Epitaxial overgrowth of platinum on palladium nanocrystals
Majiong
Jiang
a,
Byungkwon
Lim
*b,
Jing
Tao
c,
Pedro H. C.
Camargo
b,
Chao
Ma
c,
Yimei
Zhu
c and
Younan
Xia
*b
aDepartment of Chemistry, Washington University, St. Louis, Missouri 63130, USA
bDepartment of Biomedical Engineering, Washington University, St. Louis, Missouri 63130, USA. E-mail: xia@biomed.wustl.edu; limb@seas.wustl.edu
cCondensed Matter Physics and Materials Science Department, Brookhaven National Laboratory, Upton, New York, 11973, USA
First published on 29th June 2010
Abstract
This paper describes a systematic study on the epitaxial overgrowth of Pt on well-defined Pd nanocrystals with different shapes (and exposed facets), including regular octahedrons, truncated octahedrons, and cubes. Two different reducing agents, i.e., citric acid and L-ascorbic acid, were evaluated and compared for the reduction of K2PtCl4 in an aqueous solution in the presence of Pd nanocrystal seeds. When citric acid was used as a reducing agent, conformal overgrowth of octahedral Pt shells on regular and truncated octahedrons of Pd led to the formation of Pd-Pt core-shell octahedrons, while non-conformal overgrowth of Pt on cubic Pd seeds resulted in the formation of an incomplete octahedral Pt shell. On the contrary, localized overgrowth of Pt branches was observed when L-ascorbic acid was used as a reducing agent regardless of the facets expressed on the surface of Pd nanocrystal seeds. This work shows that both the binding affinity of a reducing agent to the Pt surface and the reduction kinetics for a Pt precursor play important roles in determining the mode of Pt overgrowth on Pd nanocrystal surface.
1. Introduction
Recently, seed-mediated synthesis of bimetallic nanocrystals consisting of noble metals such as Pt, Pd, Au, and Ag has advanced remarkably, making it possible to generate a wide variety of bimetallic nanostructures with controllable morphologies and compositions. Notable examples of bimetallic nanostructures prepared via heterogeneous, seeded growth include polyhedral nanocrystals with a core-shell structure,1–6 nanorods consisting of nanoparticles deposited at the tips of preformed nanorods,7 and nanodendrites characterized by a dense array of branches made of one metal on a core of another metal.8–12 For the rational design and synthesis of bimetallic nanocrystals with desired compositions, morphologies, and thus properties, understanding the heterogeneous nucleation and growth mechanisms is essential but still at a rudimentary stage. In a seed-mediated synthesis of bimetallic nanocrystals, the mode of heterogeneous nucleation and growth has generally been considered to be mainly determined by physical parameters such as lattice mismatch between core and deposited metals. The roles of other parameters such as surface capping, type of facets exposed on the core nanocrystal, and the reduction kinetics remain largely unexplored.Platinum is a key catalyst invaluable to many types of reactions such as CO oxidation in a catalytic converter, nitric acid production, and petroleum cracking, as well as oxygen reduction reaction (ORR) in a proton-exchange membrane fuel cell.13–18 Since Pt is extremely rare and expensive, it is necessary to maximize the activity of a Pt-based catalyst by engineering its composition and/or morphology in order to reduce the amount of Pt required in a process where it is essential. To this end, the overgrowth of Pt on a single-crystal surface or nanocrystal made of other metals such as Pd and Au has been studied,4,8–11,19 and has shown great improvement in catalytic activity. For instance, a Pt monolayer deposited on a Pd {111} single-crystal surface has shown an enhanced catalytic activity for the ORR relative to pure Pt {111} surface.20 In addition, we have recently demonstrated that a dense array of Pt branches anchored to a Pd nanocrystal core with a truncated octahedral shape provides a much enhanced activity for the ORR than the commercial Pt catalysts.9 Further control of Pt overgrowth on the surface of Pd nanocrystals with well-defined morphologies is expected to provide a promising route to the development of Pt-based catalysts or electrocatalysts with greatly improved activity and cost-effectiveness.
In this study, we systematically investigated the epitaxial overgrowth of Pt on well-defined Pd nanocrystals with different shapes such as regular octahedrons, truncated octahedrons, and cubes. Owing to the negligible lattice mismatch between Pd and Pt (only 0.77%), Pd nanocrystals can be considered as ideal substrates for the epitaxial overgrowth of Pt. Despite previous works on the seeded growth of Pd-Pt bimetallic nanocrystals,4,9–11 there is still no correlation between the final morphology and the capping effect, reduction kinetics, and facets exposed on the seed. This work clearly demonstrates that the mode of epitaxial growth of Pt on Pd nanocrystals can be controlled by employing a surface capping agent and/or manipulating the kinetics for Pt reduction. In practice, such controls can be achieved by judicious selection of a reducing agent involved in the synthesis. Furthermore, the use of Pd nanocrystals with different shapes as the seeds allows us to investigate the behavior of Pt overgrowth as a function of the facets expressed on the surface of Pd nanocrystals. This work not only provides a simple route to the synthesis of Pd-Pt bimetallic nanocrystals with controllable morphologies but also greatly advances our understanding of the mechanisms underlying the heterogeneous nucleation and growth of Pt on Pd nanocrystal surface.
2. Experimental
2.1. Synthesis of Pd nanocrystals
Octahedral nanocrystals of Pd were synthesized by heating 11 mL of an aqueous solution containing poly(vinyl pyrrolidone) (PVP, 105 mg, MW = 55,000, Aldrich), citric acid (60 mg, Aldrich), and Na2PdCl4 (57 mg, Aldrich) at 90 °C in air under magnetic stirring for 26 h. Truncated octahedral nanocrystals of Pd were synthesized by heating 11 mL of an aqueous solution containing PVP (105 mg), L-ascorbic acid (60 mg, Aldrich), and Na2PdCl4 (57 mg) at 100 °C in air under magnetic stirring for 3 h. Nanocubes of Pd were synthesized by heating 11 mL of an aqueous solution containing PVP (105 mg), L-ascorbic acid (60 mg), KBr (300 mg, Fisher), and Na2PdCl4 (57 mg) at 80 °C in air under magnetic stirring for 3 h.2.2. Synthesis of Pd-Pt bimetallic nanocrystals
For the synthesis of Pd-Pt nanocrystals with a core-shell structure, 1 mL of a suspension of the as-prepared Pd nanocrystals was washed with deionized water three times. Then, 1 mL of the suspension and 6 mL of an aqueous solution containing PVP (35 mg) and citric acid (300 mg) were added into a 25-mL, three-necked flask. The mixture was heated to 90 °C in air under magnetic stirring. Meanwhile, K2PtCl4 (13.5 mg, Aldrich) was dissolved at room temperature in 3 mL of deionized water. The aqueous solution of K2PtCl4 was then rapidly injected into the flask by pipette. The reaction mixture was heated at 90 °C in air for 3 h, and then cooled down to room temperature. The synthesis of Pd-Pt nanocrystals with branched Pt nanostructures was performed under the same experimental conditions as in the citric acid-mediated synthesis except that L-ascorbic acid (60 mg) was used as a reducing agent with the increase in the added amount of K2PtCl4 from 13.5 to 27 mg.2.3. Characterization
TEM studies were done using a FEI Tecnai G2 Spirit microscope operated at 120 kV. HRTEM images were obtained with a JEOL 2100F microscope operated at an accelerating voltage of 200 kV. HAADF-STEM images were recorded with a Hitachi HD-2700 microscope.3. Results
Palladium nanocrystals with well-defined shapes, including regular octahedrons, truncated octahedrons, and cubes, were prepared by reducing Na2PdCl4 with a small organic acid such as citric acid or L-ascorbic acid in an aqueous solution as reported previously.21,22 Specifically, Pd octahedrons with edge lengths of 10–15 nm were prepared with citric acid as a reducing agent, while Pd truncated octahedrons (∼9 nm in average size) and nanocubes (∼10 nm in average edge length, including ∼20% of nanobars with an aspect ratio >1) were synthesized with L-ascorbic acid as a reducing agent in the absence and presence of bromide ions, respectively. In the synthesis of Pd nanocubes, bromide ions served as the capping agent to promote the formation of {100} facets. These Pd samples were washed with water to remove the excess stabilizing and capping agent(s) before being used as seeds for Pt overgrowth.We first investigated Pt overgrowth on the surface of Pd octahedrons (Fig. 1a) upon the reduction of K2PtCl4 by citric acid in an aqueous solution. Conformal overgrowth was observed for Pt, producing Pd-Pt core-shell octahedrons. Both transmission electron microscopy (TEM) and high-angle annular dark-field scanning TEM (HAADF-STEM) analyses revealed that the resultant Pd-Pt nanocrystals retained an overall octahedral morphology and were still uniform in terms of shape (Fig. 1, b and c). The energy-dispersive X-ray spectroscopy (EDS) line scanning analysis confirmed a core-shell structure for these Pd-Pt octahedrons (Fig. 1d). Fig. 1e shows a high-resolution TEM (HRTEM) image of a single Pd-Pt core-shell octahedron recorded along the [011] zone axis, in which the Pt shell appears to be slightly darker than the Pd core due to the contrast from the difference in atomic number between Pt and Pd. The image clearly shows that the lattice fringes are coherently extended from the Pd core to the Pt shell, indicating an epitaxial relationship between these two metals. The thickness of the Pt shell was extremely thin, on the scale of 1–2 nm. These results clearly demonstrate the capability to generate octahedral Pt shells via a conformal, epitaxial overgrowth of Pt on the {111} faces of Pd octahedrons (Fig. 1f).
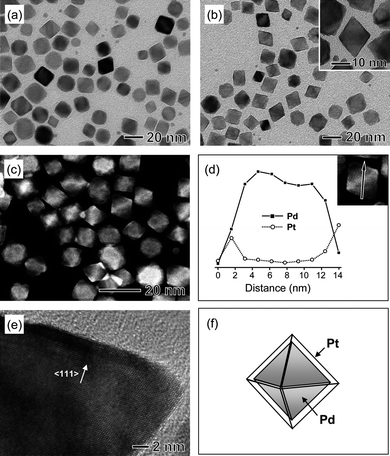 | ||
| Fig. 1 (a) TEM image of Pd octahedrons used as the seeds. (b) TEM and (c) HAADF-STEM images of Pd-Pt core-shell octahedrons synthesized by reducing K2PtCl4 with citric acid in the presence of the octahedral Pd seeds shown in (a). (d) Compositional line profiles of Pd and Pt recorded along the line shown in the HAADF-STEM image (inset). (e) HRTEM image of a Pd-Pt core-shell octahedron. (f) Schematic drawing of a Pd-Pt core-shell octahedron containing a Pd octahedron as the core. The gray color represents the {111} facets of a Pd octahedron. | ||
We also applied truncated octahedrons of Pd (Fig. 2a) as seeds for Pt overgrowth with citric acid as a reducing agent. Unlike a regular octahedron enclosed exclusively by {111} facets, a truncated octahedron is bounded by eight {111} facets and six {100} facets. Conformal overgrowth of Pt was also observed in this case, but interestingly the final product was dominated by an octahedral morphology rather than a truncated one (Fig. 2, b and c). The EDS line scanning analysis also revealed a core-shell structure for this new type of Pd-Pt octahedrons obtained from the truncated octahedral Pd seeds (Fig. 2d). The HRTEM image of a single Pd-Pt octahedron clearly shows that it is a piece of single crystal with the exposed facets being {111} planes (Fig. 2e). Taken together, we can conclude that the resultant Pd-Pt nanocrystals had a core-shell structure consisting of a truncated octahedral Pd core and an octahedral Pt shell (Fig. 2f) when citric acid was used as the reducing agent.
 | ||
| Fig. 2 (a) TEM image of Pd truncated octahedrons used as the seeds. (b) TEM and (c) HAADF-STEM images of Pd-Pt core-shell octahedrons synthesized by reducing K2PtCl4 with citric acid in the presence of the truncated octahedral Pd seeds shown in (a). (d) Compositional line profiles of Pd and Pt recorded along the line shown in the HAADF-STEM image (inset). (e) HRTEM image of a Pd-Pt core-shell octahedron. (f) Schematic drawing of a Pd-Pt core-shell octahedron containing a Pd truncated octahedron as the core. The gray and black colors represent the {111} and {100} facets of a Pd truncated octahedron, respectively. | ||
More interestingly, the overgrowth of Pt on cubic Pd seeds (Fig. 3a) gave Pd-Pt nanocrystals in a “peanut-like” morphology rather than core-shell nanocubes, as shown in Fig. 3, b and c. The EDS line scanning analysis revealed that the surface of each cubic Pd core was completely covered by a Pt shell (Fig. 3d and Fig. 4). The HRTEM image in Fig. 3e clearly shows that both the main and concave-type faces of a Pt shell are predominantly terminated by {111} planes. On the basis of both morphological and structural analyses, this unconventional nanostructure could be described as a bimetallic nanocrystal consisting of a cubic Pd core and an incomplete octahedral Pt shell with concave truncation at the edges (Fig. 3f). TEM analysis of the Pd-Pt sample taken at an early stage of the synthesis revealed that the deposition of Pt atoms was initiated at multiple sites on a cubic Pd seed, primarily at the edges and corners, leading to the formation of Pt bumps on the surface (Fig. 5). These observations indicate that the overgrowth of incomplete octahedral Pt shells on cubic Pd seeds occurred non-conformally through multiple nucleation of Pt on each Pd nanocube.
 | ||
| Fig. 3 (a) TEM image of Pd nanocubes used as the seeds. (b) TEM and (c) HAADF-STEM images of Pd-Pt nanocrystals synthesized by reducing K2PtCl4 with citric acid in the presence of the cubic Pd seeds shown in (a). (d) Compositional line profiles of Pd and Pt recorded along the line shown in the HAADF-STEM image (inset). (e) HRTEM image of a peanut-shaped Pd-Pt nanocrystal. (f) Schematic drawing of a Pd-Pt core-shell nanocrystal consisting of a cubic Pd core and an incomplete octahedral Pt shell with truncation at edges. The black color represents the {100} facets of a Pd nanocube. | ||
 | ||
| Fig. 4 (a) HRTEM image of a peanut-shaped Pd-Pt core-shell nanocrystal and (b–f) EDS spectra obtained from different sites marked on the HRTEM image. | ||
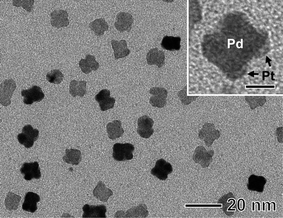 | ||
| Fig. 5 TEM image of the Pd-Pt sample prepared under the same conditions as those in Fig. 3(b) except that the reaction time was shortened to 30 min. The scale bar in the inset corresponds to 5 nm. | ||
In recent studies, we demonstrated that the fast reduction of K2PtCl4 by L-ascorbic acid in the presence of truncated octahedral Pd seeds could result in the formation of a dense array of Pt branches on each Pd seed.9,11 It was also shown that the growth of Pt branches proceeded via the attachment of small Pt nanoparticles that had been formed via homogeneous nucleation in the solution.11 In the current work, Pt branches was also observed to form for Pd-Pt nanocrystals when prepared with L-ascorbic acid as a reducing agent in the presence of Pd seeds with other shapes such as regular octahedron and cube (see Fig. 6). As shown in Fig. 7, both homogeneous and heterogeneous nucleation occurred at an early stage of the synthesis, and the growth of Pt branches also proceeded via the attachment of initially formed, small Pt particles. The interfaces between the Pd core and the Pt branches are coherent in both the Pd-Pt nanostructures derived from the regular octahedrons and cubes of Pd (Fig. 6, b and d), implying that epitaxial growth of Pt branches is essentially independent of the facets expressed on the surface of the Pd nanocrystal seeds.
 | ||
| Fig. 6 Electron microscopy characterization of Pd-Pt bimetallic nanocrystals synthesized by reducing K2PtCl4 with L-ascorbic acid in the presence of (a, b) octahedral and (c, d) cubic Pd seeds. (a, c) TEM and (b, d) HRTEM images. | ||
 | ||
| Fig. 7 TEM images showing the morphological evolution of Pd-Pt nanoparticles synthesized by reducing K2PtCl4 with L-ascorbic acid in the presence of cubic Pd seeds. | ||
4. Discussion
In the conformal overgrowth of octahedral Pt shells on regular octahedrons of Pd, citric acid seems to play an important role by stabilizing the Pt {111} surface. If two metals have a very close lattice match as in the case of Pd and Pt (Pd and Pt have a lattice mismatch of only 0.77%), the mode of heterogeneous nucleation and growth is largely affected by correlation of surface and interfacial energies between the two metals. According to the thermodynamic analysis established by Bauer,23,24 layered growth (Frank-van der Merwe mechanism) is favored when γs = γd + γi, while island growth (Volmer-Weber mechanism) can take place when γs < γd + γi, where γs, γd, and γi refer to the specific surface energies of the substrate, deposit, and substrate-deposit interface, respectively. Since Pt has a relatively higher surface energy relative to Pd,25 these thermodynamic conditions predict an island growth mode for Pt deposition on Pd surface. As demonstrated in this work, however, citric acid favors the conformal overgrowth of octahedral Pt shells on Pd octahedrons. This might be explained by the preferential interaction of citric acid with the Pt {111} surface, which could lower the surface energy of Pt layers deposited on Pd octahedrons whose surfaces are exclusively enclosed by {111} facets, thus promoting the layered growth.The formation of octahedral Pt shells on Pd truncated octahedrons is quite intriguing. According to Wulff's theorem, a metal nanocrystal with a face-centered cubic (fcc) structure tends to evolve into a truncated octahedral shape in an attempt to minimize the total surface energy.26 In a typical nanocrystal synthesis, however, impurities or capping agents can change the order of free energies of different facets through the preferential interaction with a metal surface, altering their relative growth rates and thus the final shape of a nanocrystal.26 The observed shape evolution from truncated octahedron to regular octahedron in Fig. 2 indicates that the Pt overgrowth was enhanced along the <100> directions, which in turn supports that citric acid acted as a capping agent for this directed Pt overgrowth through the preferential interaction with the Pt {111} surface. This kind of surface capping may drive the deposition of Pt atoms primarily onto the poorly passivated {100} faces, allowing the growth along the <100> directions to dominate. In this way, an octahedral shell terminated by {111} planes could be formed, even though the formation of a truncated octahedral shell is more favored in terms of the total surface energy per volume.
However, the interaction of citric acid with the Pt {100} surfaces might not be effective to stabilize Pt layers deposited on the {100}-terminated Pd nanocube. In this case, the initial deposition of Pt atoms would less likely occur in a layered growth mode due to a relatively higher surface energy of Pt. Instead, Pt deposits nucleated on the cubic Pd seed could grow non-conformally into discrete bumps that are spatially separated from each other, and eventually form a single, incomplete octahedral shell through overlap and fusion between them with continued growth in their sizes. Our results suggest that the effective stabilization of deposited Pt layer is crucial in achieving conformal overgrowth of a Pt shell that takes the same morphology as a Pd nanocrystal core.
It is worth emphasizing that the reduction kinetics also has a significant impact on the nucleation and growth modes for Pt. In the L-ascorbic acid-mediated synthesis, the degree of supersaturation for Pt atoms would drastically increase due to the high rate of Pt reduction associated with a strong reducing power of L-ascorbic acid,22 which could facilitate homogeneous nucleation in the solution.27 Small particles have a higher chemical potential due to a large surface-to-volume ratio and tend to aggregate when poorly stabilized by a polymeric stabilizer or capping agent.28–32 Compared to L-ascorbic acid, citric acid has a relatively weak reducing power.22 Therefore, we expect that the degree of supersaturation for Pt atoms would gradually increase as the reaction proceeds, which may account for the predominance of heterogeneous nucleation and growth of Pt on the Pd seed in the citric acid-mediated synthesis.
5. Conclusion
In summary, we have investigated the epitaxial overgrowth of Pt on the well-defined surface of Pd nanocrystals with a variety of shapes such as regular octahedron, truncated octahedron, and cube. When citric acid was used as a reducing agent, the conformal overgrowth of octahedral Pt shells was observed on regular and truncated octahedrons of Pd, producing Pd-Pt core-shell octahedrons, in which citric acid played a key role in conformal overgrowth of Pt by stabilizing {111} facets of the deposited Pt layer. On the contrary, the overgrowth of Pt on cubic Pd seeds occurred non-conformally due to the poor stabilization of Pt layers deposited on the {100}-terminated Pd nanocube by citric acid. In the L-ascorbic acid-mediated synthesis, both homogeneous and heterogeneous nucleation of Pt occurred at very early stages of the reaction due to the fast reduction of a Pt precursor by L-ascorbic acid, and growth proceeded by particle attachment, generating a dense array of Pt branches on a Pd nanocrystal core. Our results show that both the surface capping and reduction kinetics can also play significant roles in determining the mode of nucleation and growth in the seed-mediated synthesis of Pd-Pt bimetallic nanocrystals.Acknowledgements
This work was supported in part by the NSF (DMR-0804088) and startup funds from Washington University in St. Louis. Part of the work research performed at the Nano Research Facility (NRF), a member of the National Nanotechnology Infrastructure Network (NNIN), which is supported by the National Science Foundation under award no. ECS-0335765.References
- M. Tsuji, N. Miyamae, S. Lim, K. Kimura, X. Zhang, S. Hikino and M. Nishio, Cryst. Growth Des., 2006, 6, 1801 CrossRef CAS.
- C. Xue, J. E. Millstone, S. Li and C. A. Mirkin, Angew. Chem., Int. Ed., 2007, 46, 8436 CrossRef.
- S. E. Habas, H. Lee, V. Radmilovic, G. A. Somorjai and P. Yang, Nat. Mater., 2007, 6, 692 CrossRef CAS.
- B. Lim, J. Wang, P. H. C. Camargo, M. Jiang, M. J. Kim and Y. Xia, Nano Lett., 2008, 8, 2535 CrossRef CAS.
- F.-R. Fan, D.-Y. Liu, Y.-F. Wu, S. Duan, Z.-X. Xie, Z.-Y. Jiang and Z.-Q. Tian, J. Am. Chem. Soc., 2008, 130, 6949 CrossRef CAS.
- B. Lim, H. Kobayashi, T. Yu, J. Wang, M. J. Kim, Z.-Y. Li, M. Rycenga and Y. Xia, J. Am. Chem. Soc., 2010, 132, 2506 CrossRef CAS.
- P. H. C. Camargo, Y. Xiong, L. Ji, J. M. Zuo and Y. Xia, J. Am. Chem. Soc., 2007, 129, 15452 CrossRef CAS.
- S. Zhou, K. McIlwrath, G. Jackson and B. Eichhorn, J. Am. Chem. Soc., 2006, 128, 1780 CrossRef.
- B. Lim, M. Jiang, P. H. C. Camargo, E. C. Cho, J. Tao, X. Lu, Y. Zhu and Y. Xia, Science, 2009, 324, 1302 CrossRef CAS.
- Z. Peng and H. Yang, J. Am. Chem. Soc., 2009, 131, 7542 CrossRef CAS.
- B. Lim, M. Jiang, T. Yu, P. H. C. Camargo and Y. Xia, Nano Res., 2010, 3, 60 Search PubMed.
- B. Lim and Y. Xia, Angew. Chem. Int. Ed., 2010 Search PubMed in press.
- J. Kua and W. A. Goddard III, J. Am. Chem. Soc., 1999, 121, 10928 CrossRef CAS.
- J. K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jónsson, J. Phys. Chem. B, 2004, 108, 17886 CrossRef CAS.
- N. Tian, Z.-Y. Zhou, S.-G. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732 CrossRef CAS.
- B. Lim, X. Lu, M. Jiang, P. H. C. Camargo, E. C. Cho, E. P. Lee and Y. Xia, Nano Lett., 2008, 8, 4043 CrossRef CAS.
- C. Wang, H. Daimon, T. Onodera, T. Koda and S. Sun, Angew. Chem., Int. Ed., 2008, 47, 3588 CrossRef CAS.
- G. Ertl, Handbook of Heterogeneous Catalysis, Wiley-VCH, Weinheim, 2008 Search PubMed.
- M. Min, C. Kim, Y. I. Yang, J. Yi and H. Lee, Phys. Chem. Chem. Phys., 2009, 11, 9759 RSC.
- J. Zhang, M. B. Vukmirovic, Y. Xu, M. Mavrikakis and R. R. Adzic, Angew. Chem., Int. Ed., 2005, 44, 2132 CrossRef CAS.
- B. Lim, Y. Xiong and Y. Xia, Angew. Chem., Int. Ed., 2007, 46, 9279 CrossRef CAS.
- B. Lim, M. Jiang, J. Tao, P. H. C. Camargo, Y. Zhu and Y. Xia, Adv. Funct. Mater., 2009, 19, 189 CrossRef CAS.
- E. Bauer, Z. Kristallogr., 1958, 110, 372.
- E. Bauer and H. Poppa, Thin Solid Films, 1972, 12, 167 CrossRef CAS.
- Q. Jiang, H. M. Lu and M. Zhao, J. Phys.: Condens. Matter, 2004, 16, 521 CrossRef CAS.
- Y. Xia, Y. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 60 CrossRef CAS.
- S. Auer and D. Frenkel, Nature, 2001, 409, 1020 CrossRef CAS.
- T. O. Ely, C. Amiens and B. Chaudret, Chem. Mater., 1999, 11, 526 CrossRef.
- I. Pastoriza-Santos and L. M. Liz-Marzán, Langmuir, 2002, 18, 2888 CrossRef CAS.
- T. D. Ewers, A. K. Sra, B. C. Norris, R. E. Cable, C.-H. Cheng, D. F. Shantz and R. E. Schaak, Chem. Mater., 2005, 17, 514 CrossRef CAS.
- H. Zheng, R. K. Smith, Y.-w. Jun, C. Kisielowski, U. Dahmen and A. P. Alivisatos, Science, 2009, 324, 1309 CrossRef CAS.
- B. Lim, J. Wang, P. H. C. Camargo, C. M. Cobley, M. J. Kim and Y. Xia, Angew. Chem., Int. Ed., 2009, 48, 6304 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
