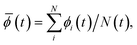Signal transmission, conversion and multiplication by polar molecules confined in nanochannels
Yusong
Tu
abc,
Ruhong
Zhou
*de and
Haiping
Fang
*a
aShanghai Institute of Applied Physics, Chinese Academy of Sciences, P.O. Box 800-204, Shanghai, 201800, China. E-mail: fanghaiping@sinap.ac.cn
bInstitute of Systems Biology, Shanghai University, Shanghai, China
cGraduate School of the Chinese Academy of Sciences, Beijing, 100080, China
dIBM Thomas J. Watson Research Center, Yorktown Heights, NY 10598, USA. E-mail: ruhongz@us.ibm.com
eDepartment of Chemistry, Columbia University, New York, NY 10027, USA
First published on 27th August 2010
Abstract
The mechanism of signal transmission, conversion and multiplication at molecular level has been of great interest lately, due to its wide applications in nanoscience and nanotechnology. The interferences between authentic signals and thermal noises at the nanoscale make it difficult for molecular signal transduction. Here we review some of our recent progress on the signal transduction mediated by water and other polar molecules confined in nanochannels, such as Y-shaped carbon nanotubes. We also explore possible future directions in this emerging field. These studies on molecular signal conduction might have significance in future designs and applications of nanoscale electronic devices, and might also provide useful insights for a better understanding of signal conduction in both physical and biological systems.
 Yusong Tu | Yusong Tu obtained his Master’s degree in 2005 from Guangxi Normal University, and received his Ph.D. degree in 2009 from Shanghai Institute of Applied Physics, Chinese Academy of Sciences. In the same year, he was appointed as a Lecturer in the Institute of Systems Biology, Shanghai University. His current research interests focus on the nanoscale molecular signal conduction mediated by polar molecules, and water anomalies and their effects on biological molecules. He has achieved eight journal publications during his M.Sc. and Ph.D. studies. |
 Ruhong Zhou | Ruhong Zhou is a Research Scientist at Computational Biology Center, IBM Research, and an Adjunct Professor at Chemistry Department, Columbia University. He received his Ph.D. in chemistry from Columbia University in 1997. His current research interests include protein folding, protein–protein interactions, ligand–receptor binding, and methodology developments for computational biology and bioinformatics. He has more than 90 journal publications and 10 patents, and has delivered 100+ invited talks worldwide. He has won the IBM Outstanding Technical Achievement Award in 2008 and 2005, the Hammett Award in 1997, and the ACS DEC Award in 1995. |
 Haiping Fang | Haiping Fang is a Research Scientist at the Shanghai Institute of Applied Physics, Chinese Academy of Sciences. He received his Ph.D. in theoretical physics from the Institute of Theoretical Physics, Chinese Academy of Sciences in 1994. His current research interests include water transportation across nanoscale and biological channels, nanoscale molecular signal conduction mediated by polar molecules confined in nanochannels, nanobubbles and water anomalies, and nanoparticle–protein interactions. He has more than 90 journal publications and 3 patents. He has won the 100 Talents Program of The Chinese Academy of Sciences in 2002, National Science Fund for Distinguished Young Scholars in 2008, and Shanghai Leading Academic Discipline Project in 2009. |
1. Introduction
The exploitation of new methods to transmit and amplify signals is of great importance. Charles Kao was awarded the 2009 Nobel Prize in Physics due to his groundbreaking work concerning the transmission of light in fibers for optical communication.1 With the development of new technology at both the microscale and nanoscale, signal transmission, conversion, and multiplication at nanoscale and/or molecular-scale has become critical in many applications, such as molecular switches, nano-gates, and biosensors.2–9 On the other hand, the signal transmission in the biological systems, particularly in various neural systems, is still far from fully understood.In the past two decades, there has been a great progress in the field of molecular electronics. Nanoscale structures or molecules have been utilized as the elements in electronic devices,10–13 such as nanowires, switches, rectifiers, and logic circuits, and the integration of these molecular-based devices and other nanoscale structures has led to a number of demonstrations of new and useful applications.14,15 Particularly, electrical transportation,16 electrical and polarity switching,10–13 and chemical and biomolecular sensors17,18 have been developed based on nanowires or nanotubes. Biosensors for DNA diagnostics, gene analysis, fast detection of warfare agents, and forensic applications have also been achieved by electrochemical method15 and microarrays18 together with the particular behaviour of biomolecules confined in the nanoscale space.19 In biology, signal representation is often related to the electrical change, although its molecule details are mostly unknown. For example, in central nerve systems, the most common mechanism for signaling between neurons is the neurotransmitter-releasing chemical synapse, but faster and simpler signaling can be achieved with electrical synapses, specialized junctions (Gap junctions) that mediate electrical coupling between neurons.20–23 Electrical coupling mediated by electrical synapses is an important feature of local inhibitory circuits and plays a fundamental role in detection and promotion of synchronous activity (firing pattern) within the neocortex.24–26 In fact, weak coupling may lead to antiphasic or asynchronous firing.20,21,27 We note that the signal transmission along intercellular and intracellular pathways and between the exterior and interior of a cell may be mediated by protein allostery,28–30 which may lead to redistribution of the charges and re-orientations of charge dipoles in the proteins, but the exact molecular mechanism is still unclear.
At the nanoscale, the thermal noise plays an important role which often significantly reduces the signal strength during the transmission, conversion and multiplication. More critically, the interferences between signals can be very strong if the signals are transmitted by charges. The interaction potential between two charges decreases with respect to r−1, where r is the distance between those two charges. At the nanoscale, this slow decrease will result in strong interaction between two charges (signals). This can be partly understood from the well-known fact that the interaction cannot be neglected between two atoms within one nanometre distance although the van der Waals interaction between them decreases with respect to r−6, much faster than r−1. In Fig. 1, we show a diagram of the function F(r, n) = An(r/r0)−n with respect to r for r0 = 1 Å and n = 1, 3, 6, where An is set as a constant = 1.
 | ||
| Fig. 1 Curves for function F(r, n) = An(r/r0)−n for r0 = 1 Å and n = 1, 3, 6, and An = 1. | ||
On the other hand, the dipole–dipole interaction potential has a form of r−3, decreasing much faster than the charge–charge interaction (r−1) with distance, as shown in Fig. 1. If we use the dipole to transmit a signal, the interaction between different dipole signals will be much weak.
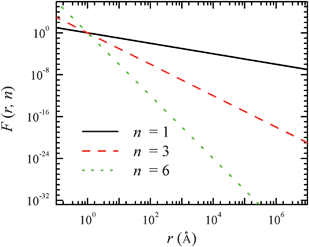
The property and applications due to electric dipoles have been extensively studied for several decades. The most notable one is the Ising model.31,32 Recently, the behaviour of one-dimensional dipole chains has been discussed, including the energy transfer in a dipole chain,33 the design of a logical AND port using dipole chains with a junction,34 and the initiation of the orientation of one-dimensional dipole chains by a local field.35 The water molecules confined in nanochannels with suitable radii, in which the water molecules form one-dimensional chains, provide an excellent example for the transmission of signals due to water dipole interactions. The water molecules display “concerted” orientations (water dipoles ordered cooperatively inside the carbon nanotube)36–41 as in the example shown in Fig. 2. If we can “tune” the orientation of one water molecule using a single charge at one end, we might be able to control the orientations of other water molecules in the same channel or other connected channels. In other words, we have a molecular level “signal transmission”, i.e., converting a charge signal to water dipole orientation signal (at one end), and then transmitting the water dipole orientation signal to other ends, which might be converted back to a charge signal again. Furthermore, if we use Y-shaped nanochannels, i.e., three nanochannels connected with each other to form a Y-junction, we can even achieve a “signal multiplication”, which means that a water-dipole-orientation signal can be multiplied into many water-dipole-orientation signals.36 It is also observed that the signal transmission and multiplication, via water molecules confined in nanoscale channels, can effectively shield from thermal fluctuations.
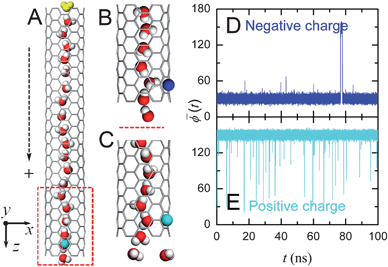 | ||
Fig. 2 (A) A schematic snapshot of the system with a single channel in side view (the xz plane) together with the trajectories (D, E) of average dipole angle ![[small phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d6.gif) (t) of the water orientations. The snapshots shown in (B, C) correspond to the red dashed part of the system in another side view (yz plane). Blue and green spheres denote the negative and positive charges, respectively, which result in different orientations of the water molecules facing the charges. The water molecule facing the external charge is named “monitored-water”. The single-walled carbon nanotube is represented by gray lines and its positive direction is shown by the black dashed arrowhead. Water molecules are shown with oxygen in red and hydrogen in gray, and the first water molecule outside the channels opposite to charge signal input is shown in yellow. The dipole direction of each water molecule is not shown, but is along the mid-line between the two O–H chemical bonds of the water molecule. Water molecules outside the nanotubes are omitted in the figure. (t) of the water orientations. The snapshots shown in (B, C) correspond to the red dashed part of the system in another side view (yz plane). Blue and green spheres denote the negative and positive charges, respectively, which result in different orientations of the water molecules facing the charges. The water molecule facing the external charge is named “monitored-water”. The single-walled carbon nanotube is represented by gray lines and its positive direction is shown by the black dashed arrowhead. Water molecules are shown with oxygen in red and hydrogen in gray, and the first water molecule outside the channels opposite to charge signal input is shown in yellow. The dipole direction of each water molecule is not shown, but is along the mid-line between the two O–H chemical bonds of the water molecule. Water molecules outside the nanotubes are omitted in the figure. | ||
It should be pointed out that this short feature article is not aimed to be a complete review of this subject, but rather a brief overview of our recent progress, including some potential future directions, with respect to nanoscale signal transmission, conversion and multiplication, mediated by water and other polar molecules confined in nanoscale channels.
2. Single transmission in a single channel with an appropriate radius
Fig. 2A shows a typical (6, 6) uncapped armchair single-walled carbon nanotube (SWNT) with a width of 0.81 nm and a length of 5.13 nm. When we attach a charge at the canter of a second carbon ring of the nanotube, the direction of the dipole orientation of water molecule facing the charge (“monitored-water” hereafter) is confined. In this study, we applied molecular dynamics simulations, which are widely used in nanoscale and molecular scale simulations for both physical and biological systems,36,39,42–48 to investigate this interesting phenomenon. We found that different signs of the charge q resulted in different orientations of the monitored-water (see Fig. 2B and C). Moreover, we found that the monitored-water determined all the water orientations in the nanotube, upward or downward in concert.36 In order to describe the water dipole orientation in the nanotube quantitatively, an angle ϕi between the ith water molecule and the nanotube axis is defined as:36ϕi = acos(![[p with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0070_20d1.gif) i·û/| i·û/|![[p with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0070_20d1.gif) i|) i|) |
![[p with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0070_20d1.gif) i is the dipole of the ith water molecule and û is the axis unit vector of the nanotube. The averaged dipole angle
i is the dipole of the ith water molecule and û is the axis unit vector of the nanotube. The averaged dipole angle ![[small phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d6.gif) (t) is computed by
(t) is computed bywhere the average runs over all the water molecules inside a nanotube at time t, and N(t) is the number of water molecules within this tube. The averaged dipole angle is used to characterize the water-mediated signal transmission. The results are shown in Fig. 2D and E. It is clear that
![[small phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d6.gif) falls into very different ranges most of the time for a positive or negative signal charge: 110° <
falls into very different ranges most of the time for a positive or negative signal charge: 110° < ![[small phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d6.gif) < 170° for q = +e, and 10° <
< 170° for q = +e, and 10° < ![[small phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d6.gif) < 70° for q = −e, indicating that the water molecules within each nanotube are well ordered (i.e., in concert). Thus, the charge (+e/−e) signal at the one end of nanotube can be readily distinguished from the dipole orientation (upward/downward) of water molecules at the other end of nanotube.
< 70° for q = −e, indicating that the water molecules within each nanotube are well ordered (i.e., in concert). Thus, the charge (+e/−e) signal at the one end of nanotube can be readily distinguished from the dipole orientation (upward/downward) of water molecules at the other end of nanotube.
We now study the time delay of the water orientations in response to a switch in the charge signal. In the aforementioned systems, we recorded the water orientation states every 10 ns after the first 30 ns of simulation time. We performed new simulations from these states, but with the charge polarity switched. The averaged angles ![[small phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d6.gif) (t) for four such scenarios are shown in Fig. 3A and B. The time delay associated with the branch tubes was 3.2 ns on average, with a minimal duration of 0.04 ns (Fig. 3B) and the maximal one 9.2 ns to respond to the +e → −e signal switch (which is slower), and approximately 0.07 ns only to respond to the −e → +e switch (which is much faster). This obvious disparity in the response time results from the interaction of the monitored-water with the signal charge. The negative charge attracts two hydrogen atoms of the monitored-water and limits the mobility of oxygen atom to a certain extent. Positive charge attracts the oxygen atom and two hydrogen atoms have more freedom to rotate or swing, which enable an easier switch for the configurations. This point can also be easily seen from Fig. 2D and E, as we observe more fluctuations in the case with a positive signal charge. We note that the short (average 0.07–3.2 ns) response time delay implies that the signal with frequency of GHz range can be expected.
(t) for four such scenarios are shown in Fig. 3A and B. The time delay associated with the branch tubes was 3.2 ns on average, with a minimal duration of 0.04 ns (Fig. 3B) and the maximal one 9.2 ns to respond to the +e → −e signal switch (which is slower), and approximately 0.07 ns only to respond to the −e → +e switch (which is much faster). This obvious disparity in the response time results from the interaction of the monitored-water with the signal charge. The negative charge attracts two hydrogen atoms of the monitored-water and limits the mobility of oxygen atom to a certain extent. Positive charge attracts the oxygen atom and two hydrogen atoms have more freedom to rotate or swing, which enable an easier switch for the configurations. This point can also be easily seen from Fig. 2D and E, as we observe more fluctuations in the case with a positive signal charge. We note that the short (average 0.07–3.2 ns) response time delay implies that the signal with frequency of GHz range can be expected.
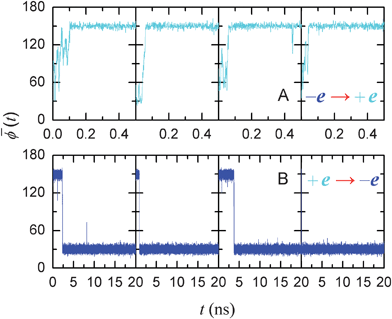 | ||
Fig. 3 Signal switching in an SWNT. Trajectories of average dipole angle ![[small phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d6.gif) (t) of the water orientations in an SWNT for the charge signal switching from negative to positive (A) and switching from positive to negative (B) for four typical cases with different initial states. The dipole orientations in the SWNT fully respond in approximately 0.07 ns when the charge switches from negative to positive and in 3.2 ns in the case of positive-to-negative transition. (t) of the water orientations in an SWNT for the charge signal switching from negative to positive (A) and switching from positive to negative (B) for four typical cases with different initial states. The dipole orientations in the SWNT fully respond in approximately 0.07 ns when the charge switches from negative to positive and in 3.2 ns in the case of positive-to-negative transition. | ||
In fact, it only takes tens of picoseconds to achieve the flipping of the orientation of the entire water chain inside the nanochannels; most of the time in the response delay is used for the re-orientation of the monitored-water and its neighbouring water molecules. Fig. 4 shows a typical example of the process of the reorientation of water molecules in the nanochannel in response to a +e → −e signal switch. We observed that the re-orientation of the water chain was carried out by flipping over the dipole of water molecules one by one, from the bottom to the top (see Fig. 2). Thus, during this process, we can trace the motion of this flipping forefront point, which is defined as the position of the water molecule (along the nanochannel axial direction), whose dipole angle ϕ falls into the ranges of [70°, 110°] and, while its two nearest neighbor molecules have their dipole angles falling into [110°, 70°] and [10°, 70°], respectively. If there is no such water molecule at any given moment, we take the position of the last-turning molecule as the current forefront. We have also plotted the value of ![[small phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d6.gif) as a function of the time. Clearly, the turning forefront is consistent with the value of
as a function of the time. Clearly, the turning forefront is consistent with the value of ![[small phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d6.gif) .
.
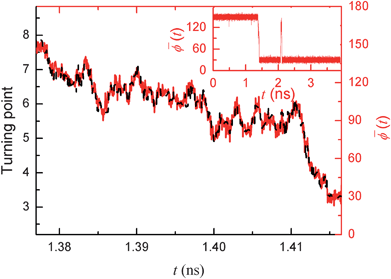 | ||
Fig. 4 A typical re-orientation process of water molecules in nanochannel in response to a +e → −e signal switch (a data point every 50 fs). During this process, red solid line represents the detailed trajectory of average dipole angle ![[small phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d6.gif) (t) adopting the red top and right axes (inset shows its whole trajectory in a longer period). This trajectory indicates that the re-orientation of the whole water chain is carried out by turning over orientations of water molecules one by one. The black dashed line shows this turning position in nanochannel as a time function using the dark left and bottom axes. (t) adopting the red top and right axes (inset shows its whole trajectory in a longer period). This trajectory indicates that the re-orientation of the whole water chain is carried out by turning over orientations of water molecules one by one. The black dashed line shows this turning position in nanochannel as a time function using the dark left and bottom axes. | ||
3. Signal multiplication with Y-shaped nanochannels
Signal multiplication at the nanoscale is also very important. Recently, Y-shaped nanotubes have been successfully fabricated by several different methods, including alumina templates,49 chemical vapor deposition of products generated from a pyrolysis of metallocenes,50–52 nano-welding of overlapping isolated nanotubes using high-intensity electron beams,53 and spontaneous growth of nanotubes mats using Ti-doped Fe catalysts.54 In ref. 16, we have constructed a computational model of Y-shaped carbon nanotube, called Y-SWNT, as shown in Fig. 5. Explicitly, the Y-SWNT was obtained by joining three SWNTs symmetrically, with an angle of 120° among them. Interestingly, we found that the behaviour of the water orientations inside one branch channel could be controlled by water orientations in another branch channel. In the following, for easy discussion, we named the three branch tubes as the main, first branch and second branch tubes, which were denoted by MT, BT1, and BT2, respectively, as shown in the figure.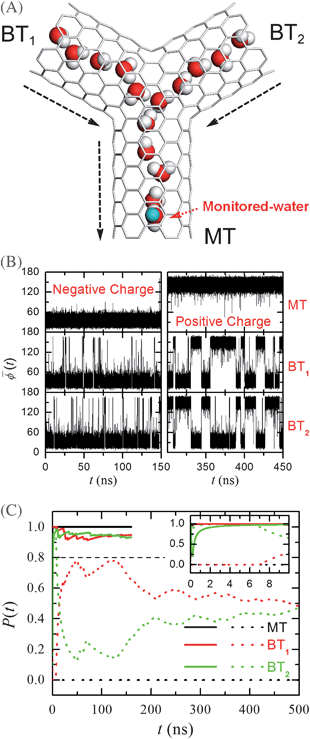 | ||
Fig. 5 (A) A schematic snapshot of the simulation system in side-view (the xz plane). The Y-SWNT features a main tube (MT) and two branch tubes (BT1, BT2) positioned in the same plane (the xz plane). The angle between each two neighbouring SWNTs is 120°. Small angular changes do not affect the results. The single-walled carbon nanotubes are represented by grey lines. Water molecules are shown with oxygen in red and hydrogen in grey. Water molecules outside the nanotubes are omitted in the figure. The green sphere represents the imposed charge. The lengths of MT, BT1 and BT2 are 1.44 nm, 1.21 nm and 1.21 nm, respectively. (B) The average dipole angle ![[small phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d6.gif) (t) of the water orientations inside the MT, BT1 and BT2 for a negative charge (left) and a positive charge (right). (C) The probabilities of the dipole orientations P(t) in different tubes for a negative charge (solid lines) and a positive charge (dashed lines). P(t) for a negative charge converges to about 1.0 within a few nanoseconds. (Reprinted from ref. 36. Copyright 2007, Proceedings of National Academy of Sciences USA). (t) of the water orientations inside the MT, BT1 and BT2 for a negative charge (left) and a positive charge (right). (C) The probabilities of the dipole orientations P(t) in different tubes for a negative charge (solid lines) and a positive charge (dashed lines). P(t) for a negative charge converges to about 1.0 within a few nanoseconds. (Reprinted from ref. 36. Copyright 2007, Proceedings of National Academy of Sciences USA). | ||
It is clear that the water chains in different nanotubes interact at the Y-junction. This usually affects the water dipole orientations in different tubes. Surprisingly, water dipoles are still in concert, just like those in the single channels. Using a similar approach described above, an external charge q was attached at the centre of a second carbon ring of the MT as shown in Fig. 5A to initiate a signal of water dipole orientations. Similar to the single channels, the water molecules in the MT have the upward and downward concerted dipole orientation for q = −e and q = +e, respectively. Remarkably, the water orientations in both the BTs followed the water orientations in the MT when water orientations in the MT were downward, while the water orientations in both the BTs fluctuated between upward and downward when the water orientations in the MT were upward. This can be seen more clearly from the time-dependent behaviour of ![[small phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d6.gif) (t) as shown in Fig. 5B. Therefore, from the water orientations in each branch tube of the Y-shaped channel, we can easily distinguish the sign of the imposed charge at the MT. In other words, the charge signal at the bottom of the MT is “converted” into water dipole signal, and then transmitted to the two Y-branch tops and “multiplied” (from one signal to two).
(t) as shown in Fig. 5B. Therefore, from the water orientations in each branch tube of the Y-shaped channel, we can easily distinguish the sign of the imposed charge at the MT. In other words, the charge signal at the bottom of the MT is “converted” into water dipole signal, and then transmitted to the two Y-branch tops and “multiplied” (from one signal to two).
To characterize the water-mediated signal multiplication, the probability of occurrence in the range of 10° < ![[small phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d6.gif) < 70°, denoted by P(t), was computed. We can expect that P(t) approaches 1 in both BTs when the water orientations in the MT are downward (after a sufficiently long time). In contrast, P(t) approaches 0.5 in both BTs when the water orientations in the MT are upward, since the water orientations in both BTs fluctuate between upward and downward with a roughly equal probability. If we set PC as a value between 0.5 and 1, we expect that P > PC in the BTs corresponds to the downward water orientations in the MT, and that P < PC in the BTs implies the upward water orientations in the MT after a sufficiently long time. We consider the rapid convergence of the case for the downward water orientations in the MT, PC = 0.8 was set, which is a little larger than the average value of 0.5. From Fig. 5C we can see that P > PC in both BTs for any time t > 1 ns for the downward water orientations in the MT, while P < PC in both BTs for any time t > 8 ns for the upward water orientations in the MT. Thus, the charge signal at the MT can be readily distinguished from the value of P(t) in each BT within a time interval of ∼8 ns with an appropriate threshold PC = 0.8.
< 70°, denoted by P(t), was computed. We can expect that P(t) approaches 1 in both BTs when the water orientations in the MT are downward (after a sufficiently long time). In contrast, P(t) approaches 0.5 in both BTs when the water orientations in the MT are upward, since the water orientations in both BTs fluctuate between upward and downward with a roughly equal probability. If we set PC as a value between 0.5 and 1, we expect that P > PC in the BTs corresponds to the downward water orientations in the MT, and that P < PC in the BTs implies the upward water orientations in the MT after a sufficiently long time. We consider the rapid convergence of the case for the downward water orientations in the MT, PC = 0.8 was set, which is a little larger than the average value of 0.5. From Fig. 5C we can see that P > PC in both BTs for any time t > 1 ns for the downward water orientations in the MT, while P < PC in both BTs for any time t > 8 ns for the upward water orientations in the MT. Thus, the charge signal at the MT can be readily distinguished from the value of P(t) in each BT within a time interval of ∼8 ns with an appropriate threshold PC = 0.8.
It is of interest to further examine the water orientations inside the branched tubes (BTs). Let's first consider a single channel without any external charge. There is a hydrogen atom pointing out at the topmost (or bottommost) end when water molecules inside the channel have a concerted upward (or downward) orientation inside the single channel. One upward → downward switch of the water orientations inside the nanochannel will result in an “apparent” transportation of an upward-pointing hydrogen atom at the topmost-end of the tube to a downward-pointing hydrogen atom at its bottommost-end.
Now we consider what happens to the water orientations inside a Y-shaped nanochannel. There is the similar bottom → upper transportation of only one hydrogen atom in the MT, after the downward → upward switch of its concerted water orientation. This causes a different situation in the BTs. There is the transportation of only half a hydrogen atom on the average in each BT, due to the conservation of the hydrogen atom (the total number of the hydrogen transportation is also one for both BTs). Different from the above simple single-tube case, this creates asymmetry for the Y-shaped tube, since the average half hydrogen atom “transportated” in BTs can be either zero or one at any moment. Thus, the situation will not be always a simple upward → downward (or downward → upward) orientation switch in each BT following that in the MT. In other words, if the water orientations in both BTs follow the downward water orientations as in the MT, these same water orientations in both BTs might not follow the upward water orientations as in the MT on the other hand. Interestingly, this is exactly what we have observed in simulations. For example, the downward water orientations in both BTs were observed when the water orientations in the MT were downward (corresponding to a negative monitored charge), while the upward water orientation in the MT (corresponding to a positive monitored charge) resulted in a fluctuation of the water orientations between the downward and upward orientations with an roughly equal probability in the BTs. This behaviour can be partly explained from the interactions of water molecules at the Y-junction. When the water orientations are downward in the MT, the O atom of the uppermost water molecule in the MT attracts two H atoms from the two bottommost water molecules in the BTs; on the other hand, when the water orientations are upward in the MT, one of the H atoms of the uppermost water molecule in the MT attracts both O atoms in the bottommost water molecules in the BTs. The interaction in the former case is much stronger than interaction in the latter case. Consequently, the average value of P(t) in the branch tubes is ∼0.5 for a concerted upward water orientation in the MT (corresponding to a positive monitored charge).
The time delay of water orientation in response to a switch in the charge signal has also been investigated.36 The time delay associated with the BTs was found to be slow, ∼40 ns on average with a maximal duration of 150 ns, to respond to the downward → upward signal switch of the water orientations in the MT, but much faster, ∼4 ns only, to respond to the upward → downward switch of the water orientations in the MT. This is because the downward water orientations in the MT (where one O atom controls two H atoms at the Y-junction) is much more stable than the case for the upward water orientations in the MT (where one H atom controls two O atoms at the Y-junction). It takes longer to shift from a more stable state to a less stable one. Compared to the time delay for switches in a single nanochannel (0.07–3.2 ns), the response times in the Y-channels are also generally longer, particularly for the downward → upward switch.
It should be noted that the signal multiplication capability is not very sensitive to the angles of the Y-shaped tube (the angles between SWNTs are currently at 120°, 120°, 120°). We have tried T-shaped tubes (with an angle of 90°, 90°, 180°) and other slightly different angles, and the results are more or less the same in terms of water orientation propagations.
4. Discussion
The orientation of the monitored-water, and consequently, the orientations of the all water molecules in the nanotubes can be controlled by a single charge with the same magnitude as the charge of one electron. This is a remarkable capability from signal transmission and conversion point of view. Then, what is the minimal charge needed for this capability? Numerical analysis shows that the magnitude of this signal charge needs to be larger than 0.6e—our data, using a charge from 0.6e to 1.0e, indicate that the water orientations can be well controlled with such a single charge. From the previous discussion,39 it is clear that the orientation of the monitored-water can be “fixated” only when the electrostatic interaction energy between the external charge and the monitored-water is comparable to the interaction energy of the monitored-water with either side of its neighboring water molecules. Since the imposed charge is placed at the center of a second carbon ring of the nanotube, which has a distance of about 4 Å from the centreline of the nanotube (close to the distance between two water neighbouring molecules), the charge required to control the orientation of the monitored-water should probably be larger than the partial charge on each hydrogen atom (e.g. 0.417e in TIP3P water model). This is consistent with our numerical observation.The technique to output the water dipole signal is very important for the practical applications. It is clear that there is a hydrogen (oxygen) atom with a partial positive (negative) charge at the end of the nanochannel when the water dipole chain is pointing outward (inward). This partial charge can trigger (or control) a neighbouring polar molecule or charge outside the channel (e.g. see Fig. 2A, for the yellow-colored first water molecule outside the channel). Since all water molecules share the same dipole orientation as the first water molecule inside the nanochannels the overall water dipole moment might be large enough to be detected. This dipole moment has a value of several Debyes36 and can interact with the electric field. We note that the semiconductor or conductor properties of some nanotubes may screen the dipole moment interacting with electric field. Insulator nanochannels, which may be fabricated in the future, might be better for this application.
We note that water is not the only polar molecule with a dipole moment. Thus, we expect other polar molecules, such as urea or ethanol, might have the same capability to transmit and multiply signals inside nanotubes. These small molecules, especially water, have some unusual and important physical and chemical properties, including their interactions with proteins.44,55–57 In fact, as a fundamental property of all cells, cell polarity have already played a central role in signal transmission and controlled a variety of polarized cell behaviours.58–62 Similar to the monitored-charge we used in this paper, a polarizing signal initiates polar distribution of signalling molecules and leads to polarity establishment and maintenance through the cytoskeleton and vesicular trafficking.59 For example, during planar cell polarity (PCP) signalling, core PCP proteins are sorted asymmetrically along the polarization axis, where PCP refers to coordinated polarization of cells within the plane of a cell sheet; this sorting is thought to direct coordinated downstream morphogenetic changes across the entire tissue.61–64 We also note that there have been other modes of achieving signal transmission, such as using the injection of finite duration vibrational signals encoding information into a biomolecular wire of the polypeptide glycine1000.65
Despite these efforts, there is still much more to be done for this relatively new field. Future directions might include, but are not limited to, the following forefronts.
(i) The concerted orientations of the dipole molecule chains inside the nanochannels are maintained by the dipole–dipole interactions. It is expected that the “in phase” strength between those dipole orientations becomes weak as the number of dipole molecules increases. This is very important in practice for the long-range transmission of the dipole signals. It has been shown that the correlation of the motion of the water molecules in a one-dimensional water chain confined in the SWNT decays as the number of water molecules in this water chain increases.66 How to achieve long-correlation in the dipole orientation in practice is a huge challenge.
(ii) Is there an energy transfer and how does the energy transfer process during such a dipole signal transmission, conduction and multiplications? These questions are still unclear.
(iii) Water molecules are very small. The devices and sensors based on water can be very small. However, the very small size of the water molecules can at the same time make the experimental realization and applications very difficult. The exploration of other larger polar molecules, such as urea, ethanol and glycerol, to be used as media in the nanotubes is highly needed and desired.
(iv) What other methods can ignite the initial dipole orientation of the monitored-water or some other molecules? Examples might be external electric fields, and/or other polar molecules connected to this monitored-water. Finally, we should note that most of the progresses discussed in this short feature article are based on molecular dynamics simulations so far. Experimental validations are therefore much needed.
5. System and methods
The molecular dynamics simulations for the single water channel were carried out in NVT ensemble at a constant temperature of 300 K using a Berendsen thermostat,42 and in constant volumes (Lx × Ly × Lz = 6.01 nm × 6.01 nm × 11.00 nm in SWNT systems with 12922 water molecules using the molecular modelling package Gromacs 4.0.543,45 (For simulations in the Y-SWNT or 3Y-SWNT systems, all settings are referred to ref. 36.) The device was constrained at the center of simulation boxes, by using the position restraints (the whole tube length is partitioned evenly into three segments with an interval of 1.71 nm, and thus four rings of carbon atoms are constrained) solvated with water molecules with constant density. We adopted the particle-mesh Ewald method67 to model long-range electrostatic interactions. We applied periodic boundary conditions in all directions. A time step of 2 fs was used, and data were collected every 0.5 ps. In all of our simulation results, the TIP3P water model was applied and the carbon atoms were modelled as uncharged Lennard-Jones particles with a cross-section of σCC = 0.34 nm, and σCO = 0.3275 nm, and a potential well depth of εCC = 0.3612 kJ mol−1, and εCO = 0.4802 kJ mol−1.37 The system was first minimized with conjugate gradient method for 10 000 steps, and then equilibrated with molecular dynamics for 5 ns at 300 K before data collection. An external signal charge of magnitude 1.0e was then introduced to the center of the second carbon ring of the main tubes (constrained by using the position restraints during the simulations), and its opposite charge for neutralization was constrained near the edge of each box at coordinates (0.00 nm, 3.00 nm, 0.00 nm), which is far enough to have a meaningful influence on the signal charge (our numerical data also confirm this). These charges were introduced to the system after initial equilibration in order to observe the response time of water dipole reorientation (shown in Fig. 2) upon the charge signal.Acknowledgements
This work was partially supported by grants from Chinese Academy of Sciences, National Natural Science Foundation of China under grant No. 10825520, the National Basic Research Program of China under grant No. 2007CB936000, Shanghai Leading Academic Discipline Project under grant No B111 and Shanghai Supercomputer Center of China. RZ acknowledges support from the IBM BlueGene Science Program.References
- K. C. Kao and G. A. Hockham, Dielectric-Fibre Surface Waveguides for optical frequencies, Proc. IEE, 1966, 133, 1151–1158 Search PubMed.
- R. W. Wagner and J. S. Lindsey, A molecular photonic wire, J. Am. Chem. Soc., 1994, 116, 9759–9760 CrossRef CAS.
- C. P. Collier, et al. Electronically Configurable Molecular-Based Logic Gates, Science, 1999, 285, 391–394 CrossRef CAS.
- A. Nitzan and M. A. Ratner, Electron Transport in Molecular Wire Junctions, Science, 2003, 300, 1384–1389 CrossRef CAS.
- G. Strack, M. Pita, M. Ornatska and E. Katz, Boolean Logic Gates that Use Enzymes as Input Signals, ChemBioChem, 2008, 9, 1260–1266 CrossRef CAS.
- K. Kwok and J. Ellenbogen, Moletronics: future electronics, Mater. Today, 2002, 5, 28–37 CrossRef CAS.
- H. Q. Xu, Nanotubes: The logical choice for electronics?, Nat. Mater., 2005, 4, 649–650 CrossRef CAS.
- S. Litvinchuk, et al. Synthetic pores with reactive signal amplifiers as artificial tongues, Nat. Mater., 2007, 6, 576–580 CrossRef CAS.
- D. R. Koenig, E. M. Weig and J. P. Kotthaus, Ultrasonically driven nanomechanical single-electron shuttle, Nat. Nanotechnol., 2008, 3, 482–485 CrossRef CAS.
- P. R. Bandaru, C. Daraio, S. Jin and A. M. Rao, Novel electrical switching behaviour and logic in carbon nanotube Y-junctions, Nat. Mater., 2005, 4, 663–666 CrossRef CAS.
- A. Bachtold, P. Hadley, T. Nakanishi and C. Dekker, Logic circuits with carbon nanotube transistors, Science, 2001, 294, 1317–1320 CrossRef CAS.
- R. Fan, M. Yue, R. Karnik, A. Majumdar and P. D. Yang, Polarity switching and transient responses in single nanotube nanofluidic transistors, Phys. Rev. Lett., 2005, 95, 086607 CrossRef.
- F. Chen, J. Hihath, Z. Huang, X. Li and N. J. Tao, Measurement of Single-Molecule Conductance, Annu. Rev. Phys. Chem., 2007, 58, 535–564 CrossRef CAS.
- J. R. Heath, Molecular Electronics, Annu. Rev. Mater. Res., 2009, 39, 1–23 CrossRef CAS.
- T. Drummond, M. Hill and J. Barton, Electrochemical DNA sensors, Nat. Biotechnol., 2003, 21, 1192–1199 CrossRef CAS.
- G. D. Yuan, et al. p-Type ZnO Nanowire Arrays, Nano Lett., 2008, 8, 2591–2597 CrossRef CAS.
- J. Kong, et al. Nanotube Molecular Wires as Chemical Sensors, Science, 2000, 287, 622–625 CrossRef CAS.
- Y. Cui, Q. Wei, H. Park and C. Lieber, Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species, Science, 2001, 293, 1289 CrossRef CAS.
- D. Li, S. Song and C. Fan, Target-Responsive Structural Switching for Nucleic Acid-Based Sensors, Acc. Chem. Res., 2010, 43, 631–641 CrossRef CAS.
- M. Bennett and R. Zukin, Electrical Coupling and Neuronal Synchronization in the Mammalian Brain, Neuron, 2004, 41, 495–511 CrossRef CAS.
- B. Connors and M. Long, Electrical Synapses In The Mammalian Brain, Annu. Rev. Neurosci., 2004, 27, 393–418 CrossRef CAS.
- M. Bartos, I. Vida and P. Jonas, Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks, Nat. Rev. Neurosci., 2007, 8, 45–56 CrossRef CAS.
- G. Söhl, S. Maxeiner and K. Willecke, Expression And Functions Of Neuronal Gap Junctions, Nat. Rev. Neurosci., 2005, 6, 191 CrossRef.
- S. Hormuzdi, et al. Impaired Electrical Signaling Disrupts Gamma Frequency Oscillations in Connexin 36-Deficient Mice, Neuron, 2001, 31, 487–495 CrossRef CAS.
- Y. Amitai, et al. The spatial dimensions of electrically coupled networks of interneurons in the neocortex, J. Neurosci., 2002, 22, 4142 CAS.
- G. Zoidl and R. Dermietzel, On the search for the electrical synapse: a glimpse at the future, Cell Tissue Res., 2002, 310, 137–142 CrossRef.
- B. Pfeuty, G. Mato, D. Golomb and D. Hansel, Electrical synapses and synchrony: the role of intrinsic currents, J. Neurosci., 2003, 23, 6280 CAS.
- R. G. Smock and L. M. Gierasch, Sending Signals Dynamically, Science, 2009, 324, 198–203 CrossRef CAS.
- C.-J. Tsai, A. del Sol and R. Nussinov, Allostery: Absence of a Change in Shape Does Not Imply that Allostery Is Not at Play, J. Mol. Biol., 2008, 378, 1–11 CrossRef CAS.
- C. Tsai, A. Sol and R. Nussinov, Protein allostery, signal transmission and dynamics: a classification scheme of allosteric mechanisms, Mol. BioSyst., 2009, 5, 207–216 RSC.
- J. Sethna, Statistical mechanics: entropy, order parameters, and complexity, Oxford University Press, USA, 2006 Search PubMed.
- O. Dudko, Statistical Mechanics: Entropy, Order Parameters, and Complexity, J. Stat. Phys., 2007, 126, 429–430 CrossRef.
- J. J. de Jonge, M. A. Ratner, S. W. de Leeuw and R. O. Simonis, Molecular Dipole Chains III: Energy Transfer, J. Phys. Chem. B, 2004, 108, 2666–2675 CrossRef CAS.
- J. J. de Jonge, M. A. Ratner, S. W. de Leeuw and R. O. Simonis, Controlling the Energy Transfer in Dipole Chains, J. Phys. Chem. B, 2006, 110, 442–446 CrossRef CAS.
- J. J. de Jonge, M. A. Ratner and S. W. de Leeuw, Local Field Controlled Switching in a One-Dimensional Dipolar Array, J. Phys. Chem. C, 2007, 111, 3770–3777 CrossRef CAS.
- Y. Tu, et al. Water-mediated signal multiplication with Y-shaped carbon nanotubes, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 18120–18124 CrossRef CAS.
- G. Hummer, J. C. Rasaiah and J. P. Noworyta, Water conduction through the hydrophobic channel of a carbon nanotube, Nature, 2001, 414, 188–190 CrossRef CAS.
- J. Köfinger, G. Hummer and C. Dellago, Macroscopically ordered water in nanopores, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 13218–13222 CrossRef.
- J. Y. Li, et al. Electrostatic gating of a nanometer water channel, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 3687–3692 CrossRef CAS.
- X. J. Gong, et al. A charge-driven molecular water pump, Nat. Nanotechnol., 2007, 2, 709–712 CrossRef CAS.
- R. H. Wan, J. Y. Li, H. J. Lu and H. P. Fang, Controllable Water Channel Gating of Nanometer Dimensions, J. Am. Chem. Soc., 2005, 127, 7166–7170 CrossRef CAS.
- H. J. C. Berendsen, J. P. M. Postma, W. F.v. Gunsteren, A. DiNola and J. R. Haak, Molecular dynamics with coupling to an external bath, J. Chem. Phys., 1984, 81, 3684–3690 CrossRef CAS.
- H. J. C. Berendsen, D. van der Spoel and R. van Drunen, GROMACS: A message-passing parallel molecular dynamics implementation, Comput. Phys. Commun., 1995, 91, 43–56 CrossRef CAS.
- L. Hua, R. Zhou, D. Thirumalai and B. Berne, Urea denaturation by stronger dispersion interactions with proteins than water implies a 2-stage unfolding, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 16928 CrossRef CAS.
- E. Lindahl, B. Hess and D. van der Spoel, GROMACS 3.0: a package for molecular simulation and trajectory analysis, J. Mol. Model., 2001, 7, 306–317 CAS.
- R. Zhou, Exploring the protein folding free energy landscape: coupling replica exchange method with P3ME/RESPA algorithm, J. Mol. Graphics Modell., 2004, 22, 451–463 CrossRef CAS.
- L. Hua, X. H. Huang, R. H. Zhou and B. J. Berne, Dynamics of water confined in the interdomain region of a multidomain protein, J. Phys. Chem. B, 2006, 110, 3704–3711 CrossRef CAS.
- P. Liu, X. Huang, R. Zhou and B. J. Berne, Hydrophobic aided replica exchange: an efficient algorithm for protein folding in explicit solvent, J. Phys. Chem. B, 2006, 110, 19018–19022 CrossRef CAS.
- C. Papadopoulos, A. Rakitin, J. Li, A. S. Vedeneev and J. M. Xu, Electronic Transport in Y-Junction Carbon Nanotubes, Phys. Rev. Lett., 2000, 85, 3476 CrossRef CAS.
- B. C. Satishkumar, P. J. Thomas, A. Govindaraj and C. N. R. Rao, Y-junction carbon nanotubes, Appl. Phys. Lett., 2000, 77, 2530–2532 CrossRef CAS.
- W. Z. Li, J. G. Wen and Z. F. Ren, Straight carbon nanotube Y junctions, Appl. Phys. Lett., 2001, 79, 1879–1881 CrossRef CAS.
- F. L. Deepak, A. Govindaraj and C. N. R. Rao, Synthetic strategies for Y-junction carbon nanotubes, Chem. Phys. Lett., 2001, 345, 5–10 CrossRef CAS.
- M. Terrones, et al. Molecular Junctions by Joining Single-Walled Carbon Nanotubes, Phys. Rev. Lett., 2002, 89, 075505 CrossRef CAS.
- N. Gothard, et al. Controlled Growth of Y-Junction Nanotubes Using Ti-Doped Vapor Catalyst, Nano Lett., 2004, 4, 213–217 CrossRef CAS.
- P. Ball, Water as an active constituent in cell biology, Chem. Rev., 2008, 108, 74–108 CrossRef CAS.
- R. H. Zhou, X. H. Huang, C. J. Margulis and B. J. Berne, Hydrophobic collapse in multidomain protein folding, Science, 2004, 305, 1605–1609 CrossRef CAS.
- P. Liu, X. Huang, R. Zhou and B. Berne, Observation of a dewetting transition in the collapse of the melittin tetramer, Nature, 2005, 437, 159–162 CrossRef CAS.
- L. Søgaard-Andersen, Cell polarity, intercellular signalling and morphogenetic cell movements in Myxococcus xanthus, Curr. Opin. Microbiol., 2004, 7, 587–593 CrossRef CAS.
- Z. Yang, Cell polarity signaling in Arabidopsis, Annu. Rev. Cell Dev. Biol., 2008, 24, 551–575 CrossRef CAS.
- S. Fuller-Espie, P. Hoffman Towler, D. Wiest, I. Tietjen and L. Spain, Transmembrane polar residues of TCR beta chain are required for signal transduction, Int. Immunol., 1998, 10, 923 CrossRef CAS.
- C. Jones, et al. Ciliary proteins link basal body polarization to planar cell polarity regulation, Nat. Genet., 2008, 40, 69–77 CrossRef CAS.
- A. Ganner, et al. Regulation of ciliary polarity by the APC/C, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 17799 CrossRef CAS.
- J. Axelrod, Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling, Genes Dev., 2001, 15, 1182 CAS.
- B. Mitchell, R. Jacobs, J. Li, S. Chien and C. Kintner, A positive feedback mechanism governs the polarity and motion of motile cilia, Nature, 2007, 447, 97–101 CrossRef CAS.
- L. Miao and J. Seminario, Molecular dynamics simulations of signal transmission through a glycine peptide chain, J. Chem. Phys., 2007, 127, 134708 CrossRef.
- J. Y. Li, Z. X. Yang, H. P. Fang, R. H. Zhou and X. W. Tang, Effect of the Carbon-Nanotube Length on Water Permeability, Chin. Phys. Lett., 2007, 24, 2710–2713 CrossRef CAS.
- T. Darden, D. York and L. Pedersen, Particle mesh Ewald: An N [center-dot] log(N) method for Ewald sums in large systems, J. Chem. Phys., 1993, 98, 10089–10092 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |