Mesoscale crystallization of calcium phosphate nanostructures in protein (casein) micelles†
Surachai
Thachepan‡
,
Mei
Li
and
Stephen
Mann
*
Centre for Organized Matter Chemistry, School of Chemistry, University of Bristol, Bristol, BS8 1TS, UK. E-mail: s.mann@bris.ac.uk
First published on 21st May 2010
Abstract
Aqueous micelles of the multi-protein calcium phosphate complex, casein, were treated at 60 °C and pH 7 over several months. Although partial dissociation of the micelles into 12 nm sized amorphous calcium phosphate (ACP)/protein nanoparticles occurred within a period of 14 days, crystallization of the ACP nanoclusters into bundles of hydroxyapatite (HAP) nanofilaments was not observed until after 12 weeks. The HAP nanofilaments were formed specifically within the partially disrupted protein micelles suggesting a micelle-mediated pathway of mesoscale crystallization. Similar experiments using ACP-containing synthetic micelles prepared from β-casein protein alone indicated that co-aligned bundles of HAP nanofilaments were produced within the protein micelle interior after 24 hours at temperatures as low as 35 °C. The presence of Mg2+ ions in the casein micelles, as well as a possible synergistic effect associated with the multi-protein nature of the native aggregates, could account for the marked inhibition in mesoscale crystallization observed in the casein micelles compared with the single-component β-casein constructs.
Introduction
An important emerging area of nanoscience concerns the equilibrium and non-equilibrium transformation of nanoscale components into complex, functional structures that extend across multiple length scales.1 Typically, these processes are determined by the interplay of kinetic and thermodynamic factors involved with the spatially confined reorganization of metastable hybrid mesostructures comprising disordered assemblages of inorganic and organic components.2In situ transformation of the colloidal aggregates into complex hierarchical nanostructures often takes place via non-classical crystallization pathways in which the oriented attachment of hybrid nanoscale units is of key mechanistic importance.3 The metastable hybrid aggregates are usually produced in the presence of interactive surfactants or polymers, which influence the nucleation and phase transformation of the amorphous precursors,4 and facilitate spatial delineation of these processes within 3-D interlinked networks of polymer hydrogels,5–9 through dynamical exchange of microemulsion water droplets,10–12 or by nanoscale encapsulation and intercalation within polyelectrolyte–inorganic clusters.13–17Given the generality of these studies, we are interested in exploring whether analogous mesoscale transformations can occur in native bioinorganic proteins exhibiting self-assembling structuration, hybrid composition and latent metastability. In this regard, many studies have been undertaken on nanoscale crystallization in biomimetic ferritins,18–20 but these transformations do not show inherent complexity because of the distinct structural demarcation between the inorganic core nanoparticle and surrounding polypeptide shell. As a consequence, the inner surface of the shell can facilitate nucleation of an amorphous/crystalline inorganic cluster but plays a minimal role in the subsequent growth or transformation of the core nanoparticle. In contrast, the milk protein complex, casein, which comprises a heterogeneous mixture of four single-chain phosphoserine (PSer)-rich proteins (αS1-casein (Mr = 23k, 40 wt%), αS2-casein (25k, 10 wt%), β-casein (24k, 40 wt%) and κ-casein (19k, 10 wt%)), is a biological analogue of a mineralized block copolymer micelle, and as such is a more realistic counterpart to the synthetic constructs previously used to investigate the meso-crystallization and assembly of nanostructured hybrids.
Unlike most other proteins, the caseins have no specific secondary structure due to a high proline content (∼9 wt%), and therefore possess extremely open and flexible secondary and tertiary conformations.21,22 Moreover, in each case, the casein proteins are amphiphilic molecules with structures based on distinct domains of disparate charge or hydrophobicity. For example, the αS1-casein molecule has a tri-block arrangement comprising a hydrophilic PSer-rich central loop located between two hydrophobic end-domains, whereas β-casein is a hydrophobic–hydrophilic diblock.23 κ-Casein is also in the form of a diblock with a predominantly hydrophobic N-terminal region and highly charged C-terminus but with only one PSer residue. Mixtures of the casein proteins therefore undergo spontaneous self-assembly into 100–150 nm diameter aggregates (micelles) that strongly sequester Ca2+ ions along with inorganic phosphate to produce bioinorganic mesostructures that are stable as aqueous dispersions.24 Whilst there remains some dispute over the detailed nature of the internal structure of the casein micelles—models involving a heterogeneous sub-micellar structure,25 or a homogeneous micelle network cross-linked by either calcium phosphate nanoclusters26,27 or a combination of inorganic clusters and protein–protein hydrophobic interactions23 have been proposed—there is a general consensus that self-assembly of the micelle is stabilized in the presence of calcium phosphate and that κ-casein is preferentially located at the micelle surface.28 As a consequence, the interior of the casein micelles consists of a disordered continuous mesostructure comprising a matrix of protein/protein conjugates and calcium phosphate/protein nanoconstructs; the latter possibly containing localized domains of 2 nm sized protein-bound clusters of amorphous calcium phosphate (ACP).29,30
Significantly, this structural arrangement is analogous to that observed in many of the hybrid aggregates responsible for complex nanostructuration in synthetic systems involving microemulsions or various polyelectrolytes (see ref. 10–17 for examples). Indeed, the similarity is particularly striking for the sequestration of Ca2+ and HPO42− ions into colloidal aggregates of poly(ethylene oxide)-b-alkylated poly(methacrylic acid) (PEO-b-PMMA-C12) micelles, which subsequently undergo a series of complex mesoscale transformations to produce nested aggregates of calcium phosphate/polymer hybrid nanofilaments.31 Interestingly, previous studies have indicated that prolonged heating of casein micelles at temperatures below 100 °C results in preferential dissociation of κ-casein along with disassembly of some of the α- and β-casein proteins,32 suggesting that it may be feasible to use these micelles as a self-organized reaction medium for promoting the crystallization of calcium phosphate nanostructures. In this regard, here we demonstrate that thermal treatment of casein dispersions can produce mesoscale transformations of the native ACP nanoclusters into bundles of crystalline hydroxyapatite (HAP) nanofilaments that remain spatially confined within the partially disrupted protein micelles. We show that similar micelle-mediated transformations also take place in synthetic micelles prepared from β-casein alone, albeit at a much increased rate. The studies suggest that co-assembly of the four different proteins in the native micelles, possibly along with the presence of Mg2+ ions, results in considerable stabilization with respect to in situ reorganization and crystallization of the ACP nanoclusters, and suggest that calcium phosphate/β-casein micelles could have considerable potential as functional bionanoconstructs with novel properties.
Materials and methods
Casein micelles were typically prepared by centrifugation of skimmed milk (1 mL) at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 60 min. The white gel-like precipitate was subjected to two cycles of washing and centrifugation with deionized water, and then re-dispersed in deionized water to a total volume of 10 mL. The resultant dispersions were passed through a modified size exclusion Sephadex-G50 (0.5 g dry weight) column,29 and the fraction collected between 5 and 15 min used for further experiments.
000 rpm for 60 min. The white gel-like precipitate was subjected to two cycles of washing and centrifugation with deionized water, and then re-dispersed in deionized water to a total volume of 10 mL. The resultant dispersions were passed through a modified size exclusion Sephadex-G50 (0.5 g dry weight) column,29 and the fraction collected between 5 and 15 min used for further experiments.
Thermally induced crystallization of calcium phosphate casein nanoclusters was carried out in screw-capped glass vials at 60 °C and pH = 7. A dispersion of casein micelles in deionized water was incubated in a temperature-controlled oven without stirring, and samples periodically removed for characterization for periods of over 3 months. In some cases, the collected samples were centrifuged at 3000 rpm for 2–5 minutes to separate the casein micelles from nanoparticulate products.
Calcium phosphate mineralization reactions were also undertaken using freshly prepared stock solutions of β-casein (2 g L−1, Mr = 24k, Sigma). Reaction mixtures were prepared in screw-capped glass vials by dilution of 1 mL of the stock solution of β-casein with deionized water (600 µL), followed by addition of aqueous CaCl2 solution (30 mM, 200 µL), and then aqueous Na2HPO4 (30 mM, 200 µL) with shaking. Typically, the final concentrations of β-casein, CaCl2 and Na2HPO4 were 42 µM (1 g L−1), 3 mM and 3 mM, respectively. Thermally induced transformation of the calcium phosphate mineralized β-casein micelles was undertaken as for the native casein micelles except that the former were equilibrated at the desired temperature (4, 35 or 60 °C) for 30 minutes prior to the addition of CaCl2 followed by Na2HPO4, and then left unstirred for various periods of time.
Samples for transmission electron microscopy (TEM), energy-dispersive X-ray (EDX) analysis and selected area electron diffraction (SAED) were collected directly from the casein or mineralized β-casein dispersions, and deposited onto carbon-coated copper grids. Excess liquid was removed by paper-blotting from the backside of the grid, and the samples left to dry in the air. In some cases, the sample grid was stained by 1% uranyl acetate solution. Samples for powder X-ray diffraction (PXRD) profiles (Bruker D8) were collected directly from the casein dispersions, mounted on a silicon wafer sample holder, and dried at room temperature under vacuum. Diffraction data were collected for 2θ values from 5 to 55°. FTIR spectroscopy was undertaken using a Perkin Elmer Spectrum One spectrometer fitted with Spectrum Analysis software. 31P NMR spectra of concentrated casein micelles prepared in the presence of EDTA at pH 9.5 to chelate excess Ca2+ ions and deprotonate all the different phosphoserine environments33 were recorded at room temperature using a JEOL Lambda 300 multinuclear NMR spectrometer.
Results and discussion
TEM images of unstained samples of purified casein micelles prepared by size exclusion gel chromatography of skimmed milk showed discrete, electron dense, spheroidal particles of variable contrast (Fig. 1a). The particles were often closely packed to produce semi-ordered arrays that were formed by evaporation-induced assembly during air drying onto the TEM grids. Particle size measurements gave an average diameter of 123 nm (σ = 14) with a range extending from approximately 70 to 300 nm (Fig. 1b). EDX analyses of the unstained samples gave peaks corresponding to Ca (3.7, 4.0 keV) and P (2.0 keV), indicating the presence of calcium phosphate associated with the samples (Fig. 1c), and confirming that the observed particles were casein micelles. SAED and PXRD profiles revealed only diffuse rings or a broad peak centred at 2θ = 30°, respectively, consistent with a disordered micelle structure comprising nanoclusters of ACP. FTIR spectra showed protein absorption bands at 1650 (amide I), 1540 (amide II), 3300 (N–H str.) and 700–500 cm−1 (N–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, N–C
O, N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bending). In addition, phosphate stretching and bending modes were observed in regions of 1200–900 and 630–500 cm−1, respectively, and were attributed to non-crystalline calcium phosphate and phosphate or phosphoserine anions complexed to calcium ions. 31P NMR spectra showed a sharp singlet at δ = −0.1 ppm and a broad multiplet centred at δ = ∼0.3 ppm, which were assigned to inorganic phosphate and phosphoserine, respectively.
O bending). In addition, phosphate stretching and bending modes were observed in regions of 1200–900 and 630–500 cm−1, respectively, and were attributed to non-crystalline calcium phosphate and phosphate or phosphoserine anions complexed to calcium ions. 31P NMR spectra showed a sharp singlet at δ = −0.1 ppm and a broad multiplet centred at δ = ∼0.3 ppm, which were assigned to inorganic phosphate and phosphoserine, respectively.
 | ||
| Fig. 1 (a) TEM image of unstained casein micelles after size exclusion chromatography, scale bar = 1000 nm. (b) Histogram of the size distribution and (c) corresponding EDX spectrum showing Ca and P peaks (Cu peaks arise from the copper grid). | ||
Micelle-mediated crystallization of the ACP nanoclusters of casein was investigated by thermal treatment of the dispersions at 60 °C and pH =7. In general, the heated micelles were stable and showed no macroscopic precipitation over a prolonged period of up to 12 weeks. TEM images of samples taken after 1 day of heating showed similar images to the unheated micelles except that the average size was reduced from 123 to 106 nm (σ = 12). The reduction in average size of the casein micelles suggested that a more compact structure was produced by heat-induced dehydration. In contrast, TEM studies of heated samples collected after 2 days showed a mixture of casein micelles along with discrete low electron-dense nanoparticles that were 15 nm (σ = 3) in mean size (ESI, Fig. S1†). Continued heating to 6 days had minimal effect on the structure or texture of the casein micelles but increased the number of nanoparticles considerably (Fig. 2a), some of which appeared to be associated with the surface of the casein micelles (Fig. 2b). The nanoparticle size was effectively unchanged (mean = 12 nm (σ = 2)) when compared with the mean diameter determined after 2 days. Significantly, EDX analysis on concentrated nanoparticle dispersions separated from the casein micelles by centrifugation showed peaks for Ca (3.7, 4.0 keV) and P (2.0 keV) (Fig. 2c), and corresponding electron diffraction analyses gave only diffuse background rings, suggesting that the disaggregated structures consisted of disordered calcium phosphate/protein nanoconjugates.
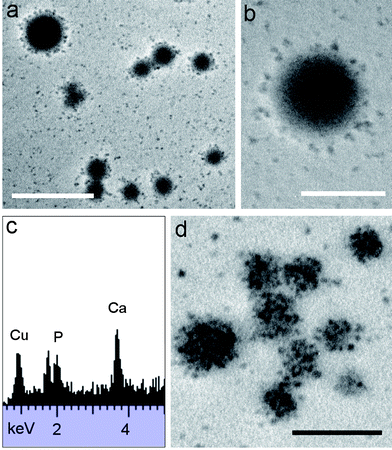 | ||
| Fig. 2 (a and b) TEM images of casein micelles incubated at 60 °C for 6 days showing intact micelles along with dissociation of calcium phosphate/protein nanoparticles. (c) EDX analysis of dissociated nanoparticles after heating for 6 days at 60 °C. (d) TEM image after 14 days of hydrothermal treatment showing modification in texture of the micelle interior. Scale bars: (a) = 500 nm and (b and d) 200 nm. | ||
Continued heating at 60 °C of the casein micelle dispersions for 2 weeks produced dissociated nanoparticles along with significant changes in the interior of the casein micelles, which appeared heterogeneous in texture due to the presence of electron-dense aggregated nanoparticles of relatively high contrast (Fig. 2d). Examination of high magnification TEM images suggested that the internal organization of the casein micelle was partially remodelled at this stage (ESI, Fig. S2†). Particle size measurements indicated that the mean dimension of the aggregated nanoparticles was the same as that for the dissociated clusters (14 nm (σ = 3) in each case), and EDX and SAED analyses of either the casein micelles or the electron-dense fragments confirmed the presence of Ca and P, and gave no evidence of crystallinity.
The above results indicated that the protein-embedded amorphous calcium phosphate nanoclusters associated with the casein micelles were remarkably stable with respect to thermally induced crystallization. Indeed, the dispersion had to be heated for approximately 12 weeks at 60 °C before any evidence of phase transformation was observed. TEM studies of samples collected after 3 months showed a marked decrease in the number of dissociated nanoparticles, as well as a reduction in the electron density and texture of the casein micelles. In addition, a large number of individual or bundled nanofilaments of variable length were observed associated specifically with the partially degraded micelles (Fig. 3), suggesting that the casein proteins played a key role in controlling the nucleation and initial growth of the nanowires. Moreover, the concurrent depletion of the discrete electron-dense particles and evolution of the filament bundles were consistent with a micelle-mediated pathway. EDX analyses of the filament bundles revealed the presence of Ca and P, and significantly, corresponding electron diffraction studies gave powder ring patterns with d-spacings (hkl) at 3.51 (002), 2.84 (211) and 1.71 (004) Å, corresponding to crystalline HAP (ESI, Fig. S3†). Particle size histograms gave an average bundle length and width of 218 nm (σ = 45) and 35 nm (σ = 7), respectively, and similar measurements of individual HAP filaments indicated that the widths were relatively uniform with a mean thickness of 9 nm (σ = 1) but that the lengths were highly variable (mean = 82 nm (σ = 42)) (ESI, Fig. S4†). As the mean thickness of the filaments was comparable to the size of the dissociated ACP/casein nanoclusters, the initial stages of crystallization possibly take place through the linear aggregation of the precursor hybrid nanoparticles.
 | ||
| Fig. 3 TEM images of casein micelles after heating at 60 °C for 12 weeks showing formation of (a) isolated, as well as (b) bundled HAP nanofilaments in association with the protein aggregates (imaged as low density diffuse objects in the micrograph). Scale bars = 200 nm. | ||
Similar experiments were undertaken using micelles prepared from the protein, β-casein, as a potential model for nucleation and phase transformation of calcium phosphate within the multi-component native casein micelles. Above a critical micelle concentration of 0.5 g L−1,34 β-casein is known to self-assemble into surfactant-like micelles with a hydrodynamic diameter of 20–25 nm.34–36 Addition of low concentrations of aqueous CaCl2 and Na2HPO4 to a solution of β-casein micelles at pH = 7 and temperatures of up to 20 °C for 24 hours produced a stable colloidal suspension of electron dense nanoparticles that were either discrete or aggregated into higher-order micelles (Fig. 4a and b). The individual nanoparticles were 15 nm (σ = 3) in average diameter and susceptible to damage in the electron beam of the TEM. The presence of Ca (3.7, 4.0 keV), P (2.0 keV), S (2.3 keV) and Cl (2.6 keV) was confirmed by EDX analysis spectrum (ESI, Fig. S5†), and electron diffraction studies failed to detect any evidence of crystallinity, suggesting that the nanoparticles were ACP/β-casein constructs. Analogous results were obtained for the larger micellar aggregates, which were polydisperse with an average size of 100 nm (σ = 30). Interestingly, control experiments at room temperature for 24 hours using identical concentrations of CaCl2 and Na2HPO4 but in the absence of β-casein micelles produced extensive aggregates of 10–150 nm sized plate-like crystals of HAP (ESI, Fig. S6†), indicating that the nucleation and growth of calcium phosphate were significantly constrained by sequestration and confinement within the protein micelles.
 | ||
| Fig. 4 TEM images of ACP/β-casein micelles after 24 hours at 20 °C. (a) Low magnification image showing large micellar aggregates and discrete nanoparticles, and (b) high magnification image showing individual calcium phosphate/protein nanoparticles. Scale bars = 200 nm. | ||
Heating of ACP/β-casein micelles at 35 or 60 °C for 24 hours resulted in an increased yield of the larger aggregated particles. TEM studies indicated that the particles were highly electron dense, spheroidal in shape, 160 nm in average size and highly polydisperse (σ = 48) (Fig. 5a). Significantly, high magnification images revealed that the electron density was associated with bundles of co-aligned nanofilaments that were less than 8 nm in thickness and specifically located within the interior of the micelles (Fig. 5b). EDX analysis revealed the presence of Ca (3.7, 4.0 keV) and P (2.0 keV), and selected area electron diffraction studies confirmed the presence of crystalline HAP (Fig. 5b, inset).
 | ||
| Fig. 5 TEM images of calcium phosphate/β-casein micelles after 24 hours at 60 °C showing (a) electron dense micellar aggregates with internal structuration (arrows). (b) High magnification image showing presence of bundles of HAP nanofilaments within the micelle interiors. Inset shows corresponding electron diffraction pattern with d-spacings at (hkl) indices corresponding to 3.51 (002), 2.84 (211), 1.99 (222), 1.85 (213) and 1.73 (004) Å planes of HAP. Scale bars: (a) = 500 nm and (b) = 100 nm. | ||
Formation of micellar aggregates from β-casein, as well as the absence of bulk calcium phosphate precipitation when exposed to Ca2+ and HPO42− ions, indicated that the β-casein aggregates were morphologically similar to the native casein micelles. However, at temperatures above 35 °C, transformation into nanocrystalline filaments of HAP occurred within 24 hours in the former, whilst the casein micelles remained unchanged for several weeks even at 60 °C. The much greater stability of the native micelles with respect to crystallization of the protein-embedded ACP nanoclusters could be due to the presence of Mg2+ ions in the native casein micelle,37,38 or originate from a synergistic effect involving the co-assembly of four different proteins, or both. The latter possibility is consistent with the lower Ca2+ binding capacity of the β-casein micelle, which comprises five PSer amino acids per molecule, compared with αS1 and αS2-caseins in the native micelles that have eight or twelve PSer residues per polypeptide chain, respectively.39 Nevertheless, our results showed that thermally induced crystallization of the HAP nanofilaments occurred specifically within the interior of the β-casein micelles, indicating that nucleation and growth from the ACP precursor phase remained strongly associated with the protein matrix. Whilst we could not rule out the possibility of de novo nucleation within the β-casein micelles of the HAP nanofilaments particularly at high temperatures, experiments in which mineralized samples prepared at 4 °C for 24 hours, and then subsequently aged for a further 24 hours at temperatures of 35 or 60 °C, revealed that the crystalline nanofilaments were formed by phase transformation specifically within the confined medium of the micelles (ESI, Fig. S7†).
Conclusions
In this paper we have shown that thermal treatment of a dispersion of casein micelles at 60 °C slowly leads to transformation of the protein-embedded ACP nanoclusters into single or bundled nanofilaments of crystalline HAP that are associated specifically with the partially disaggregated casein micelles. This process proceeds initially via compaction of the micelles within a few days after incubation, followed by progressive dissociation of the ACP–casein complexes over several weeks. Dissociation and re-aggregation of the hybrid conjugates result in intramicellar reorganization and eventually to in situ nucleation of the crystalline HAP filaments. Significantly, the close association of the HAP filaments and the casein micelle suggests that the protein matrix is crucial in controlling both the rate and structural evolution of the transformation process.The above scenario is strongly supported by the temperature-dependent study of β-casein-mediated formation of calcium phosphate. At temperatures below 20 °C, discrete micelles as well as higher-order aggregates comprising an ACP/β-casein hybrid construct spontaneously self-assemble in the presence of aqueous Ca2+ and HPO42− ions. However, unlike the heterogeneous casein micelles, the β-casein structures readily transform within 24 h at temperatures of ∼35 °C and above to produce crystalline HAP nanofilaments that are bundled into co-aligned mesostructures in association with the protein matrix. The high metastability of this system compared with the native casein micelles highlights the possible synergistic stabilizing efficiency associated with mixtures of the αS1-, αS2-, β- and κ-casein proteins. Clearly, this cooperative behaviour has functional advantages for the biological delivery of high concentrations of calcium and phosphate via a relatively soluble amorphous inorganic nanophase, as well as preventing ectopic calcification of the mammary gland,40 and providing a natural vehicle for bioactives.41 On the other hand, the calcium phosphate/β-casein constructs are more similar in their instability to synthetic polymer/surfactant-based counterparts known to produce a wide range of complex nanostructures via coupled mesoscale transformations.10–17,31 Moreover, the inherent metastability of the calcium phosphate/β-casein could be exploited for potential uses such as in calcification/remineralization agents (regenerative enamel, synthetic bone pastes, for example), pharmaceutical materials (storage and release of drugs, vitamins, etc.), or as hybrid nanostructures for in vitro or in vivo delivery of signaling molecules or plasmids/vectors for cell differentiation and transfection, respectively.42,43 We hope to address the potential of these bionanoconstructs in our future work.
References
- S. Mann, Nat. Mater., 2009, 8, 781–792 CrossRef CAS.
- H. Cölfen and S. Mann, Angew. Chem., Int. Ed., 2003, 42, 2350–2365 CrossRef.
- H. Cölfen and M. Antonietti, Angew. Chem., Int. Ed., 2005, 44, 5576–5591 CrossRef.
- S. Mann, Angew. Chem., Int. Ed., 2000, 39, 3392–3406 CrossRef CAS.
- A. Sugawara, T. Ishii and T. Kato, Angew. Chem., Int. Ed., 2003, 42, 5299–5303 CrossRef CAS.
- H. Imai, S. Tatara, K. Furuichi and Y. Oaki, Chem. Commun., 2003, 1952–1953 RSC.
- P. Simon, W. Carrillo-Cabrera, P. Formánek, C. Göbel, D. Geiger, R. Ramlau, H. Tlatlik, J. Buder and R. Kniep, J. Mater. Chem., 2004, 14, 2218–2224 RSC.
- A. E. Voinescu, M. Kellermeier, B. Bartel, A. M. Camerup, A. K. Larsson, D. Touraud, W. Kunz, L. Kienle, A. Pfitzner and S. T. Hyde, Cryst. Growth Des., 2008, 8, 1515–1521 CrossRef CAS.
- J. M. Garcia-Ruiz, E. Melero-Gracia and S. T. Hyde, Science, 2009, 323, 362–365 CrossRef CAS.
- M. Li and S. Mann, Adv. Funct. Mater., 2002, 12, 773–779 CrossRef CAS.
- M. Li, B. Lebeau and S. Mann, Adv. Mater., 2003, 15, 2032–2035 CrossRef CAS.
- S. Thachepan, M. Li, S. A. Davis and S. Mann, Chem. Mater., 2006, 18, 3557–3561 CrossRef CAS.
- A. Bigi, E. Boanini, D. Walsh and S. Mann, Angew. Chem., Int. Ed., 2002, 41, 2163–2166 CrossRef CAS.
- M. Li, H. Cölfen and S. Mann, J. Mater. Chem., 2004, 14, 2269–2276 RSC.
- S.-H. Yu, H. Cölfen, K. Tauer and M. Antonietti, Nat. Mater., 2005, 4, 51–55 CrossRef CAS.
- T. X. Wang, H. Cölfen and M. Antonietti, J. Am. Chem. Soc., 2005, 127, 3246–3247 CrossRef.
- Y. Oaki and H. Imai, Adv. Funct. Mater., 2005, 15, 1407–1414 CrossRef CAS.
- R. M. Kramer, C. Li, D. C. Carter, M. O. Stone and R. R. Naik, J. Am. Chem. Soc., 2004, 126, 13282–13286 CrossRef CAS.
- T. Ueno, M. Suzuki, T. Goto, T. Matsumoto, K. Nagayama and Y. Watanabe, Angew. Chem., Int. Ed., 2004, 43, 2527–2530 CrossRef CAS.
- M. Li, C. Viravaida and S. Mann, Small, 2007, 3, 1477–1481 CrossRef CAS.
- C. Holt and L. Sawyer, J. Chem. Soc., Faraday Trans., 1993, 89, 2683–2692 RSC.
- Y. D. Livney, A. L. Schwan and D. G. Dalgleish, J. Dairy Sci., 2004, 87, 3638–3647 CrossRef CAS.
- D. S. Horne, Int. Dairy J., 1998, 8, 171–177 CrossRef CAS.
- E. Dickinson, Soft Matter, 2006, 2, 642–652 RSC.
- P. Walstra, Int. Dairy J., 1999, 9, 189–192 CrossRef CAS.
- H. M. Farrell, E. L. Malin, E. M. Brown and P. X. Qi, Curr. Opin. Colloid Interface Sci., 2006, 11, 135–147 CrossRef CAS.
- C. Holt, C. G. de Kruif, R. Tuinier and P. A. Timmins, Colloids Surf., A, 2003, 213, 275–284 CrossRef CAS.
- S. G. Anema and Y. Li, Food Sci. Technol., 2000, 33, 335–343 Search PubMed.
- R. L. J. Lyster, S. Mann, S. B. Parker and R. J. P. Williams, Biochim. Biophys. Acta, 1984, 801, 315–317 CrossRef CAS.
- Q. Z. Dong and L. X. Gu, Eur. Polym. J., 2002, 38, 511–519 CrossRef CAS.
- M. Antonietti, M. Breulmann, C. G. Göltner, H. Cölfen, K. K. Wong, D. Walsh and S. Mann, Chem.–Eur. J., 1998, 4, 2493–2500 CrossRef CAS.
- S. G. Anema, J. Agric. Food Chem., 1998, 46, 2299–2305 CrossRef CAS.
- J. Belloque and M. Ramos, J. Dairy Res., 2002, 69, 411–418 CAS.
- J. E. O'Connell, V. Y. Grinberg and C. G. de Kruif, J. Colloid Interface Sci., 2003, 258, 33–39 CrossRef CAS.
- E. Leclerc and P. Calmettes, Phys. Rev. Lett., 1997, 78, 150–153 CrossRef CAS.
- C. G. de Kruif and V. Y. Grinberg, Colloids Surf., A, 2002, 210, 183–190 CrossRef CAS.
- F. Gaucheron, Reprod., Nutr., Dev., 2005, 45, 473–483 CrossRef CAS.
- A. L. Boskey and A. S. Posner, Mater. Res. Bull., 1974, 9, 907–914 CrossRef CAS.
- C. Holt, J. Dairy Sci., 1998, 81, 2994–3003 CrossRef CAS.
- E. Smyth, R. A. Clegg and C. Holt, Int. J. Dairy Technol., 2004, 57, 121–126 CrossRef CAS.
- Y. D. Livney, Curr. Opin. Colloid Interface Sci., 2010, 15, 73–83 CrossRef CAS.
- R. Gonzalez-McQuire, D. Green, K. Partridge, R. O. C. Oreffo, S. Mann and S. A. Davis, Adv. Mater., 2007, 19, 2236–2240 CrossRef CAS.
- J. C. Babister, L. A. Hails, R. O. C. Oreffo, S. A. Davis and S. Mann, Biomaterials, 2009, 30, 3174–3182 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Particle size histograms, TEM, EDX and electron diffraction data. See DOI: 10.1039/c0nr00158a |
| ‡ Present address: Department of Chemistry, Kasetsart University, Bangkok, Thailand. |
| This journal is © The Royal Society of Chemistry 2010 |
