger than that in traditional piezoelectric ceramics.
BNNTs have a high thermal conductivity (Table 1). In fact, BNNTs were predicted to have a larger thermal conductivity at low temperatures45 than CNTs under the same conditions of phonon mean free path as CNTs. This will make BNNTs useful for nanoelectronics applications due to the demand for heat dissipation in nanoelectronics operations. On the other hand, BNNTs have a higher chemical stability than CNTs at elevated temperatures.41,46,47 It was shown that purified BNNTs can survive up to 1100 °C in air.47 In fact, because of their excellent thermal and chemical stability, boron nitride has been used as high-temperature ceramic for years.
| Boron nitride nanotubes (BNNTs) | Carbon nanotubes (CNTs) | |
|---|---|---|
| Color | White | Black |
| Electrical properties | Almost constant wide band gap (∼6.0 eV)13 | Semimetallic or semiconducting, depending on the chirality, diameter, and number of tubular walls39,40 |
| Mechanical properties (Young's modulus) | ∼1.18 TPa6 | ∼1.25 TPa5 |
| Chemical stability | Stable up to 1100 °C in air41 | Stable up to ∼500 °C in air41 |
| Thermal conductivity | ∼350 W mK−1 (for outer diameter of 30–40 nm tube)42 | ∼ 300 W mK−1 (diameter of 30–40 nm).43 >3000 W mK−1 (diameter of ∼14 nm).43 |
| Optical properties | Applicable in the deep-UV regime13 | Applicable in the near-IR regime44 |
Chemically functionalized BNNTs are important for various applications in nanotechnology. Because of their similar structural properties, BNNTs provide an alternative choice to CNTs for mechanical reinforcement and improving thermal conductivity in polymeric composites. Intensive research on BNNTs’ functionalization have been reported by Zhi et al. and a review of their works can be found in literature.48 In general, non-covalent functionalization of BNNTs is simpler by π–π interaction, similar to that of CNTs. However, covalent functionalization is more challenging due to the chemical inertness of BNNTs. Recent work shows that surface modification of BNNTs49 or alignment of BNNTs in polymer films50 improve the thermal conductivity of the polymer films.43
Optical applications for BNNTs are highly anticipated since BN powder and thin films have already been demonstrated as a promising candidate for deep-blue and UV applications. Cathodeluminescence (CL) of an individual multiwalled BNNT shows a strong band at 320 nm (∼3.9 eV) and a weak peak at 223 (∼5.3 eV).51 Later, optical absorption measurement showed that BNNTs could have an optical band gap at ∼210 nm (∼5.9 eV) and two sub-band absorptions at ∼260 nm (∼4.8 eV) and ∼325 nm (∼3.8 eV).52 The optical band gap of high-quality BNNTs was recently found to be ∼6.0 eV (absorption peak at ∼205 nm) without sub-band absorption.13 Owing to their wide band-gap, pristine BNNTs are insulators.13 However, it was recently found that bending of an individual BNNT could convert the insulating behavior into that of a semiconductor.53,54
Recent theory predicts that a single-walled BNNT embedded in silicon nitride membrane can conduct water molecules while rejecting ions, thus producing an efficient desalinator.55 Single-walled BNNT (5, 5) may achieve 100% salt rejection at concentrations as high as 1 M. On the other hand, Lee et al. discovered superhydrophobic behavior of BNNTs grown on an Si substrate.56 Partially vertically aligned BNNT films were found to exhibit strong water repellency (Fig. 3). It was found that nanoscopic surface roughness was the origin of this superhydrophobicity since BN thin films were hydrophilic materials. Randomly orientated BNNTs57 and BN nanosheets58 were later reported, confirming the same kind of behavior. Since BNNTs are chemically and structurally robust, they could be useful as a self-cleaning, protective and anticorrosive surface under rigorous chemical and thermal conditions.
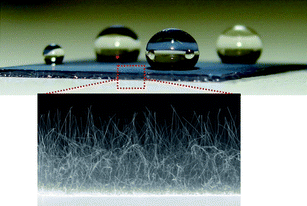 | ||
| Fig. 3 Nearly spherical water droplets on surfaces coated with BNNTs indicating the superhydrophobicity. The zoomed-in image shows the cross-sectional view of the BNNTs on the substrate. | ||
Recent experiments showed that BNNTs could be noncytotoxic, suggesting that they are emerging as new biocompatible materials for biomedical, therapeutic or diagnostic applications.36,59,60 Furthermore, incorporating BNNTs into biodegradable polymers, such as polycaprolactone (PLC) and polylactic acid (PLA), with improved mechanical properties and biocompatibility, was demonstrated to display increased osteoblast cell viability and its potential application in orthopedic scaffolds.61 BNNTs were also demonstrated as nanotools to enable cell electropermeabilization for enhanced cellular uptake of molecules with very low electric fields.62 Cell permeabilization is a technique for introducing various molecules and drugs into cells, which currently requires high-voltage generators. In addition, it is proposed that BNNTs may be the boron carriers in boron neutron capture therapy (BNCT).63 If this is true, BNNTs delivered into cancer cells may absorb neutrons from an external neutron beam source and generate localized alpha radiation to kill the tumors. In addition, it was recently shown that uniformly distributed Fe3O4 nanoparticles could be formed on the surfaces of BNNTs.35 This technique introduced magnetic behavior into BNNTs and might find a wide range of potential applications in magnetorheological devices, microelectromechanical systems (MEMS) and magnetically targeted drug delivery.
3. The synthesis of BNNTs
The growth of BNNTs has been demonstrated by various techniques, including arc-discharge,64 laser vaporization,65–67 the BN substitution method from CNT templates,68 chemical vapor deposition (CVD) using borazine,69,70 induction heating boron oxide CVD (BOCVD),71,72 high-temperature ball milling,73 and combustion of Fe4N/B powders.74 The BOCVD approach has been used for the mass production of well crystallized multi-walled BNNTs by the use of an induction furnace with a high growth temperature gradient. The high-temperature ball milling technique is a potential mass production approach, but results in bamboo-like structures. In 2005, Wang et al. showed that BNNTs could be directly grown on substrates by a plasma-enhanced pulsed-laser deposition (PE-PLD) technique at 600 °C.8 It is apparent that the use of plasma has eliminated the need for high growth temperatures and has led to the first success in the vertically-aligned growth mode. Impurity-free and highly crystallized BNNTs can be grown at desired patterns as defined by the Fe catalyst films. However, all the techniques mentioned so far involved either specific instrumentation, high growth temperatures (>1300 °C), and/or dangerous chemistry.4. Recent advancement on low-temperature growth and high-yield synthesis
4.1. Low-temperature and patterned growth by plasma techniques
Low temperature synthesis of BNNTs is important for experimental investigation of the properties and applications of BNNTs. As discussed, the PE-PLD technique was the first success in patterned growth of high-quality BNNTs at 600 °C. The as-grown BNNTs are impurity-free and have capped tips and highly-crystalline structures. However, the lengths of these BNNTs are relatively short (up to one micrometre). The patterned growth of BNNTs was recently demonstrated by a microwave plasma chemical vapor deposition approach. The B2H6–NH3–H2 gas system and an Ni-coated SiO2 substrate were used.9 BNNTs produced by this approach at 800 °C were still low in density and crystallinity. A similar approach was reported later using B2H6, NH3, O2, and Ar gases and an Al/Fe or Fe/SiO2–Al2O3 catalyst.10,11 A quartz tube furnace was used as a reactor with an integrated inductively coupled plasma (ICP) setup. Again, however, this plasma approach produced low density and relatively defective BNNTs at 900 °C.4.2. Patterned growth by thermal annealing and catalytic CVD
Patterned growth of BN nanorods using Fe(NO3)3 catalyst was also demonstrated by thermal annealing of milled boron carbide powders in flowing nitrogen at 1300 °C.75 However, the solution-based catalyst caused spatial diffusion during annealing and the growth region was not well defined. Like the products of ball milling,73 these BN nanorods were bamboo-like in structure.As discussed so far, there are no simple techniques for the growth of high-quality BNNTs such as the popular thermal CVD for the growth of CNTs. Recently, Lee et al. succeeded in growing BNNTs in a conventional tube furnace by thermal CVD at 1100–1200 °C.52 This was obtained by the growth vapor trapping (GVT) approach and the well-developed BOCVD chemical route. This success has provided a convenient technique for growing BNNTs in a regular tube furnace without complicated chemical reactions. Later, Lee et al. demonstrated that high quality BNNTs could be grown on substrates with desired patterns.13 The experimental setup for this catalytic chemical vapor deposition (CCVD) technique is illustrated in Fig. 4. For patterned growth of BNNTs, the substrate was coated with a 30 nm thick Al2O3 film, followed by a 10 nm thick MgO, Ni or Fe film, using a pulsed laser deposition (PLD) technique, with a shadow mask to define the growth areas. Subsequently, they were placed on the top of a combustion boat, in which B, MgO and FeO precursors (molar ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were filled. The assembly was then loaded inside a closed-end quartz tube to be heated up in a horizontal tube furnace at 1200 °C with an ammonia flow of 200 to 350 sccm for 30 min. The closed-end quartz tube was to avoid direct NH3 flow through the reaction zone and thus helped to trap the growth species to generate the so-called GVT approach. As a result, the as-grown BNNTs by this controlled CCVD approach had high purity and structural quality with an optical band gap of ∼6 eV without significant sub-band levels. This approach has made the synthesis of BNNTs as convenient as the growth of CNTs.14
1) were filled. The assembly was then loaded inside a closed-end quartz tube to be heated up in a horizontal tube furnace at 1200 °C with an ammonia flow of 200 to 350 sccm for 30 min. The closed-end quartz tube was to avoid direct NH3 flow through the reaction zone and thus helped to trap the growth species to generate the so-called GVT approach. As a result, the as-grown BNNTs by this controlled CCVD approach had high purity and structural quality with an optical band gap of ∼6 eV without significant sub-band levels. This approach has made the synthesis of BNNTs as convenient as the growth of CNTs.14
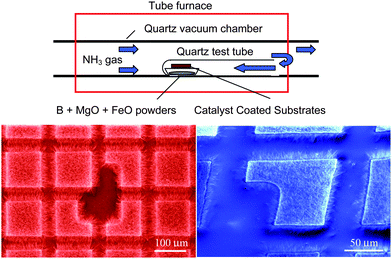 | ||
| Fig. 4 Experimental setup for catalytic chemical vapor deposition and the resultant patterned growth. | ||
4.2. High-yield synthesis
In addition to the BOCVD, a new mass production approach was recently reported via the pressurized vapor/condenser method (PVC).12 In brief, a 1 kW free-electron laser (wavelength ∼1.6 μm) or a kW-class CO2 laser (wavelength ∼10.6 μm) was used as the evaporation source of a boron target inside a chamber filled with nitrogen gas (pressure ∼2–20 bars). This will produce an upward stream of hot boron vapor (∼4000 °C) in the high-pressure nitrogen gas. Condensation of the boron vapors will form liquid boron droplets which serve as nucleation sites for the growth of BNNT networks that interlock to form fibrils. As shown in Fig. 5(a), a cotton-like fibril of BNNTs can be finger-twisted into a yarn with a diameter of about one millimetre. In fact, the as-grown products are an entangled network of branching BNNTs and tube bundles as shown in Fig. 5(b) and 5(c). These BNNTs often linked and anchored by boron nanodroplets (dark particles in Fig. 5(d)). Fig. 5(e) shows transmission electron microscopy (TEM) images. As shown, most of these BNNTs have 2 to 5 walls, although single-walled BNNTs can also be found. It is apparent that the PVC approach is based on spontaneous nucleation (or condensation) of the boron nanoparticles and thus the diameters of BNNTs are not controllable.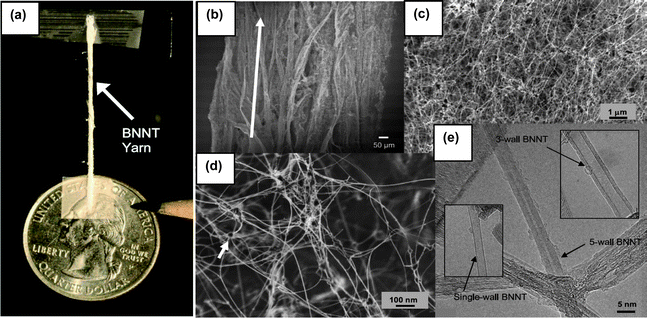 | ||
| Fig. 5 (a) A ∼1 mm diameter, 3 cm long BNNT yarn spun directly from as grown BNNT raw material. Scanning (b)–(d) and transmission (e) electron microscope images showing the structures of the as grown BNNT fibrils at increasing magnifications (modified from ref. 12). | ||
5. Conclusion and perspective
In addition to their attractive properties, research on CNTs has become popular due to the fact that many laboratories are capable of producing their own samples for various investigations. As discussed, the progress of research on BNNTs is still limited by the availability of BNNT samples for widespread investigation of their properties and applications. Many novel properties were predicted without sufficient experimental verification. It is apparent that effective growth of BNNTs is important for further research development. As evidenced, the success of growing large quantity of BNNTs by BOCVD has led to a series of experimental researches on the properties and applications of BNNTs.15,19,32,35,47–51,76–80 However, the specific design of the induction furnace system for BOCVD is not commonly accessible and thus hinders widespread use of the technique by other research groups.Recent progress on the synthesis of BNNTs has started to generate a new understanding of the properties of BNNTs and is likely to promote more research interest in the field. For example, the GVT approach52 has enabled the effective growth of BNNTs in resistive tube furnaces that are regularly use for the synthesis of CNTs81–84 and ZnO nanostructures.85–87 This success has begun to initiate experimental study of the chemical,88 electrical,53 superhydrophobicity,56 and patterned growth13 of BNNTs. The GVT approach is expected to gain popularity, as the technique enables the growth of BNNTs much like the synthesis of CNTs.14 On the other hand, the PVC approach seems to be the most effective growth technique for mass production of BNNTs.12 The next step needed will be to untangle the BNNT fibril from the chemically inert boron nanoparticles. It is apparent that significant boron nitride powders might be contained in the as-grown samples, as PVC is a physical vapor evaporation approach. Solutions for these will lead toward large scale application of BNNTs in reinforcement purposes, for example in polymeric composites. Obviously, research interest in the synthesis of BNNTs will continue, especially toward the controlled growth of single-walled BNNTs, which can only be produced so far by laser evaporation.
As evidenced, there is growing interest on using BNNTs for biological and chemical applications.59,60,63,89 In addition to their physical properties (mechanical, thermal, and electrical, magnetic, etc.), a new understanding of the non-physical behaviors of BNNTs will be the next frontier for future BNNT research.
Acknowledgements
Yoke Khin Yap acknowledges supports from the National Science Foundation CAREER award (award number 0447555) and the U. S. Department of Energy, the Office of Basic Energy Sciences (Grant No. DE-FG02-06ER46294).References
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- X. Blase, A. Rubio, S. G. Louie and M. L. Cohen, Europhys. Lett., 1994, 28, 335–340 CrossRef CAS.
- A. Rubio, J. L. Corkill and M. L. Cohen, Phys. Rev. B: Condens. Matter, 1994, 49, 5081–5084 CrossRef CAS.
- C. H. Lee, V. K. Kayastha, J. Wang, and Y. K. Yap, in B–C–N Nanotubes and Related Nanostructures, Lecture Notes in Nanoscale Science and Technology, ed. Y. K. Yap, Springer, New York, 2009, ch. 1, p. 1–22 Search PubMed.
- A. Krishnan, E. Dujardin, T. W. Ebbesen, P. N. Yianilos and M. M. J. Treacy, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 14013 CrossRef.
- N. G. Chopra and A. Zettl, Solid State Commun., 1998, 105, 297–300 CrossRef CAS.
- J. Wang, C. H. Lee, Y. Bando, D. Golberg, and Y. K. Yap, in B–C–N Nanotubes and Related Nanostructures, Lecture Notes in Nanoscale Science and Technology, ed. Y. K. Yap, Springer, New York, 2009, ch. 2, p. 23–44 Search PubMed.
- J. Wang, V. K. Kayastha, Y. K. Yap, Z. Fan, J. G. Lu, Z. Pan, I. N. Ivanov, A. A. Puretzky and D. B. Geohegan, Nano Lett., 2005, 5, 2528–2532 CrossRef CAS.
- L. Guo and R. N. Singh, Nanotechnology, 2008, 19, 065601 CrossRef.
- C. Y. Su, Z. Y. Juang, K. F. Chen, B. M. Cheng, F. R. Chen, K. C. Leou and C. H. Tsai, J. Phys. Chem. C, 2009, 113, 14681–14688 CrossRef CAS.
- C. Y. Su, W. Y. Chu, Z. Y. Juang, K. F. Chen, B. M. Cheng, F. R. Chen, K. C. Leou and C. H. Tsai, J. Phys. Chem. C, 2009, 113, 14732–14738 CrossRef CAS.
- M. W. Smith, K. C. Jordan, C. Park, J.-W. Kim, P. T. Lillehei, R. Crooks and J. S. Harrison, Nanotechnology, 2009, 20, 505604 CrossRef.
- C. H. Lee, M. Xie, V. Kayastha, J. Wang and Y. K. Yap, Chem. Mater., 2010, 22, 1782–1787 CrossRef CAS.
- C. Sealy, Nano Today, 2010, 5, 80–81 CrossRef.
- D. Golberg, Y. Bando, C. C. Tang and C. Y. Zhi, Adv. Mater., 2007, 19, 2413–2432 CrossRef CAS.
- M. Terrones, J. M. Romo-Herrera, E. Cruz-Silva, F. López-Urías, E. Muñoz-Sandoval, J. J. Velázquez-Salazar, H. Terrones, Y. Bando and D. Golberg, Mater. Today, 2007, 10, 30–38 CrossRef.
- R. Arenal, X. Blase and A. Loiseau, Adv. Phys., 2010, 59, 101–179 CrossRef CAS.
- E. Hernández, C. Goze, P. Bernier and A. Rubio, Phys. Rev. Lett., 1998, 80, 4502 CrossRef CAS.
- Q. Huang, Y. Bando, X. Xu, T. Nishimura, C. Zhi, C. Tang, F. Xu, L. Gao and D. Golberg, Nanotechnology, 2007, 18, 485706 CrossRef.
- G. Mpourmpakis and G. E. Froudakis, Catal. Today, 2007, 120, 341–345 CrossRef CAS.
- P. Wang, S. Orimo, T. Matsushima, H. Fujii and G. Majer, Appl. Phys. Lett., 2002, 80, 318–320 CrossRef CAS.
- C. Tang, Y. Bando, X. Ding, S. Qi and D. Golberg, J. Am. Chem. Soc., 2002, 124, 14550–14551 CrossRef CAS.
- C. Liu, Y. Y. Fan, M. Liu, H. T. Cong, H. M. Cheng and M. S. Dresselhaus, Science, 1999, 286, 1127–1129 CrossRef CAS.
- R. Czerw, S. Webster, D. L. Carroll, S. M. C. Vieira, P. R. Birkett, C. A. Rego and S. Roth, Appl. Phys. Lett., 2003, 83, 1617–1619 CrossRef CAS.
- Y. Li, P. S. Dorozhkin, Y. Bando and D. Golberg, Adv. Mater., 2005, 17, 545–549 CrossRef CAS.
- Y. Bando, K. Ogawa and D. Golberg, Chem. Phys. Lett., 2001, 347, 349–354 CrossRef CAS.
- Y. Miyamoto, A. Rubio, M. L. Cohen and S. G. Louie, Phys. Rev. B: Condens. Matter, 1994, 50, 4976 CrossRef CAS.
- Y.-H. Kim, K. J. Chang and S. G. Louie, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 205408 CrossRef.
- K. H. Khoo, M. S. C. Mazzoni and S. G. Louie, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 201401 CrossRef.
- C. W. Chen, M. H. Lee and S. J. Clark, Nanotechnology, 2004, 15, 1837–1843 CrossRef CAS.
- M. Ishigami, J. D. Sau, S. Aloni, M. L. Cohen and A. Zettl, Phys. Rev. Lett., 2005, 94, 056804 CrossRef.
- C. Tang, Y. Bando, Y. Huang, S. Yue, C. Gu, F. Xu and D. Golberg, J. Am. Chem. Soc., 2005, 127, 6552–6553 CrossRef CAS.
- R. Q. Wu, L. Liu, G. W. Peng and Y. P. Feng, Appl. Phys. Lett., 2005, 86, 122510 CrossRef.
- F. Li, Z. Zhu, X. Yao, G. Lu, M. Zhao, Y. Xia and Y. Chen, Appl. Phys. Lett., 2008, 92, 102515 CrossRef.
- Y. Huang, J. Lin, Y. Bando, C. C. Tang, C. Y. Zhi, Y. G. Shi, E. Takayama-Muromachi and D. Golberg, J. Mater. Chem., 2010, 20, 1007–1011 RSC.
- G. Ciofani, V. Raffa, J. Yu, Y. Chen, Y. Obata, S. Takeoka, A. Menciassi and A. Cuschieri, Curr. Nanosci., 2009, 5, 33–38 Search PubMed.
- S. M. Nakhmanson, A. Calzolari, V. Meunier, J. Bernholc and M. Buongiorno Nardelli, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 235406 CrossRef.
- D. Yitao, W. Guo, Z. Zhang, B. Zhou and C. Tang, J. Phys. D: Appl. Phys., 2009, 42, 085403 CrossRef.
- N. Hamada, S. Sawada and A. Oshiyama, Phys. Rev. Lett., 1992, 68, 1579–1581 CrossRef CAS.
- R. Saito, M. Fujita, G. Dresselhaus and M. S. Dresselhaus, Appl. Phys. Lett., 1992, 60, 2204–2206 CrossRef CAS.
- D. Golberg, Y. Bando, K. Kurashima and T. Sato, Scr. Mater., 2001, 44, 1561–1565 CrossRef CAS.
- C. W. Chang, A. M. Fennimore, A. Afanasiev, D. Okawa, T. Ikuno, H. Garcia, D. Li, A. Majumdar and A. Zettl, Phys. Rev. Lett., 2006, 97, 085901 CrossRef CAS.
- P. Kim, L. Shi, A. Majumdar and P. L. McEuen, Phys. Rev. Lett., 2001, 87, 215502 CrossRef CAS.
- J. Chen, V. Perebeinos, M. Freitag, J. Tsang, Q. Fu, J. Liu and P. Avouris, Science, 2005, 310, 1171–1174 CrossRef CAS.
- Y. Xiao, X. H. Yan, J. X. Cao, J. W. Ding, Y. L. Mao and J. Xiang, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 205415 CrossRef.
- Y. Chen, J. Zou, S. J. Campbell and G. Le Caer, Appl. Phys. Lett., 2004, 84, 2430–2432 CrossRef CAS.
- C. Zhi, Y. Bando, C. Tang, R. Xie, T. Sekiguchi and D. Golberg, J. Am. Chem. Soc., 2005, 127, 15996–15997 CrossRef CAS.
- C. Y. Zhi, Y. Bando, C. C. Tang, Q. Huang and D. Golberg, J. Mater. Chem., 2008, 18, 3900–3908 RSC.
- T. Terao, Y. Bando, M. Mitome, C. Zhi, C. Tang and D. Golberg, J. Phys. Chem. C, 2009, 113, 13605–13609 CrossRef CAS.
- T. Terao, C. Zhi, Y. Bando, M. Mitome, C. Tang and D. Golberg, J. Phys. Chem. C, 2010, 114, 4340–4344 CrossRef CAS.
- P. Jaffrennou, J. Barjon, T. Schmid, L. Museur, A. Kanaev, J. S. Lauret, C. Y. Zhi, C. Tang, Y. Bando, D. Golberg, B. Attal-Tretout, F. Ducastelle and A. Loiseau, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 235422 CrossRef.
- C. H. Lee, J. Wang, V. K. Kayatsha, J. Y. Huang and Y. K. Yap, Nanotechnology, 2008, 19, 455605 CrossRef.
- H. M. Ghassemi, C. H. Lee, Y. K. Yap and R. S. Yassar, JOM, 2010, 62, 69–73 CrossRef CAS.
- X. D. Bai, D. Golberg, Y. Bando, C. Y. Zhi, C. C. Tang, M. Mitome and K. Kurashima, Nano Lett., 2007, 7, 632–637 CrossRef CAS.
- T. A. Hilder, D. Gordon and S. H. Chung, Small, 2009, 5, 2183–2190 CrossRef CAS.
- C. H. Lee, J. Drelich and Y. K. Yap, Langmuir, 2009, 25, 4853–4860 CrossRef CAS.
- L. H. Li and Y. Chen, Langmuir, 2010, 26, 5135–5140 CrossRef CAS.
- J. Yu, L. Qin, Y. Hao, S. Kuang, X. Bai, Y.-M. Chong, W. Zhang and E. Wang, ACS Nano, 2010, 4, 414–422 CrossRef CAS.
- X. Chen, P. Wu, M. Rousseas, D. Okawa, Z. Gartner, A. Zettl and C. R. Bertozzi, J. Am. Chem. Soc., 2009, 131, 890–891 CrossRef CAS.
- G. Ciofani, S. Danti, D. D'Alessandro, S. Moscato and A. Menciassi, Biochem. Biophys. Res. Commun., 2010, 394, 405–411 CrossRef CAS.
- D. Lahiri, F. Rouzaud, T. Richard, A. K. Keshri, S. R. Bakshi, L. Kos and A. Agarwal, Acta Biomater., 2010, 6, 3524, DOI:10.1016/j.actbio.2010.02.044.
- V. Raffa, G. Ciofani and A. Cuschieri, Nanotechnology, 2009, 20, 075104 CrossRef CAS.
- G. Ciofani, V. Raffa, A. Menciassi and A. Cuschieri, Nanoscale Res. Lett., 2009, 4, 113–121 Search PubMed.
- N. G. Chopra, R. J. Luyken, K. Cherrey, V. H. Crespi, M. L. Cohen, S. G. Louie and A. Zettl, Science, 1995, 269, 966–967 CrossRef CAS.
- R. S. Lee, J. Gavillet, M. L.d. l. Chapelle, A. Loiseau, J. L. Cochon, D. Pigache, J. Thibault and F. Willaime, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 121405 CrossRef.
- D. P. Yu, X. S. Sun, C. S. Lee, I. Bello, S. T. Lee, H. D. Gu, K. M. Leung, G. W. Zhou, Z. F. Dong and Z. Zhang, Appl. Phys. Lett., 1998, 72, 1966–1968 CrossRef CAS.
- R. Arenal, O. Stephan, J. L. Cochon and A. Loiseau, J. Am. Chem. Soc., 2007, 129, 16183–16189 CrossRef CAS.
- W. Han, Y. Bando, K. Kurashima and T. Sato, Appl. Phys. Lett., 1998, 73, 3085–3087 CrossRef CAS.
- O. R. Lourie, C. R. Jones, B. M. Bartlett, P. C. Gibbons, R. S. Ruoff and W. E. Buhro, Chem. Mater., 2000, 12, 1808–1810 CrossRef CAS.
- M. J. Kim, S. Chatterjee, S. M. Kim, E. A. Stach, M. G. Bradley, M. J. Pender, L. G. Sneddon and B. Maruyama, Nano Lett., 2008, 8, 3298–3302 CrossRef CAS.
- C. Tang, Y. Bando, T. Sato and K. Kurashima, Chem. Commun., 2002, 1290–1291 RSC.
- C. Zhi, Y. Bando, C. Tan and D. Golberg, Solid State Commun., 2005, 135, 67–70 CrossRef CAS.
- Y. Chen, L. T. Chadderton, J. FitzGerald and J. S. Williams, Appl. Phys. Lett., 1999, 74, 2960–2962 CrossRef CAS.
- T. Oku, N. Koi and K. Suganuma, J. Phys. Chem. Solids, 2008, 69, 1228–1231 CrossRef CAS.
- H. Z. Zhang, M. R. Phillips, J. D. F. Gerald, J. Yu and Y. Chen, Appl. Phys. Lett., 2006, 88, 093117–3 CrossRef.
- D. Golberg, P. M. F. J. Costa, O. Lourie, M. Mitome, X. D. Bai, K. Kurashima, C. Y. Zhi, C. C. Tang and Y. Bando, Nano Lett., 2007, 7, 2146–2151 CrossRef.
- C. Zhi, Y. Bando, C. Tang, H. Kuwahara and D. Golberg, Adv. Mater., 2009, 21, 2889–2893 CrossRef CAS.
- C. Y. Zhi, Y. Bando, T. Terao, C. C. Tang, H. Kuwahara and D. Golberg, Adv. Funct. Mater., 2009, 19, 1857–1862 CrossRef CAS.
- C. Y. Zhi, Y. Bando, C. C. Tang, S. Honda, K. Sato, H. Kuwahara and D. Golberg, Angew. Chem., Int. Ed., 2005, 44, 7932–7935 CrossRef CAS.
- C. Zhi, Y. Bando, C. Tang and D. Golberg, J. Am. Chem. Soc., 2005, 127, 17144–17145 CrossRef CAS.
- J. P. Moscatello, J. S. Wang, B. Ulmen, S. L. Mensah, M. Xie, S. Wu, A. Pandey, C. H. Lee, A. Prasad, V. K. Kayastha and Y. K. Yap, IEEE Sens. J., 2008, 8, 922–929 CrossRef CAS.
- V. K. Kayastha, S. Wu, J. Moscatello and Y. K. Yap, J. Phys. Chem. C, 2007, 111, 10158–10161 CrossRef.
- V. K. Kayastha, Y. K. Yap, Z. Pan, I. N. Ivanov, A. A. Puretzky and D. B. Geohegan, Appl. Phys. Lett., 2005, 86, 253105 CrossRef.
- V. Kayastha, Y. K. Yap, S. Dimovski and Y. Gogotsi, Appl. Phys. Lett., 2004, 85, 3265–3267 CrossRef CAS.
- S. L. Mensah, A. Prasad, J. S. Wang and Y. K. Yap, J. Nanosci. Nanotechnol., 2008, 8, 233–236 CrossRef CAS.
- S. L. Mensah, V. K. Kayastha and Y. K. Yap, J. Phys. Chem. C, 2007, 111, 16092–16095 CrossRef CAS.
- S. L. Mensah, V. K. Kayastha, I. N. Ivanov, D. B. Geohegan and Y. K. Yap, Appl. Phys. Lett., 2007, 90, 113108 CrossRef.
- S. Velayudham, C. H. Lee, M. Xie, D. Blair, N. Bauman, Y. K. Yap, S. A. Green and H. Y. Liu, ACS Appl. Mater. Interfaces, 2010, 2, 104–110 Search PubMed.
- Q. Huang, Y. Bando, L. Zhao, C. Y. Zhi and D. Golberg, Nanotechnology, 2009, 20, 415501 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
