Towards chirality-pure carbon nanotubes
Yani
Zhang
and
Lianxi
Zheng
*
School of Mechanical and Aerospace Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798. E-mail: Lxzheng@ntu.edu.sg
First published on 10th September 2010
Abstract
Current as-grown single-walled carbon nanotubes vary in diameter and chirality, which results in variations in their electronic and optical properties. Two approaches have been intensively studied to obtain chirality-pure nanotube structures and thus uniform properties for advanced applications. The first approach involves the post-synthesis separation according to the nanotubes' chiral vectors (n, m), and the second one involves direct synthes of carbon nanotubes with the same (n, m). This paper reviews the efforts along these two directions, with emphasis on the most recent progress of post-synthesis separation and the perspectives of controllable synthesis.
 Yani Zhang | Dr Yani Zhang was born in Shaanxi, China. She received her BSc degree in 2003 and her PhD degree in 2009 in Materials Science & Engineering, Northwestern Polytechnical University, China. She researched carbon fiber reinforced ceramic composites for ∼5 years in the National Key Lab of Thermostructure Composite Materials during her PhD. Currently, she is a research fellow in Nanyang Technological University (NTU), Singapore. Her research interests include carbon nanotube (CNT) materials, advanced nanotube fibers, composites, and ceramics, for functional and structural applications. |
 Lianxi Zheng | Prof. Lianxi Zheng received his BE degree in Electronic Engineering from Southeast University in China, and his Ph.D. on Physics from The University of Hong Kong. He has worked in Los Alamos National Laboratory as a director's postdoctoral fellow, and in CNT Technologies Inc. (USA) as a research scientist. In January 2008, he joined Nanyang Technological University, Singapore. His research interests include novel enabling technologies on nano-material synthesis, smart nanotube based sensors, nanoelectronics, and nanotube fibers & fiber composites. |
1. Introduction
A single-walled carbon nanotube (SWCNT) is a seamless cylinder structure that could be considered as a rolled-up graphene sheet. Owing to this unique structure, SWCNTs have excellent properties including nanoscale dimension, ballistic electron transport, high current capacity, and superior mechanical and chemical properties, making them the potential building blocks for next generation electronics.1–4 SWCNT transistors, including single-electron transistors employing metallic nanotubes5 and field-effect transistors employing semiconducting nanotubes,6 have already been demonstrated. However, the electronic and optical properties of an SWCNT strongly depend on its diameter and chiral vector (the direction in which the graphene is rolled up). Approximately 67% of SWCNTs are semiconducting and 33% of them are metallic among all possible (n, m)s,7–9 and the bandgap changes inversely with the diameter for semiconducting SWCNTs. As a result, an as-grown nanotube product from any synthesis methods, including arc discharge,10 laser ablation,11 and chemical vapor deposition (CVD),12 is a mixture of nanostructures with a broad distribution in diameter and chirality. Obtaining identical SWCNT structures, i.e. the same diameter and the same chirality, is then the major challenge for large-scale applications of SWCNTs, and has gained considerable research attention.13,14There exist two strategies for obtaining chirality-pure SWCNT structures: controllable synthesis and post-synthesis separation. In controllable synthesis, the diameter and chirality of a SWCNT are controlled through optimizing catalyst preparation and growth parameters,15–17 based on an assumption that some particular SWCNTs are thermodynamically favorable under those conditions. And, more recently, seeded growth18 was proposed and demonstrated a promising future. In post-synthesis separation strategy, SWCNTs are chemically functionalized or physically encapsulated by surfactants, and then sorted out according to their structures.19–22 By tuning the surfactant–SWCNT interaction, interested SWCNT structures could be separated from a starting mixture. This method is the most promising approach in the current stage, evidenced by the significant success in DNA–SWCNT separation.23 Among many post-synthesis approaches, selective destruction is a strategy used to destroy un-wanted SWCNTs and keep the remainder for utilization. Particular methods include selective oxidation,24,25 gas-phase etching,26,27 and electrical breakdown.28,29 This strategy is mainly used to separate nanotubes by conducting type, i.e. semiconducting from metallic, but shows limited selection on diameter and chirality and will not be discussed in this paper.
Therefore, this review paper will mainly focus on controllable synthesis and post-synthesis separations that have demonstrated a potential capability for chirality selection and diameter control, with emphasis on the most recent progress of post-synthesis separation and a relatively detailed review on the controllable synthesis.
2. Post-synthesis separation
For post-synthesis separation, SWCNTs need to be firstly dispersed in liquids, otherwise they tend to bond to each other through van der Waals forces to form bundles or large aggregates. By ultrasonically agitating an aqueous dispersion of raw SWCNTs in sodium dodecyl sulfate and then centrifuging, O'Connell30 has successfully separated individual SWCNTs from bundles by taking advantage of the different sedimentation rates between them. Inspired by this work, many researchers have explored selective chemistry with reactivity that varies as a function of an SWCNT's electronic type, diameter, and chiral vector, in order to amplify the differences between various SWCNTs and then sort them accordingly. Furthermore, extensive study has been carried out on chemical functionalization of nanotubes, and the details can be found in several good review papers.31–33 In general, chemical functionalization can be classified into two categories: covalent functionalization and non-covalent encapsulation. Covalent functionalization often induces substantial changes to the structure and properties of SWCNTs, while non-covalent functionalization has minimized perturbation of the SWCNTs. Both of them have been used for dispersing SWCNTs.Following selective functionalization, SWCNTs could then be sorted by using a variety of techniques including electrophoresis,34–38 ultracentrifugation,20,39–43 and chromatography.19,23,44–47 Electrophoresis sorts SWCNTs according to their relative mobility in response to an electrical field. This method is favorable for length sorting of SWCNTs, because short SWCNTs have small molecular weight and then move fast under the electrical field. If an AC electrical field is applied, the method can be extended to sort SWCNTs according to their conducting type, owing to the difference in the dielectric constants of metallic and semiconducting SWCNTs. Ultracentrifugation, especially density gradient ultracentrifugation, has been used to sort SWCNTs according to their buoyant densities. By introducing a density gradient, the SWCNTs will be separated into different layers in the centrifuge tube according to their buoyant densities, which are in turn determined by their diameters. Chromatography is a technique to separate interested analyte from other molecular mixture based on the differential partitioning between the “mobile phase” and the “stationary phase”. A slight difference in partition coefficient will result in differential retention of analyte on the stationary phase. This method was first introduced for the separation of DNA-encapsulated SWCNTs by Zheng,19 and showed great promise in a recent study.23
Numerous separation methods have been proposed by combining various selective surfactants with different separation techniques. For any combination, the principle of separation technique is relatively clear, but the mechanism of selective chemistry is very complicated. Therefore, in this paper we focus our discussion on the discrimination mechanism, and accordingly we can group related research work into two categories, chemical-affinity approach and surfactant-pattern approach, based on their research emphases on structure discrimination.
2.1 Affinity approach
In this approach, the structure discrimination originates from the structure-dependent chemical affinity between the surfactant molecules and SWCNT surfaces. Most of the sorting methods belong to this category, and the diameter-dependent binding has been observed for many common surfactants. One successful example is of using structure-discriminating surfactants to engineer subtle difference in SWCNTs' buoyant densities. By using a bile salt, sodium cholate (SC), to encapsulate SWCNTs, followed by density gradient ultracentrifugation, multiple regions of separated SWCNTs have been observed in the centrifuge tube.20 As shown in Fig. 1a, different colors represent different layers of SWCNTs with different structures, with the most buoyant region corresponding to the semiconducting nanotubes (with different bandgaps) and lower sediment in the gradient corresponding to the bundles or aggregates. The successful separation of semiconducting SWCNTs by diameter and bandgap is supported by the optical absorption for different transitions in the 900–1340 nm range (first-order semiconducting transitions), as shown in Fig. 1b. The spectra in Fig. 1b also illustrate that SWCNTs of increasingly larger diameters are enhanced at increasingly larger densities. This observation contradicts the mass-volume ratio of intrinsic SWCNTs, indicating the diameter-dependent affinity of surfactants to SWCNTs, with more surfactants attaching to the larger diameter SWCNTs.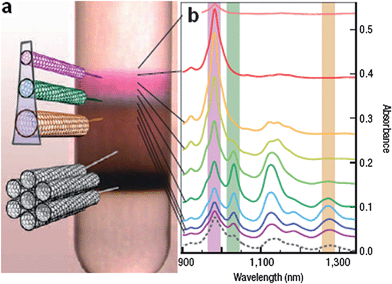 | ||
| Fig. 1 Sorting SWCNTs by diameter. (a) SC encapsulated CoMoCAT-grown SWCNTs after centrifugating. Colored bands indicate the isolated SWCNTs sorted by diameter and bandgap. Bundles, aggregates and insoluble materials deposit at lower part of centrifuge tube. (b) Adsorption spectra indicate that SWCNTs of increasing diameter are more concentrated at larger densities. (Adapted with permission from ref. 20, © Nature.) | ||
More interestingly, the density–structure relationship could be tuned by introducing a co-surfactant or changing the pH value of solution. As shown in Fig. 2, by increasing the pH to 8.5, the (7, 5) SWCNTs shift to lower buoyant density. Alternatively, by adding sodium dodecyl sulfate (SDS) to compete with the SC for non-covalent binding, (7, 5) and (9, 5)/(8, 7) SWCNTs shift to larger buoyant densities. Combining this method with multiple cycles of ultracentrifugation, it is technically reasonable to separate high purity SWCNTs according to their chiralities. Using a similar approach but different surfactant and solvent, (7, 5), (7, 6), (10, 5) and (9, 7) SWCNTs with respective enrichments of up to 90% have been separated from raw materials.22
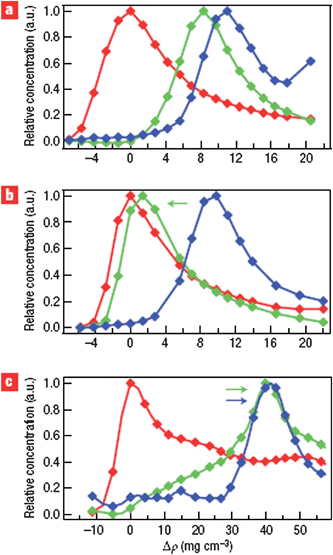 | ||
| Fig. 2 Tuning the structure–density relationship. (6, 5), (7, 5) and (9, 5)/(8, 7) SWCNTs from CoMoCAT growth are represented by red, green and blue color, respectively. (a) SC with pH 7.4. (b) SC with Ph 8.5. (c) SDS/SC with pH 7.4. (Adapted with permission from ref. 20, © Nature.) | ||
2.2 Surfactant-pattern approach
Besides chemical affinity, the structure patterns of surfactant molecules or surfactant assemblies were also used for chirality selection. It was found that geometrically constrained polyaromatic amphiphiles were selective to the chirality angle of SWCNTs, with a pentacenic-based amphiphile recognizing armchair SWCNTs and a quaterylene-based amphiphile discriminating zig-zag SWCNTs.48 The organization of several other aromatic surfactants are also capable of the enrichment of a certain few (n, m) SWCNTs. In these studies, surfactants could form a chiral pattern around SWCNTs through hydrogen bonding between adjacent moieties. The interaction between the surfactants and SWCNTs was found to be strongly dependant on the chirality of SWCNTs, resulting in the discrimination of particular chiral SWCNTs.21,23The most promising approach in this category should be DNA–SWCNT chromatography. DNA sequences can form ordered structures on SWCNTs, allowing diameter and conducting type-sorting of SWCNTs through ion exchange chromatography (IEX).19 Experimental19,23,49,50 and theoretical51,52 studies indicate that DNA–SWCNT interaction and the resulting hybrid structure are dependent on both DNA sequence and SWCNT structure. When the sequence length is comparable to the van der Waals circumference of a typical SWCNT (1 nm in diameter), the IEX separation becomes extremely sensitive to the length of the DNA strand.23 Authors have identified more than 20 DNA sequences, each of which allows purification of a particular (n, m) SWCNTs. Using these DNA sequences, 12 semiconducting SWCNTs have been successfully sorted from the starting mixture materials, evidenced by their absorption spectra shown in Fig. 3. In this study, the authors suggested that particular DNA strands could form a stable and well-ordered two-dimensional (2D) sheet through hydrogen bonding (Fig. 4a), and the 2D sheet could be further rolled up onto a particular SWCNT to form a stable barrel (Fig. 4b). This chiral and ordered DNA–SWCNT structure would minimize its van der Waals and hydrophobic interaction with IEX resin, allowing it to be separated and purified. Since each of the recognition sequences can form an ordered DNA barrel structure only on one particular (n, m) SWCNT, and a large number of sequences are available in DNA library, this method is expected to be used to separate any (n, m) SWCNTs in the future.
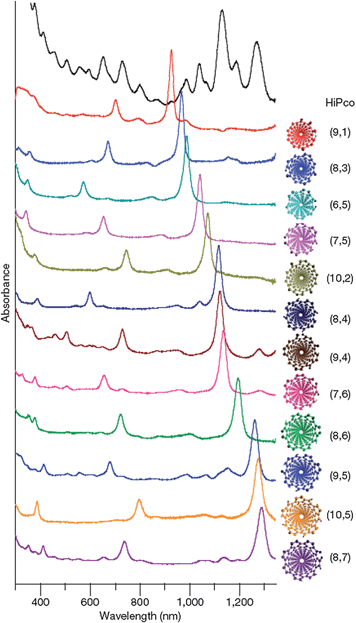 | ||
| Fig. 3 Optical absorption spectra and atomic structures of 12 purified semiconducting SWCNTs and the starting HiPco mixture. (Adapted with permission from ref. 23, © Nature.) | ||
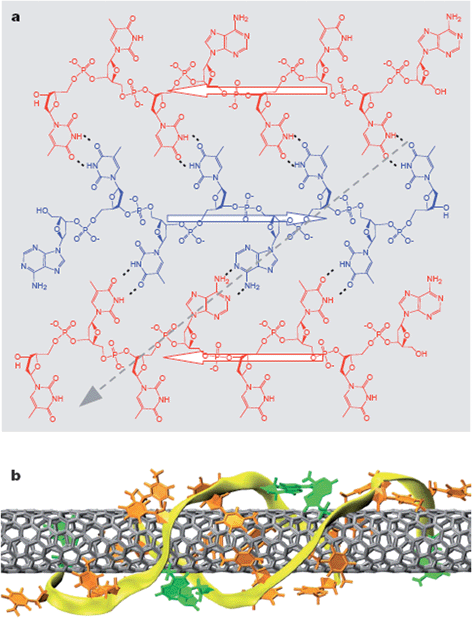 | ||
| Fig. 4 DNA–SWCNT structure. (a) 2D DNA sheet structure formed through hydrogen bonds. b) DNA barrel on a SWCNT formed by rolling up a 2D DNA sheet. (Adapted with permission from ref. 23, © Nature.) | ||
3. Controllable synthesis
In parallel to the post-synthesis separation, tremendous efforts have been paid to controllable synthesis, aiming at obtaining chirality-pure SWCNT structures from one single process step. Although it is still under studying, we are gradually gaining controllability over the location, length, direction, diameter, defect and chirality of the SWCNT growth.53–59 Here we review two important areas: diameter and chirality control through condition optimization and seeded nanotube growth.3.1. Diameter and chirality control through condition optimization
Extensive efforts towards the controllable growth of nanotubes with specific diameter, chirality, and conducting type have been made, by modifying the catalyst type/materials,60–62 support/substrate materials, synthesis temperature, gas flow rate, and carbon source feedstock/concentration.63,58 These efforts could be summarized into three groups: (i) controlling catalyst type and particle size;64 *(ii) controlling the growth conditions, such as temperature15 and pressure;65–67 and (iii) using other top-down methods.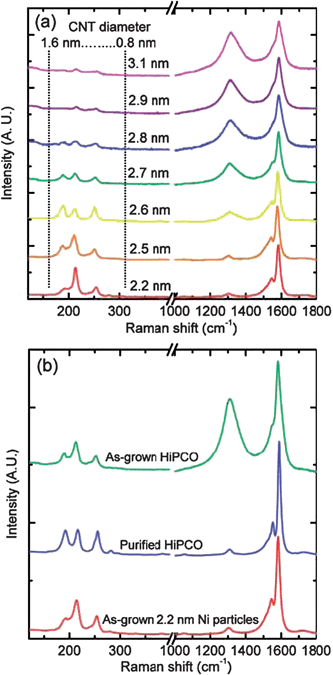 | ||
| Fig. 5 (a) Micro Raman spectra of nanotubes synthesized in a flow furnace with different Ni particle mean diameters. (b) Comparison of micro Raman spectrum of nanotubes synthesized using 2.2 nm mean diameter Ni particles with as-grown and purified commercial HiPCO product samples. (Adapted with permission from ref. 68, © American Chemical Society.) | ||
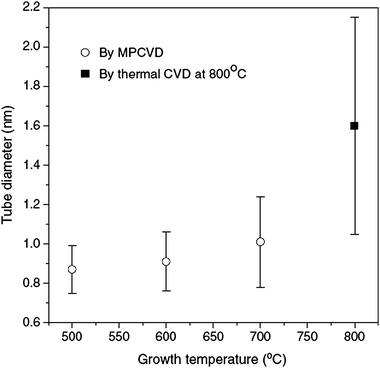 | ||
| Fig. 6 Plot of the average diameters with standard deviation for SWCNT samples grown under different temperatures (adapted with permission from ref. 77, © Elsevier). | ||
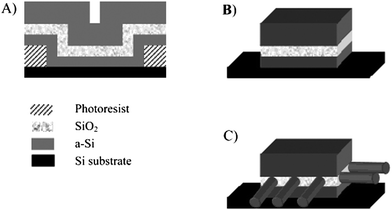 | ||
| Fig. 7 Synthetic steps for the growth of carbon nanotubes from exposed face of Si/SiO2/Si multilayer. (a) Cross-section of patterned Si/SiO2/Si multilayer. (b) Post structure after photo resist liftoff and HF etching of surface oxide. (c) nanotube growth on exposed face of SiO2 thin film. (Adapted with permission from ref. 82, © American Chemical Society.) | ||
3.2. Seeded growth
Because the diameter variations that span many chiral indices are inevitable owing to the thermal vibrations in catalyst particles at elevated growth temperatures, growing specific (n, m) SWCNTs through condition optimization was speculated to be impossible.86 Seeded growth, or nanotube cloning, was then proposed.18 In nanotube cloning, as shown in Fig. 9, a SWCNT segment with desired (n, m) would be prepared with open ends, upon which catalyst is docked. This segment, serving as a seed, is then placed into a CVD chamber for regrowth. It is expected that a new SWCNT will grow epitaxially from the seeded SWCNT under proper growth conditions, and thus preserve the structure (diameter and chirality) of the SWCNT seed.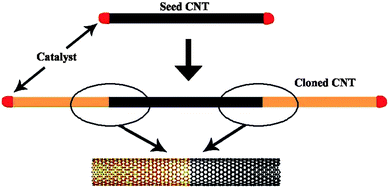 | ||
| Fig. 9 Sketch of nanotube cloning. | ||
This idea was first proposed and demonstrated by Smalley's group.18 They started from an array of open-ended SWCNTs, and initialized a second CVD growth after “docking” catalyst atoms on nanotube's open tips. The elongation of a nanotube array was observed, and the regrown SWCNTs were found to have a diameter distribution similar to that of the original SWCNTs. Following this concept, they further demonstrated the nanotube regrowth from individual SWCNTs87,88 to check whether or not such a regrowth process could be achieved at individual SWCNT level. As shown in Fig. 10, the amplification of a SWCNT can be clearly seen at the same location, indicated by the different nanotube lengths before and after regrowth. The height profiles of Atomic Force Microscopy (AFM) indicate that the diameter of amplified SWCNT is almost identical to the diameter of original seed SWCNT, demonstrating the success of regrowth. Future work is needed to clarify whether or not the amplified individual SWCNTs have preserved their original chiralities.89
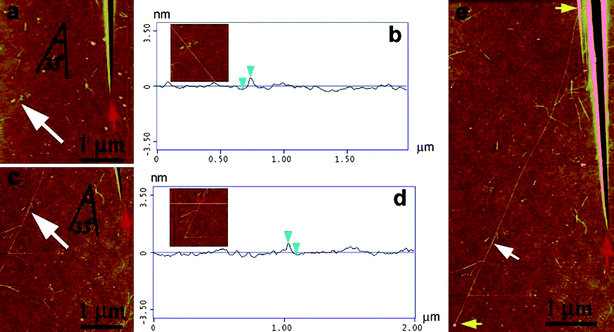 | ||
| Fig. 10 AFM images of a seed SWCNT and its amplified SWCNT. (a) The starting 200 nm SWCNT indicated by the white arrow (the red arrow is the locator inscription). (b) A height profile of the seed-SWCNT showing a diameter of 0.73 nm. (c) Amplified SWCNT after regrowth. (d) Height profile of regrown SWCNT showing a nearly identical height of 0.72 nm over several points measured along its entire length. (e) The entire length of the amplified nanotube is 6.7 μm (between the yellow arrows). (Adapted with permission from ref. 87, © American Chemical Society.) | ||
Catalyst-free regrowth was also used for SWCNT cloning.90 By using open-end SWCNTs as seeds, duplicated SWCNTs have been directly grown from parent segments via an open-end growth mechanism without involving any metal catalyst, as shown in Fig. 11. Among 600 SWCNT seeds, about 56 (9%) of them show observable regrowth. By changing the substrate from SiO2/Si to quartz, the regrowth yield has been improved from 9% to 40%. AFM height profiles show that newly grown SWCNTs have heights nearly identical to the original SWCNT seeds. More importantly, Raman spectra show the same RBM shift before and after regrowth, as shown in Fig. 11c, providing the first evidence of successful cloning at the individual SWCNT level.
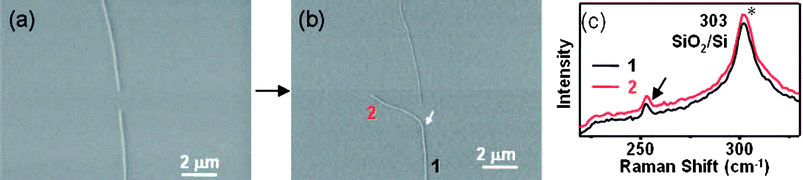 | ||
| Fig. 11 (a) SEM image of open-ended SWCNT seeds. (b) SEM image of the duplicate SWCNT grown from a SWCNT seed. The white arrow denotes the start of the regrowth. (c) Typical Raman spectroscopy across the SWCNT of the bottom in panel b, which shows the same RBM shift at 252.7 cm−1 (the G-band was not shown here, and the other peak at 303 cm−1 marked by * is from the SiO2/Si substrate) and there are no other RBM peaks at low frequency. (Adapted with permission from ref. 90, © American Chemical Society.) | ||
Although nanotube cloning has been demonstrated conceptually, the ultimate protocol would involve taking a single (n, m) SWCNT, cutting it into many short segments, using each of them as the seed for cloning, and repeating the process to obtain considerable amount chirality-pure SWCNT structure for applications.87 Moving towards this goal, several particular issues need to be taken into consideration: (i) the synthesis process itself should be extremely stable so that no defects form during the growth; (ii) the growth mode should be tip growth for catalyst-docked cloning, otherwise the catalyst particles will be inserted between the seed SWCNTs and the regrown SWCNTs and; (iii) the regrowth should be an elongation process, i.e. non-nucleation growth. Recent progress on ultralong SWCNT synthesis may offer solutions for the three above-mentioned issues.
It is well known that the electronic properties of a SWCNT depend not only on its diameter and chirality, but also strongly on the defects.57,91–95 It has been reported that a metal–semiconductor junction behaves like a rectifying diode with nonlinear transport characteristics that are strongly asymmetric with respect to bias polarity; the conductance in a metal–metal junction appears to be strongly suppressed and it displays a power-law dependence on temperature and applied voltage.57 Unfortunately most SWCNTs possess a considerable amount of defects along their length.96 Cloning alone does not guarantee identical electrical properties in final products if defects present. Therefore, developing a method of growing defect-free SWCNTs with a considerable length is an inevitable requirement for any cloning approach. Using ethanol chemical vapor deposition, our group has successfully synthesized individual SWCNTs that are up to 40 mm long.53 The growth rate is as high as 11 μm per second. We believe that oxygen atoms present in ethanol contribute to such a growth by keeping the catalyst active for longer time by removing harmful amorphous carbon.97,98 Raman spectra confirmed that almost all individual long SWCNTs maintain their diameter and chirality along their entire length, i.e. these SWCNTs are nearly defect-free. This synthesis method is especially suitable for nanotube cloning, with the high cloning efficiency offered by the ultralong length and fast growth rate of the initial seeds, and the possible identical structures guaranteed by defect-free growth.
The long SWCNTs appear to grow by a tip-growth mechanism,53 that is, the catalytic Fe particles moved with the growing SWCNT tips, evidenced by the SWCNTs' morphologies shown in Fig. 12. A base growth with a stationary catalyst particle would require the whole SWCNT to overcome the van der Waals forces with the substrate and slide on the substrate, which is clearly impossible for obtaining ultralong and straight SWCNT on an Si surface. As shown in Fig. 12, short SWCNTs or dying SWCNTs show wavy and tangled morphologies, but long SWCNTs are relatively straight along most of their length, strongly suggesting a tip growth mode in this synthesis method for ultralong SWCNTs.
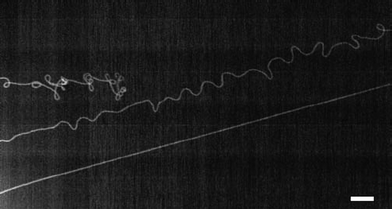 | ||
| Fig. 12 Nanotube morphologies indicate the tip growth mode (scale bar is 10 μm). (Adapted with permission from ref. 53, © Nature.) | ||
The key issue in nanotube cloning is how to keep the regrowth as an elongation process rather than nucleation of new nanotube structures. To address this issue, we have studied the nucleation kinetics of ultralong SWCNTs.99 By counting the number density of SWCNTs and studying its temperature dependence, the nucleation process of SWCNTs could be studied separately from other growth kinetics. From the Arrhenius form relation, we found that the nucleation energy of such long SWCNTs is about 2.8 eV, which is obviously larger than the diffusion energy (∼1.5 eV) of cabon atoms in metal particles.100,101 This big difference suggests that elongating an existing nanotube (through carbon diffusion) is energetically much more favorable than nucleating a new SWCNT structure. Ethanol CVD could then be a very promising technique for the seeded growth of SWCNTs to obtain controlled structures.
4. Conclusion
Comparing these two strategies of obtaining chirality-pure nanotube structures, post-synthesis separation seems to be a promising approach at the current stage. Both centrifugation and DNA chromatography have demonstrated the capability of chiral selection of CNTs. However, most of the present reports are focused on the separation of semiconducting nanotubes, with few reports on separation of metallic nanotubes. In addition, SWCNTs with a desirable chiral vector (n, m) only constitute a tiny fraction of any as-synthesized CNT aggregates, which limits nanotubes' large-scale applications even if the post-synthesis separation process is fully successful. At the same time, seeded growth (or nanotube cloning) of SWCNTs is still in the preliminary stage. Both seeded growth methods are faced currently with great challenges. For catalytic seeded growth, the biggest challenge is how to guarantee the regrowth occurs exclusively on CNT tips rather than from substrate or CNT sidewalls where it is also possible to attach the catalyst. The interaction of the catalyst with CNT seed and substrate during heating is also an issue. These issues are expected to be solved by a novel catalyst docking method. In open-end seeded growth, elongation is not a deterministic process, because the open-ended state is not energetically stable. The lower growth rate also gives a higher chance of defect formation. However, encouraged by the recent progress on the successful regrowth and defect-free synthesis of ultralong SWCNTs, we can be confident that, sooner or later, it will be possible for these methods to synthesize large quantities of SWCNTs with any pre-selected specific diameter and chirality. Nevertheless, the final solution should be the combination of synthesis control and post-synthesis separation, with controllable synthesis offering some degree of chirality-pure CNTs and post-synthesis separation providing further purification.Acknowledgements
The authors highly acknowledge the financial support of the Singapore MOE tier 1 RG 26/08 research fund and NTU SUG fund.Reference
- S. Iijima, Helical microtubules of graphitic carbon, Nature, 1991, 354, 56–58 CrossRef CAS.
- A. Thess, R. Lee, P. Nikolaev, H. Dai, P. Petit and J. Robert, et al. Crystalline ropes of metallic carbon nanotubes, Science, 1996, 273, 483–487 CrossRef CAS.
- R. H. Baughman, A. A. Zakhidov and W. A. de Heer, Carbon nanotubes—the route toward applications, Science, 2002, 297, 787–792 CrossRef CAS.
- A. Javey, J. Guo, Q. Wang, M. Lundstrom and H. J. Dai, Ballistic carbon nanotube field-effect transistors, Nature, 2003, 424, 654–657 CrossRef CAS.
- S. J. Tans, M. H. Devoret, H. J. Dai, A. Thess, R. E. Smalley, L. J. Geerligs and C. Dekker, Individual single-wall carbon nanotubes as quantum wires, Nature, 1997, 386, 474–477 CrossRef CAS.
- S. J. Tans, A. R. M. Verschueren and C. Dekker, Room-temperature transistor based on a single carbon nanotube, Nature, 1998, 393, 49–52 CrossRef CAS.
- R. Saito, M. Fujita, G. Dresselhaus and M. S. Dresselhaus, Electronic-structure of chiral graphene tubules, Appl. Phys. Lett., 1992, 60(18), 2204–2206 CrossRef CAS.
- R. Saito, G. Dresselhaus, M. S. Dresselhaus, Physical Properties of Carbon Nanotubes, Imperial College Press, London, 1998 Search PubMed.
- R. B. Weisman and S. M. Bachilo, Dependence of optical transition energies on structure for single-walled carbon nanotubes in aqueous suspension: An empirical Kataura plot, Nano Lett., 2003, 3, 1235–1238 CrossRef CAS.
- T. W. Ebbesen and P. M. Ajayan, Large-scale synthesis of carbon nanotubes, Nature, 1992, 358, 220–222 CrossRef CAS.
- T. Guo, P. Nikolaev, A. Thess, D. T. Colbert and R. E. Smalley, Catalytic growth of single-walled nanotubes by laser vaporization, Chem. Phys. Lett., 1995, 243, 49–54 CrossRef CAS.
- M. Endo, K. Takeuchi, S. Igarashi, K. Kobori, M. Shiraishi and H. W. Kroto, The production and structure of pyrolytic carbon nanotubes, J. Phys. Chem. Solids, 1993, 54, 1841–1848 CrossRef CAS.
- M. C. Hersam, Progress towards monodisperse single-walled carbon nanotubes, Nat. Nanotechnol., 2008, 3, 387–394 CrossRef.
- R. Martel, Sorting carbon nanotubes for electronics, ACS Nano, 2008, 2, 2195–2199 CrossRef CAS.
- S. Bandow, S. Asaka, Y. Saito, A. M. Rao, L. Grigorian, E. Richter and P. C. Eklund, Effect of the growth temperature on the diameter distribution and chirality of single-wall carbon nanotubes, Phys. Rev. Lett., 1998, 80, 3779–3782 CrossRef CAS.
- E. F. Kukovitsky, S. G. L'vov, N. A. Sainov, V. A. Shustov and L. A. Chernozatonskii, Correlation between metal catalyst particle size and carbon nanotube growth, Chem. Phys. Lett., 2002, 355, 497–503 CrossRef CAS.
- W. H. Chiang and S. Mohan, Linking catalyst composition to chirality distributions of as-grown single-walled carbon nanotubes by tuning NixFe1−x nanoparticles, Nat. Mater., 2009, 8(11), 882–886 CrossRef.
- Y. H. Wang, M. J. Kim, H. W. Shan, C. Kittrell, H. Fan, L. M. Ericson, W. F. Hwang, S. Arepalli, R. S. Hauge and R. E. Smalley, Continued growth of single-walled carbon nanotubes, Nano Lett., 2005, 5, 997–1002 CrossRef CAS.
- M. Zheng, A. Jagota and M. S. Strano, et al. Structure-based carbon nanotube sorting by sequence-dependent DNA assembly, Science, 2003, 302, 1545–1548 CrossRef CAS.
- M. S. Arnold, A. A. Green, J. F. Hulvat, S. I. Stupp and M. C. Hersam, Sorting carbon nanotubes by electronic structure using density differentiation, Nat. Nanotechnol., 2006, 1, 60–65 CrossRef CAS.
- S. Y. Ju, J. Doll, I. Sharma and F. Papadimitrakopoulos, Selection of carbon nanotubes with specific chiralities using helical assemblies of flavin mononucleotide, Nat. Nanotechnol., 2008, 3(6), 356–362 CrossRef CAS.
- N. Stiirzl, F. Hennrich, S. Lebedkin, S. Lebedkin and M. M. Kappes, Near monochiral single-walled carbon nanotube dispersions in organic solvents, J. Phys. Chem. C, 2009, 113, 14628–14632 CrossRef.
- X. M. Tu, S. Manohar, A. Jagota and M. Zheng, DNA sequence motifs for structure-specific recognition and separation of carbon nanotubes, Nature, 2009, 460, 250–253 CrossRef CAS.
- S. Nagasawa, M. Yudasaka, K. Hirahara, T. Ichihashi and S. Iijima, Effect of oxidation on singlewall carbon nanotubes, Chem. Phys. Lett., 2000, 328, 374–380 CrossRef CAS.
- Y. Miyata, T. Kawai, Y. Miyamoto, K. Yanagi, Y. Maniwa and H. Kataura, Chirality-dependent combustion of single-walled carbon nanotubes, J. Phys. Chem. C, 2007, 111, 9671–9677 CrossRef CAS.
- C. M. Yang, K. H. An, J. S. Park, K. A. Park, S. C. Lim, S. H. Cho, Y. S. Lee, W. Park, C. Y. Park and Y. H. Lee, Preferential etching of metallic single-walled carbon nanotubes with small diameter by fluorine gas, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 075419 CrossRef.
- G. Y. Zhang, P. F. Qi, X. R. Wang, Y. R. Lu, X. L. Li, R. Tu, S. Bangsaruntip, D. Mann, L. Zhang and H. J. Dai, Selective etching of metallic carbon nanotubes by gas-phase reaction, Science, 2006, 314, 974–977 CrossRef CAS.
- P. G. Collins, M. S. Arnold and P. Avouris, Engineering carbon nanotubes and nanotube circuits using electrical breakdown, Science, 2001, 292, 706–709 CrossRef.
- P. G. Collins, M. Hersam, M. Arnold, R. Martel and P. Avouris, Current saturation and electrical breakdown in multiwalled carbon nanotubes, Phys. Rev. Lett., 2001, 86, 3128–3131 CrossRef CAS.
- M. J. O'Connell, S. M. Bachilo and C. B. Huffman, et al. Band gap fluorescence from individual single-walled carbon nanotubes, Science, 2002, 297, 593–596 CrossRef CAS.
- A. Hirsch, Functionalization of single-walled carbon nanotubes, Angew. Chem., Int. Ed., 2002, 41, 1853–1859 CrossRef CAS.
- S. Banerjee, T. Hemraj-Benny and S. S. Wong, Covalent surface chemistry of single-walled carbon nanotubes, Adv. Mater., 2005, 17, 17–29 CrossRef CAS.
- D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chemistry of carbon nanotubes, Chem. Rev., 2006, 106, 1105–1136 CrossRef CAS.
- D. A. Heller, R. M. Mayrhofer, S. Baik, Y. V. Grinkova, M. L. Usrey and M. S. Strano, Concomitant length and diameter separation of single-walled carbon nanotubes, J. Am. Chem. Soc., 2004, 126, 14567–14573 CrossRef CAS.
- S. K. Doorn, R. E. Fields, H. Hu, M. A. Hamon, R. C. Haddon, J. P. Selegue and V. Majidi, High resolution capillary electrophoresis of carbon nanotubes, J. Am. Chem. Soc., 2002, 124, 3169–3174 CrossRef CAS.
- R. Krupke, F. Hennrich, H. von Lohneysen and M. M. Kappes, Separation of metallic from semiconducting single-walled carbon nanotubes, Science, 2003, 301, 344–347 CrossRef CAS.
- H. Q. Peng, N. T. Alvarez, C. Kittrell, R. H. Hauge and H. K. Schmidt, Dielectrophoresis field flow fractionation of single-walled carbon nanotubes, J. Am. Chem. Soc., 2006, 128, 8396–8397 CrossRef CAS.
- J. Liu, A. G. Rinzler and H. J. Dai, et al. Fullerene pipes, Science, 1998, 280, 1253–1256 CrossRef CAS.
- Y. Maeda, S. Kimura and M. Kanda, et al. Large-scale separation of metallic and semiconducting single-walled carbon nanotubes, J. Am. Chem. Soc., 2005, 127, 10287–10290 CrossRef CAS.
- Z. H. Chen, X. Du, M. H. Du, C. D. Rancken, H. P. Cheng and A. G. Rinzler, Bulk separative enrichment in metallic or semiconducting single-walled carbon nanotubes, Nano Lett., 2003, 3, 1245–1249 CrossRef CAS.
- A. A. Green and M. C. Hersam, Ultracentrifugation of single-walled nanotubes, Mater. Today, 2007, 10, 59–60 CrossRef CAS.
- N. Nair, W.-J. Kim, R. D. Braatz and M. S. Strano, Dynamics of surfactant-suspended single-walled carbon nanotubes in a centrifugal field, Langmuir, 2008, 24, 1790–1795 CrossRef CAS.
- M. S. Arnold, S. I. Stupp and M. C. Hersam, Enrichment of single-walled carbon nanotubes by diameter in density gradients, Nano Lett., 2005, 5, 713–718 CrossRef CAS.
- M. Zheng, A. Jagota, E. D. Semke, B. A. Diner, R. S. Mclean, S. R. Lustig, R. E. Richardson and N. G. Tassi, DNA-assisted dispersion and separation of carbon nanotubes, Nat. Mater., 2003, 2, 338–342 CrossRef CAS.
- D. Chattopadhyay, S. Lastella, S. Kim and F. Papadimitrakopoulos, Length separation of Zwitterion-functionalized single wall carbon nanotubes by GPC, J. Am. Chem. Soc., 2002, 124, 728–729 CrossRef CAS.
- M. Zheng and E. D. Semke, Enrichment of single chirality carbon nanotubes, J. Am. Chem. Soc., 2007, 129, 6084–6085 CrossRef CAS.
- L. Zhang, S. Zaric, X. M. Tu, X. R. Wang, W. Zhao and H. J. Dai, Assessment of chemically separated carbon nanotubes for nanoelectronics, J. Am. Chem. Soc., 2008, 130, 2686–2691 CrossRef CAS.
- R. Marquis, C. Greco, I. Sadokierska, S. Lebedkin, M. M. Kappes, T. Michel, L. Alvarez, J. L. Sauvajol, S. P. Meunier and C. Mioskowski, Superamolecular discrimination of carbon nanotubes according to their helicity, Nano Lett., 2008, 8, 1830–1835 CrossRef CAS.
- S. Manohar, A. R. Mantz, K. E. Bancroft, C. Y. Hui, A. Jagota and D. V. Vezenov, Peeling single-stranded DNA from graphite surface to determine oligonucleotide binding energy by force spectroscopy, Nano Lett., 2008, 8, 4365–4372 CrossRef CAS.
- S. Meng, P. Maregakis, C. Papaloukas and E. Kaxiras, DNA nucleoside interaction and identification with carbon nanotubes, Nano Lett., 2007, 7, 45–50 CrossRef CAS.
- R. R. Johnson, A. T. C. Johnson and M. L. Klein, Probing the structure of DNA–carbon nanotube hybrids with molecular dynamics, Nano Lett., 2008, 8, 69–75 CrossRef CAS.
- W. Martin, W. Zhu and G. Krilov, Simulation study of noncovalent hybridization of carbon nanotubes by single-stranded DNA in water, J. Phys. Chem. B, 2008, 112, 16076–16089 CrossRef CAS.
- L. X. Zheng, M. J. O'connell, S. K. Doorn, X. Z. Liao, Y. H. Zhao and E. A. Akhadov, et al. Ultralong single-wall carbon nanotubes, Nat. Mater., 2004, 3, 673–376 CrossRef CAS.
- K. H. Lee, J. M. Cho and W. Sigmund, Control of growth orientation for carbon nanotubes, Appl. Phys. Lett., 2003, 82, 448–450 CrossRef CAS.
- Y. Liu, J. Hong, Y. Zhang, R. Cui, J. Wang, W. Tan and Y. Li, Flexible orientation control of ultralong single-walled carbon nanotubes by gas flow, Nanotechnology, 2009, 20, 185601 CrossRef.
- C. Lu and J. Liu, Controlling the diameter of carbon nanotubes in chemical vapor deposition method by carbon feeding, J. Phys. Chem. B, 2006, 110(41), 20254–20257 CrossRef CAS.
- Z. Yao, H. W. C. Postma, L. Balents and C. Dekker, Carbon nanotube intramolecular junctions, Nature, 1992, 402, 273.
- B. Wang, C. H. P. Poa, L. Wei, L.-J. Li, Y. Yang and Y. Chen, (n, m) Selectivity of single-walled carbon nanotubes by different carbon precursors on Co–Mo catalyst, J. Am. Chem. Soc., 2007, 129(29), 9014–9019 CrossRef CAS.
- S. Jeon, C. Lee, J. Tang, J. Hone and C. Nuckolls, Growth of serpentine carbon nanotubes on quartz substrates and their electrical properties, Nano Res., 2008, 1, 427–433 Search PubMed.
- B. Kitiyanan, W. E. Alvarez, J. H. Harwell and D. E. Resasco, Controlled production of single-wall carbon nanotubes by catalytic decomposition of CO on bimetallic Co–Mo catalysts, Chem. Phys. Lett., 2000, 317, 497–503 CrossRef CAS.
- S. M. Chilo, L. Balzano, J. E. Herrera, F. Pompeo, D. E. Resasco and R. B. Weisman, Narrow (n, m)-distribution of single-walled carbon nanotubes grown using a solid supported catalyst, J. Am. Chem. Soc., 2003, 125, 11186–11187 CrossRef CAS.
- X. L. Li, X. M. Tu, S. Zaric, K. Welsher, W. S. Seo, W. Zhao and H. J. Dai, Selective synthesis combined with chemical separation of single-walled carbon nanotubes for chirality selection, J. Am. Chem. Soc., 2007, 129, 15770–15771 CrossRef CAS.
- S. Maruyama, R. Kojima, Y. Miyauchi, S. Chiashi and M. Kohno, Low-temperature synthesis of high-purity single-walled carbon nanotubes from alcohol, Chem. Phys. Lett., 2002, 360, 229–234 CrossRef CAS.
- S. B. Sinnott, R. Andrews, D. Qian, A. M. Rao, Z. Mao, E. C. Dickey and F. Derbyshire, Model of carbon nanotube growth through chemical vapor deposition, Chem. Phys. Lett., 1999, 315, 25–30 CrossRef CAS.
- B. Wang, L. Wei, L. Yao, L. J. Li, Y. H. Yang and Y. Chen, Pressure-induced single-walled carbon nanotube (n, m) selectivity on Co–Mo catalysts, J. Phys. Chem. C, 2007, 111, 14612–14616 CrossRef CAS.
- T. Hiraoka, S. Bandow, H. Shinohara and S. Iijima, Control on the diameter of single-walled carbon nanotubes by changing the pressure in floating catalyst CVD, Carbon, 2006, 44, 1853–1859 CrossRef CAS.
- P. Nikolaev, M. J. Bronikowski, R. K. Bradley, F. Rohmund, D. T. Colbert, K. A. Smith and R. E. Smalley, Gas-phase catalytic growth of single-walled carbon nanotubes from carbon monoxide, Chem. Phys. Lett., 1999, 313, 91–97 CrossRef CAS.
- W.-H. Chiang and R. M. Sankaran, In-flight dimensional tuning of metal nanoparticles by microplasma synthesis for selective production of diameter-controlled carbon nanotubes, J. Phys. Chem. C, 2008, 112(46), 17920–17925 CrossRef CAS.
- K. Kajiwara, S. Suzuki, H. Sato, K. Hata, Chirality-selective synthesis of carbon nanotubes by catalytic-chemical vapor deposition using quasicrystal alloys as catalysts. 10th International Conference on Quasicrystals (ICQ10) ETH, Zurich, Switzerland, 2009, 224(1–2), 5–8 Search PubMed.
- S. Inoue and Y. Kikuchi, Diameter control and growth mechanism of single-walled carbon nanotubes, Chem. Phys. Lett., 2005, 410(4–6), 209–212 CrossRef CAS.
- G. H. Jeong, S. Suzuki, Y. Kobayashi, A. Yamazaki, H. Yoshimura and Y. Homma, Size control of catalytic nanoparticles by thermal treatment and its application to diameter control of single-walled carbon nanotubes, App. Phys.Lett., 2007, 90(4), 043108-1–043108-3 Search PubMed.
- G. H. Jeong, S. Suzuki, Y. Kobayashi, A. Yamazaki, H. Yoshimura and Y. Homma, Effect of nanoparticle density on narrow diameter distribution of carbon nanotubes and particle evolution during chemical vapor deposition growth, J App. Phys., 2005, 98(12), 124311-1–124311-6 Search PubMed.
- Y. H. Miyauchi, S. H. Chiashi, Y. Murakami, Y. Hayashida and S. Maruyama, Fluorescence spectroscopy of single-walled carbon nanotubes synthesized from alcohol, Chem. Phys. Lett., 2004, 387, 198–203 CrossRef CAS.
- Y. Chen, D. Ciuparu, S. Lim, Y. Yang, G. L. Haller and L. Pfefferle, Synthesis of uniform diameter single-wall carbon nanotubes in Co-MCM-41: effects of the catalyst prereduction and nanotube growth temperatures, J. Catal., 2004, 225, 453–465 CrossRef CAS.
- D. Uparu, Y. Chen, S. Lim, G. L. Haller and L. Pfefferle, Uniform-diameter single-walled carbon nanotubes catalytically grown in cobalt-incorporated MCM-41, J. Phys. Chem. B, 2004, 108(2), 503–507 CrossRef CAS.
- J. E. Herrera, L. Balzano, A. Borgna, W. E. Alvarez and D. E. Resasco, Relationship between the structure/composition of Co–Mo catalysts and their ability to produce single-walled carbon nanotubes by CO disproportionation, J. Catal., 2001, 204, 129–145 CrossRef CAS.
- W. L. Wang, X. D. Bai, Z. Xu, S. Liu and E. G. Wang, Low temperature growth of single-walled carbon nanotubes: Small diameters with narrow distribution, Chem. Phys. Lett., 2006, 419(1–3), 81–85 CrossRef CAS.
- M. Kumar and Y. Ando, Controlling the diameter distribution of carbon nanotubes grown from camphor on a zeolite support, Carbon, 2005, 43(3), 533–540 CrossRef CAS.
- Y. Wang, Y. Q. Liu, D. C. Wei, L. C. Cao, L. Fu, X. L. Li, H. Kajiura, Y. M. Li and K. Noda, Controlled growth of single-walled carbon nanotubes at atmospheric pressure by catalytic decomposition of ethanol and an efficient purification method, J. Mater. Chem., 2007, 17(4), 357–363 RSC.
- T. Saito, S. Ohshima, T. Okazaki, S. Ohmori, M. Yumura and S. Iijima, Selective diameter control of single-walled carbon nanotubes in the gas-phase synthesis, J. Nanosci. Nanotechnol., 2008, 8(11), 6153–6157 CrossRef CAS.
- J. Kong, H. T. Soh, A. M. Cassell, C. F. Quate and H. J. Dai, Synthesis of individual single-walled carbon nanotubes on patterned silicon wafers, Nature, 1998, 395, 878–881 CrossRef CAS.
- N. Chopra, P. D. Kichambare, R. Andrews and B. J. Hinds, Control of multiwalled carbon nanotube diameter by selective growth on the exposed edge of a thin film multilayer structure, Nano Lett., 2002, 2(10), 1177–1181 CrossRef CAS.
- K. Kobayashi, R. Kitaura, Y. Kumai, Y. Goto, S. Inagaki and H. Shinohara, Synthesis of single-wall carbon nanotubes grown from size-controlled Rh/Pd nanoparticles by catalyst-supported chemical vapor deposition, Chem. Phys. Lett., 2008, 458(4–6), 346–350 CrossRef CAS.
- D. Yu and F. Liu, Synthesis of carbon nanotubes by rolling up patterned graphene nanoribbons using selective atomic adsorption, Nano Lett., 2007, 7(10), 3046–3050 CrossRef CAS.
- A. Sidorov, D. Mudd, G. Sumanasekera, P. J. Ouseph, C. S. Jayanthi and S. Y. Wu, Electrostatic deposition of graphene in a gaseous environment: a deterministic route for synthesizing rolled graphenes?, Nanotechnology, 2009, 20(5), 055611-1–055611-5.
- Z. H. Chen, X. Du, M. H. Du, C. D. Rancken, H. P. Cheng and A. G. Rinzler, Bulk separative enrichment in metallic or semiconducting single-walled carbon nanotubes, Nano Lett., 2003, 3, 1245–1249 CrossRef CAS.
- R. E. Smalley, Y. B. Li, V. C. Moore, B. K. Price, R. Colorado, H. K. Schmidt, R. H. Hauge, A. R. Barron and J. M. Tour, Single wall carbon nanotube amplification: En route to a type-specific growth mechanism, J. Am. Chem. Soc., 2006, 128, 15824–15829 CrossRef CAS.
- D. Ogrin, R. E. Anderson, R. Colorado, B. Maruyama, M. J. Pender and V. C. Moore, et al. Amplification of single-walled carbon nanotubes from designed seeds: Separation of nucleation and growth, J. Phys. Chem. C, 2007, 111, 17804–17806 CrossRef CAS.
- Z. F. Ren, Cloning carbon, Nat. Nanotechnol., 2007, 2, 17–18 CrossRef CAS.
- Y. G. Yao, C. Q. Feng, J. Zhang and Z. F. Liu, “Cloning” of single-walled carbon nanotubes via open-end growth mechanism, Nano Lett., 2009, 9, 1673–1677 CrossRef CAS.
- J. C. Charlier, T. W. Ebbesen and P. Lambin, Structural and electronic properties of pentagon–heptagon pair defects in carbon nanotubes, Phys. Rev. B: Condens. Matter, 1996, 53, 11108–11113 CrossRef CAS.
- M. Fuhrer, J. Nygard, L. Shih, M. Foreo, Y. G. Yoon and M. S. C. Mazzoni, et al. Crossed nanotube junctions, Science, 2000, 288, 494–497 CrossRef CAS.
- L. Chico, V. H. Crespi, L. X. Benedict, S. G. Louie and M. L. Cohen, Pure carbon nanoscale devices: Nanotube heterojunctions, Phys. Rev. Lett., 1996, 76, 971–974 CrossRef CAS.
- D. Orlikowski, M. B. Nardelli, J. Bernholc and C. Roland, Ad-dimers on strained carbon nanotubes: A new route for quantum dot formation?, Phys. Rev. Lett., 1999, 83, 4132–4135 CrossRef CAS.
- M. Ouyang, J. L. Huang, C.-L. Cheung and C. M. Lieber, Atomically resolved single-walled carbon nanotube intramolecular junctions, Science, 2001, 291, 97–100 CrossRef CAS.
- Y. W. Fan, B. R. Goldsmith and P. G. Collins, Identifying and counting point defects in carbon nanotubes, Nat. Mater., 2005, 4, 906–911 CrossRef CAS.
- S. Maruyama, R. Kojima, Y. Miyauchi, S. Chiashi and M. Kohno, Low-temperature synthesis of high-purity single-walled carbon nanotubes from alcohol, Chem. Phys. Lett., 2002, 360, 229–334 CrossRef CAS.
- L. X. Zheng, X. Z. Liao and Y. T. Zhu, Parametric study of carbon nanotube growth via cobalt-catalyzed ethanol decomposition, Mater. Lett., 2006, 60, 1968 CrossRef CAS.
- L. X. Zheng, B. C. Satishkumar, P. Gao and Q. Zhang, Kinetics studies of ultra-long single-walled carbon nanotubes, J. Phys. Chem. C, 2009, 113, 10896–10900 CrossRef CAS.
- W. F. Gale, T. C. Totemeier, Smithells Metals Reference Book, 7th edition, Elsevier Butterworth-Heinemann, 2004 Search PubMed.
- R. T. K. Baker, Catalytic growth of carbon filaments, Carbon, 1989, 27, 315–323 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |

