Paramagnetic nanofibrous composite films enhance the osteogenic responses of pre-osteoblast cells†
Jie
Meng‡
a,
Yu
Zhang‡
b,
Xiaojin
Qi
a,
Hua
Kong
a,
Chaoying
Wang
c,
Zhen
Xu
a,
Sishen
Xie
c,
Ning
Gu
*b and
Haiyan
Xu
*a
aInstitute of Basic Medical Sciences, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China. E-mail: xuhy@pumc.edu.cn
bSoutheastern University, Nanjing, China. E-mail: guning@seu.edu.cn
cInstitute of Physics, Chinese Academy of Sciences, Beijing, China
First published on 15th October 2010
Abstract
In this work, a paramagnetic nanofibrous composite film was fabricated with poly lactide, hydroxyapatite and γ-Fe203 nanoparticles using the electrospinning technique. The composite film significantly enhanced the proliferation, differentiation and ECM secretion of the osteoblast cells under a static magnetic field, which offers promising application potentials in bone tissue engineering and bone regeneration therapy.
Conventional surgical procedures to reconstruct lost or damaged bones mainly include the use of autografts, allografts or metallic and ceramic implants. Each of these options has its own drawbacks, such as donor site morbidity, pathogen transmission, and mismatching material properties with the native bone. As an alternative to these procedures, tissue engineering has emerged to create new tissue in vitro or to guide tissue regeneration in situ by growing cells on scaffolds.1–4
In tissue engineering, scaffolds are designed to serve as a temporary matrix to support cells’ growth and secretion of the extracellular matrix (ECM) that is required for tissue regeneration. Using biomaterial scaffolds to guide bone cell proliferation, differentiation and mineralization has led to a growing interest in the development of this regenerative tool in bone-healing therapy. Some synthetic materials have been further modified with growth factors and cellular adhesion ligands through chemical or physical immobilization to enhance cell attachment and other basic functions.5–8
Most components of the natural ECM have structural features in the nanometre dimensions, and the organization of cells and the corresponding tissue properties are found to be highly dependent on the architecture of the ECM. To fabricate scaffolds that are analogues to the natural ECM in which the cells live, electrospinning is one of the techniques that has been widely used to produce ultra-fine polymer fibres to form nanofibrous network architectures.9 The nanofibrous scaffolds thus fabricated have been proved to be advantageous for bone-associated cell proliferation as well as differentiation.10,11 A broad range of materials, from natural polymers12–15 and synthetic polymers16–19 to polymer blends or polymer-inorganic composite/hybrids,20–25 have been processed into nanofibrous scaffolds for studies on bone engineering.
The magnetic field is one factor that may be beneficial for enhancing bone tissue regeneration though mechanisms that have not yet been clarified. A few groups have reported that either static or pulse magnetic fields play a role in promoting the healing of some tissues, including bones. Singh et al. reported that a static magnetic field had a stimulating effect on the microstructure and mineralisation process of bone repair.26 Strauch et al. demonstrated that exposing bone wounds to pulsed magnetic fields of very specific configurations accelerated early wound healing in the animal model, as evidenced by significantly increased wound tensile strength at 21 days after wounding.27
Inspired by the effect of magnetic fields on wound healing as well as the nanofibrous structure on cell growth, we propose a novel paramagnetic composite material fabricated into nanofibrous nonwoven films by means of electrospinning for use in bone tissue engineering. The nanofibrous film is composed of γ-Fe203 nanoparticles coated with meso-2, 3-dimercaptosuccinic acid (γ-Fe2O3), hydroxyapatite nanoparticles (nHA) and poly (D,L-lactide) (PLA). We show that the paramagnetic nanofibrous composite films induce a significantly higher proliferation rate and faster differentiation of osteoblast cells in an inducible culture medium. The effect can be further significantly increased when a static magnetic field is applied to the cell culture environment.
To fabricate the paramagnetic nanofibrous composite film, 2.5 g of γ-Fe2O3 with an average diameter of 14 nm and 10 g of nHA with an average diameter of 50 nm were dispersed in 100 ml of N,N-dimethylacetamide (DMAc) with the aid of sonication, followed by dissolving 20 g of PLA with a molecular weight of 10 kD in the suspension. The mixture solution was processed into nanofibrous composite films (γ-Fe2O3/nHA/PLA) by electrospinning with optimum parameters. Fig. 1A presents an optical picture of the γ-Fe2O3/nHA/PLA film, which is brown, silk-like with a macro-scale area reaching several tens of centimetres squared. The combined TEM image displays the morphology of the γ-Fe2O3; most of the particles were quasi-spherical with an average diameter of 14 nm. The homogenous brown colour of the electrospun films resulted from the incorporation of γ-Fe2O3, which was also indicative of the nanoparticles dispersing well in the composite. Fig. 1B and Fig. 1C present typical morphologies of the fibres in the films. The nanofibres oriented randomly and crossed each other to organize a connected porous network. The pores formed in each layer have diameters in range of 3–20 μm. The average diameter of the fibers was statistically counted and measured more than 100 fibers randomly taken in ten of SEM images, which is 812 ± 124 nm.
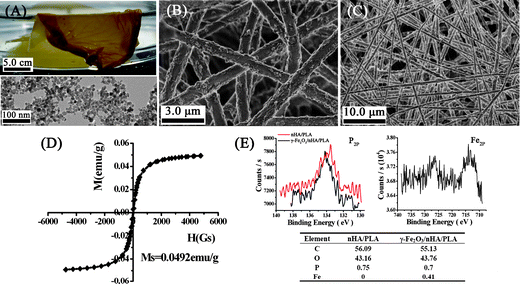 | ||
| Fig. 1 Characterisation of the nanofibrous composite film of γ-Fe2O3/nHA/PLA. A: Optical graph of the film combined with a TEM image of the γ-Fe2O3. B–C: SEM images showing that the fibres formed nonwoven mesh-like structures. D: Magnetization curve of the film. E: XPS analysis for the nanofibrous composite film of γ-Fe2O3/nHA/PLA and nHA/PLA. The inserted Table summarizes the content of the element Fe and P on the film surface. | ||
To set a control sample to figure out effect of the γ-Fe2O3 incorporated in the fibres, a nanofibrous composite film composed of nHA and PLA (nHA/PLA) was fabricated with the same proportion and electrospinning parameters. The control film was white and displayed similar architectures to that of γ-Fe2O3/nHA/PLA (Fig. S1 in the ESI‡). It was noted that the surface of the fibres of either γ-Fe2O3/nHA/PLA or nHA/PLA was somewhat rough, which should be attributed to the nHA that was embedded in the composite fibres, because fibres composed of γ-Fe2O3 and PLA have a smooth surface (Fig. S2‡).
The nanofibrous composite film of γ-Fe2O3/nHA/PLA displayed a paramagnetic property measured by a vibrating S measurement. Fig. 1D presents the hysteresis loop of the nanofibrous membrane. The saturation magnetization was determined to be 0.0492 emu g−1, and the typical characterisation of super paramagnetic behaviour was observed, showing an almost immeasurable coercive force and remanence. As the saturation magnetization of γ-Fe2O3 nanoparticles is about 67.6 emu g−1, the paramagnetic property of the γ-Fe2O3/nHA/PLA film should be contributed by the incorporation of γ-Fe2O3. The film surface chemistry of the paramagnetic nanofibrous composite and the control was analyzed by XPS. The spectra of P2p and Fe2p for the γ-Fe2O3/nHA/PLA film are displayed in Fig. 1E, and the content of the elements Fe, P, C, and O on the surface of the films is summarized in the combined Table. The analysis revealed that there was 0.41% of Fe and 0.7% of P on the surface of the γ-Fe2O3/nHA/PLA.
When the γ-Fe2O3/nHA/PLA film was immersed in PBS buffer solution (pH = 7.4) at 37 °C for 8 weeks with no fresh buffer replacement, the pH value of the PBS buffer solution decreased from 7.4 to 5.5 during that period (Fig. S3‡), which indicated that some lactic acid entered into the solution due to PLA degradation. However, the degradation in general was minimal, the γ-Fe2O3/nHA/PLA films maintained their integrity over the 8 weeks, and their mass loss was too little to detect (data not shown).
MC3T3-E1 is a pre-osteoblast cell line that differentiates into osteoblasts when cultivated in inductive culture medium. The osteoblasts then undergo a proliferation and differentiation course over time.28 In the first period, the osteoblasts actively proliferate and express genes for extracellular matrix molecules. At the end of the first period, the proliferation decreases. In the second period, the proliferation goes to the end, and the extracellular matrix development and maturation of the osteoblasts begin. In this period, one of indicative features is that alkaline phosphatase activity of the osteoblasts is increased as well as proliferation is decreased. In the third period, the mineralization process begins, osteoblasts synthesize and deposit various bone proteins.
In the present work, 4 groups were set in the cells’ viability assay: group I, nHA/PLA film; group II, nHA/PLA film under a magnetic field; group III, γ-Fe2O3/nHA/PLA film; and group IV, γ-Fe2O3/nHA/PLA film under a magnetic field. The magnetic field was applied to the cultured cells by using a lab-developed device. The distribution of magnetic field strength in the device space was measured by a Teslameter. The culture plates were placed in the area with a magnetic field strength of 0.9–1.0 mT (Fig. S4‡). Fig. 2A displays osteoblasts cells proliferation on the different composite films with or without the applied magnetic field. Statistical analysis of the differences for viable cell numbers in the groups over culture time is summarized in Table 1.
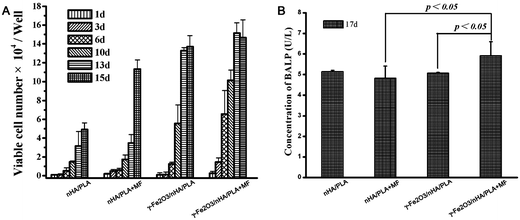 | ||
| Fig. 2 Proliferation and ALP secretion of the pre-osteoblast cells seeded on different nanofibrous films. “+MF” means applying a static magnetic field of 0.9–1.0 mT to the cells. | ||
As shown in Fig. 2A, the cells seeded on the γ-Fe2O3/nHA/PLA films exhibited a significantly higher proliferation rate than those on the nHA/PLA films over the experimental period (compare group I with group III). This clearly indicates that the paramagnetic nanoparticles incorporated in the films are beneficial to the growth of bone cells. A recent publication29 reported that introduction of paramagnetic nanoparticles to CaP bioceramics could promote bone formation and growth in vitro and in vivo, which supported our observations.
When a magnetic field of 0.9–1.0 mT was applied, the proliferation rate for the cells on the nHA/PLA films increased markedly in comparison to those without a magnetic field within 8 days of culture (compare group I with group II). For comparison between group III and group IV, the magnetic field enhanced the proliferation significantly in the experimental period. This suggests that the applied static magnetic field played a positive role on the cells’ proliferation, which is consistent with the results reported in the literature that static magnetic fields stimulated bone tissue regeneration.26,27 Furthermore, the degree of variation between groups II and IV was greater than that between groups I and II (see Table 1). All together this indicates that the group IV has the strongest effect of promoting cell proliferation among the four groups, or we could consider that the paramagnetic nanoparticles of γ-Fe2O3 in the fibres and the applied static magnetic field acted in a coordinated way to boost the cells’ proliferation.
It could be noticed that proliferation of the osteoblast cells on the γ-Fe2O3/nHA/PLA films under the magnetic field decreased a little on day 15 of culture; and the osteoblasts in the γ-Fe2O3/nHA/PLA films without a magnetic field showed a lower increase on the 15th day of culture compared with those cultured on the other days. In contrast, cells growing on the nHA/PLA film had not yet shown a decreasing tendency of proliferation at the same time point either with or without applying magnetic fields.
According to the time course of osteoblast functions mentioned before, from the data of the proliferation assay two points could be extracted: (1) osteoblast cells in the group II and IV have higher proliferation rates within 13 days than those in the group I and group III, respectively, which meant the applied magnetic field had a promotion effect on cell proliferation; (2) osteoblast cells in group IV reached the end of first period faster than those in the other groups, which implied that the applied magnetic field is beneficial to the osteobalst cells’ differentiation as well. Hence, Fig. 2A not only shows proliferation tendency of the osteoblasts induced by the different composite films with or without magnetic fields, but also reflects differentiation of the osteoblasts indirectly.
ALP is one of the key substances that indicate whether osteoblasts have entered the period of extracellular matrix development and maturation. The amount of ALP produced by the cells growing in the different films with or without applying the magnetic fields was further examined using a mouse bone alkaline phosphatase (BALP) ELISA kit (USCNLIFE, China). As shown in Fig. 2B, without applying the magnetic field, cells growing on the γ-Fe2O3/nHA/PLA films produced similar level of ALP to those on the nHA/PLA films. However, cells growing on the γ-Fe2O3/nHA/PLA films produced a markedly higher amount of ALP than those on the nHA/PLA films under the magnetic fields after 17 days of culture. In addition, it could be also noted that for cells growing on the γ-Fe2O3/nHA/PLA films, the applied magnetic field enhanced ALP production significantly, while for those on the nHA/PLA films, the magnetic field did not make an obvious difference. This indicated that more osteoblasts entered into the second period due to induction of the γ-Fe2O3/nHA/PLA films with applying the magnetic field in comparison with those growing on the nHA/PLA films under the magnetic field. The γ-Fe2O3 nanoparticles incorporated in the PLA and the magnetic field played enhancement roles in a synergistic way.
In bone tissue engineering, ECM deposition by osteoblast cells is one of the most crucial factors that lead to the ultimate formation of new bone tissue. The ECM proteins function as a substratum for bone cell adhesion and serve as a scaffold for mineralization. In the current work, the constructs of the cells/nanofibrous composite films of γ-Fe2O3/nHA/PLA and nHA/PLA were observed with SEM after culture with osteogenesis induction medium for 21 days with the application of a magnetic field (Fig. 3). In general, cells growing on the γ-Fe2O3/nHA/PLA films (Fig. 3A–D) revealed many typical globular round cells which integrated with the nanofibres. In particular, Fig. 3B–D shows that the cells growing on the films of γ-Fe2O3/nHA/PLA were surrounded by a thick substance. Because there was not any of this substance on the surface of the original films, it is considered that the substance was produced by the osteoblasts. However, many of the cells on the nHA/PLA films exhibited a fibroblast-like morphology (black arrow point in Fig. 3E), there was a less-thick substances deposited on most of the area of the films (Fig. 3E–F). These observations suggest that the paramagnetic nanofibrous composite films of γ-Fe2O3/nHA/PLA might enhance new ECM secretion of the osteoblasts under a magnetic field.
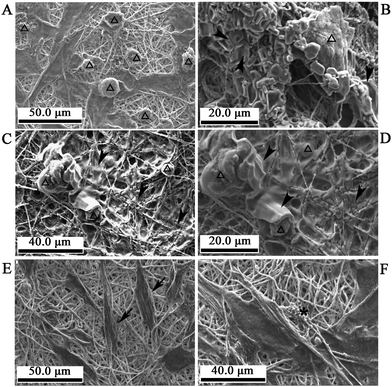 | ||
| Fig. 3 SEM images of the cells on the nanofibrous composite films of γ-Fe2O3/nHA/PLA or nHA/PLA in the inducible osteogenic supplements for 21 days with applying magnetic fields. A–D: Cells on the γ-Fe2O3/nHA/PLA films. Many of the cells show a globular morphology (hollow arrow head) and are integrated with the nanofibres. There are thick substances deposited on the films of some areas as well as surrounding the cells (solid arrow head). E–F: Cells on the nHA/PLA films. Many of the cells have a fibroblast-like morphology (solid arrow); there are less thick substances deposited on most of the area of these films (star). | ||
Together with the above data, we have shown that the novel paramagnetic nanofibrous composite film of γ-Fe2O3/nHA/PLA obviously enhanced the osteogenic responses of the osteoblast cells under a static magnetic field of 0.9–1.0 mT. We would suggest that the films offer promising application potentials for bone tissue engineering and bone regeneration. Comprehensive investigations are necessary to understand the mechanism of the interactions of the paramagnetic film and the static magnetic field with the cells.
Hydroxyapatite nanoparticles have been used as fillers in various polymers to improve bone conductivity as well as to reinforce mechanical properties in bone engineering. The nanoparticles of γ-Fe2O3 are becoming one of the important imaging contrast agents and are being investigated intensely in vivo. Theoretically the two kinds of nanoparticles can be uptaken by the tissues and undergo metabolism. So far few literature examples have reported obvious damage induced by nanoparticles of hydroxyapatite or γ-Fe2O3 incorporated in degradable polymeric composites for bone engineering, however, the associated risks should be paid attention, and thus comprehensive evaluations are very necessary in further studies.
Experimental
Preparation and characterisation of electrospun nanofibrous films
Nanoparticles of γ-Fe2O3 coated with meso-2, 3-dimercaptosuccinic acid (DMSA) were prepared according to our previous procedure.30 Briefly, 200 mL aqueous ammonia solution (1.5M) was added into 500 mL aqueous solution containing FeCl3 (0.1 M) and FeSO4 (0.06 M) with vigorous stirring at room temperature for 30 min. The resulting Fe3O4 nanoparticles were washed and dispersed in water at 3 mg mL−1 (pH = 3.0), then oxidized into reddish-brown γ-Fe2O3 nanoparticles under aeration (with air) at 95 °C. The γ-Fe2O3 nanoparticles were mixed with DMSA to obtain γ-Fe2O3 nanoparticles coated with DMSA. The particle size and morphology was determined by transmission electronic microscopy (TEM, JEOL, JEM-200EX). The magnetic property was measured by a vibrating sample magnetometer (VSM, Lakeshore 7407). Hydroxyapatite nanoparticles (nHA) were purchased from Nanjing Emperor Nano Material Co., Ltd (purity: 97%, major diameter: 20 nm). Poly (DL-lactide) (PLA) with an average molecular weight of 10 kDa was purchased from Chengdu Dikang Biomedical Co., Ltd.To fabricate nanofibrous composite films, 2.5 g of γ-Fe2O3 coated with DMSA and 10.0 g of nHA were dispersed in 100 mL of N, N-dimethylacetamide (DMAc) by sonication to form a homogenous suspension. Then 20 g of PLA was dissolved to form a viscous solution. The resulting solution was subjected to an electrospinning device and processed with the following optimum parameters: needle inner diameter was 0.9 mm, distance between the needle's tip and the collector was 25 cm, and voltage was 15 kV. Electrospun fibres were collected by a flat aluminium plate and treated in a vacuum oven at room temperature for 48 h. The obtained films were adhered tightly on polyurethane films and trimmed to fit the size of wells in the cell-culture plate. Control films composed of 10% of nHA and 20% of PLA were prepared under the same processing conditions. All samples for cellular experiments were sterilized with ethylene oxide. The morphology of the electrospun films was observed by SEM. The surface chemistry of the films was analyzed with XPS. The magnetic property of the films was measured by a vibrating sample magnetometer (VSM, Lakeshore 7407).
The static magnetic field to cell culture
Two pieces of magnet were fixed to a metal box to establish a magnetic field in the box space. The magnetic field strength distribution was measured using a Teslameter (SG-3-A, Beijing). The culture plates were placed in the area with a constant magnetic field strength of 0.9–1.0 mT to allow the cells to live under the same magnetic conditions.Cell proliferation assays
The mouse pre-osteoblast cell line MC3T3-E1 was purchased from the Centre for Cell Culture, Chinese Academy of Medical Sciences. In the experiments, freshly confluent flasks of MC3T3-E1 were incubated with trypsin/EDTA for 2 min and then resuspended in the culture medium. The cells were seeded at a density of 1.2 × 104 cells cm−2 onto the nanofibrous composite films set in the 96-well cell-culture plate and cultivated in an inducible medium containing dexamethasone, ascorbic acid, and beta-glycerophosphate. The medium was exchanged once every 3 days. The number of attached cells was determined at each designated time using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS assay, Promega). The absorbance was measured at 490 nm (UV-2450, Shimadzu), which was calculated to cell numbers using a calibration curve established according to the instruction of the MTS assay.ALP activity of cells cultured on the nanofibrous composite films
MC3T3-E1 cells were seeded on the nanofibrous composite films with a density of 1.2 × 104 cells cm−2. The cell culture supernatants were collected after 21 days and centrifuged at 1000 × g for 20 min. A mouse bone alkaline phosphate (BALP) ELISA Kit (USCNLIFE) was used to determine the concentrations of BALP in the cell culture supernatants. The absorbance at 450 nm was measured and transferred into the concentration of BALP according to the calibration curve.Observation of osteoblastic morphologies
MC3T3-E1 cells were seeded at a density of 1 × 104 cells cm−2 on the nanofibrous composite films set in 24-well culture plates and placed in a humidified incubator with 5% CO2 at 37 °C for 21 days. The film/cell constructs were removed from the wells and rinsed with PBS buffer solution (pH = 7.4) twice. The constructs were fixed in 8% glutaraldehyde for 30 min, followed by dehydration through a conventional graded series of ethanol with 3 min for each step. The dehydrated samples were subjected to scanning electron microscopy (SEM, Hitachi S-5200) for morphology observation.Statistical analysis
All measurements for the cellular experiments were collected in triplicate and expressed as mean ± standard deviation (SD). Single-factor analysis of variance (ANOVA) was employed to assess the statistical significance of the results. Differences were considered statistically significant at p < 0.05.Notes and references
- R. Langer and J. Vacanti, Science, 1993, 260, 920 CrossRef CAS.
- P. X. Ma, Mater. Today, 2004, 7, 30 CrossRef CAS.
- E. M. Christenson, K. S. Anseth, J. J. J. P. van den Beucken, C. K. Chan, B. Ercan and J. A. Janson, J. Orthop. Res., 2007, 25, 11 CrossRef CAS.
- W. D. Chan, H. Perinpanayagam, H. A. Goldberg, G. K. Hunter, S. J. Dixon, G. C. J. Santos and A. S. Rizkalla, J. Can. Dent. Assoc., 2009, 75, 373 Search PubMed.
- D. S. Benoit and K. S. Anseth, Biomaterials, 2005, 26, 5209 CrossRef CAS.
- G. Gómez, S. Korkiakoski, M. M. González, S. Länsman, V. Ellä, T. Salo, M. Kellomäki, N. Ashammakhi and E. Arnaud, J. Craniofacial Surg., 2006, 17, 935 CrossRef.
- H. Shin, K. Zygourakis, M. C. Farach-Carson, M. J. Yaszemski and A. G. Mikos, J. Biomed. Mater. Res., 2004, 69a, 535 Search PubMed.
- A. Tachibana, Y. Nishikawa, M. Nishino, S. Kaneko, T. Tanabe and K. Yamauchi, J. Biosci. Bioeng., 2006, 102, 425 CrossRef CAS.
- J. H. Jang, O. Castano and H. W. Kim, Adv. Drug Delivery Rev., 2009, 61, 1065 CrossRef CAS.
- H. Yoshimoto, Y. M. Shin, H. Terai and J. P. Vacanti, Biomaterials, 2003, 24, 2077 CrossRef CAS.
- K. M. Woo, J. H. Jun, V. J. Chen, J. H. Seo, J. H. Baek, H. M. Ryoo, G. S. Kim, M. J. Somerman and P. X. Ma, Biomaterials, 2007, 28, 335 CAS.
- Y. R. V. Shin, C. N. Chen, S. W. Tsai, Y. J. Wang and O. K. Lee, Stem Cells, 2006, 24, 125 Search PubMed.
- C. Meechaisue, P. Wutticharoenmongkol, R. Waraput, T. Huangjing, N. Ketbumrung, P. Pavasant and P. Supaphol, Biomed. Mater., 2007, 2, 181 Search PubMed.
- M. Li, H. J. Jing, G. D. Botsaris and D. L. Kaplan, J. Mater. Res., 2005, 20, 3374 CrossRef.
- S. Y. Shin, H. N. Park, K. H. Kim, M. H. Lee, Y. S. Choi, Y. J. Park, Y. M. Lee, I. C. Rhyu, S. B. Han, S. J. Lee and C. P. Chung, J. Periodontol., 2005, 76, 1778 CrossRef CAS.
- I. Wimpenny, K. Hampson, Y. Yang, N. Ashammakhi and N. R. Forsyth, Tissue Eng., Part C, 2010, 16, 503 Search PubMed.
- M. Ehrbar, M. P. Lutolf, S. C. Rizzi, J. A. Hubbell and F. E. Weber, Bone, 2008, 42, S72 CrossRef.
- A. S. Badami, M. R. Kreke, M. S. Thompson, J. S. Riffle and A. S. Goldstein, Biomaterials, 2006, 27, 596 CrossRef CAS.
- K. Sombatmankhong, N. Sanchavanakit, P. Pavasant and P. Supaphol, Polymer, 2007, 48, 1419 CrossRef CAS.
- H. W. Kim, H. S. Yu and H. H. Lee, J. Biomed. Mater. Res. A, 2007, 87, 25.
- J. H. Song, H. E. Kim and H. W. Kim, J. Mater. Sci.: Mater. Med., 2008, 19, 2925 CrossRef CAS.
- H. W. Kim, J. H. Song and H. E. Kim, Adv. Funct. Mater., 2005, 15, 1988 CrossRef CAS.
- Y. Zhang, J. R. Venugopal, A. El-Turki, S. Ramakrishna, B. Su and C. T. Lim, Biomaterials, 2008, 29, 4314 CrossRef CAS.
- H. W. Kim, H. H. Lee and J. C. Knowles, J. Biomed. Mater. Res. A, 2007, 84, 875.
- X. Erisken, D. M. Kalyon and H. Wang, Biomaterials, 2008, 29, 4065 CrossRef.
- P. Singh, R. C. YashRoy and M. Hoque, Indian J. Biochem. Biophys, 2006, 43, 167 Search PubMed.
- B. Strauch, M. K. Patel, J. A. Navarro, M. Berdichevsky, H. L. Yu and A. A. Pilla, Plast. Reconstr. Surg., 2007, 120, 425 Search PubMed.
- T. J. Webster. Nanotechnology for the Regeneration of Hard and Soft Tissues. World Scientific Publishing Co. Pte.Ltd. 2007. P9–10 Search PubMed.
- Y. Wu, W. Jiang, X. Wen, B. He, X. Zeng, G. Wang and Z. Gu, Biomed. Mater., 2010, 5, 015001 Search PubMed.
- Z. P. Chen, Y. Zhang, S. Zhang, J. G. Xia, J. W. Liu, K. Xu and N. Gu, Colloids Surf., A, 2008, 316, 210 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: TEM images; pH data; magnetic field distribution; BALP data. See DOI: 10.1039/c0nr00178c |
| ‡ The first two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2010 |
