Luminescence resonance energy transfer from an upconverting nanoparticle to a fluorescent phycobiliprotein
Fiorenzo
Vetrone
ab,
Rafik
Naccache
a,
Christopher G.
Morgan
b and
John A.
Capobianco
*a
aDepartment of Chemistry and Biochemistry, Concordia University, 7141 Sherbrooke St. W., Montreal, QC H4B 1R6, Canada. E-mail: capo@vax2.concordia.ca; Fax: +1 514-848-2868
bBiomedical Sciences Research Institute, School of Environmental and Life Sciences, University of Salford, Salford, M5 4WT, UK
First published on 10th May 2010
Abstract
Water dispersible upconverting polyethylenimine (PEI)-capped NaYF4 nanoparticles co-doped with trivalent erbium (Er3+) and ytterbium (Yb3+) were prepared via solvothermal synthesis with an 18 nm average particle diameter. These upconverting nanoparticles can be used to sensitize a light-harvesting phycobiliprotein (R-Phycoerythrin) via luminescence resonance energy transfer (LRET).
1. Introduction
The recent development of novel highly sensitive and specific luminescent probes with optical properties superior to organic dyes and fluorescent proteins has attracted a diverse group of researchers in a number of key areas. Luminescent nanoparticles are emerging as useful tools in diagnostic medicine and therapeutics and are finding widespread applications. In cancer research, for example, semiconductor quantum dots (QDs) have been used experimentally in animal models for imaging of various carcinomas.1,2 Alternatively, luminescent nanoparticles can also be prepared from inorganic insulating phosphors, where emission typically originates from the dopant ions. In commercial phosphors, trivalent lanthanide ions (Ln3+) such as terbium (Tb3+) and europium (Eu3+) are widely used as dopants on account of high quantum yield and narrow spectral emission. For Ln3+-doped materials the supporting matrix can be used to absorb incident radiation and transfer it radiationlessly to the dopant ion, or else the matrix can act passively, simply providing a favorable environment for efficient Ln3+ emission. In fact, the matrix plays a fundamental role in determining the efficiency of the luminescence, which is dependent on the vibrational energy of the host. Ln3+-doping in low energy matrices, such as fluorides, generally results in higher luminescence efficiencies.3 At the nanoscale, the surface properties of the nanoparticles are also particularly important in determining luminescence efficiency.Currently studied luminescent nanoparticles typically rely on single photon excitation with high energy light (such as blue or UV) with emission at lower energies (i.e. Stokes emission). However, some lanthanide ions (particularly Er3+ and Tm3+) have another interesting characteristic; in that they can be excited with low energy light (typically NIR) and in turn, emit higher energy light such as visible or UV (anti-Stokes emission).4 Unlike most other two-photon absorption (TPA) materials, where excitation is via “virtual” excited states, excitation of Ln3+ ions such as Er3+ and Tm3+ proceeds via “real” electronic states of finite lifetime and thus high power, ultrafast lasers are not required for efficient excitation. This multiphoton process, known commonly as “upconversion”, can be quite useful in a number of applications including displays, diagnostics, imaging, therapeutics and nanomedicine and thus has attracted a great deal of interest.5–8 Excitation with NIR light has a number of advantages relative to excitation with UV or visible light. For example, the NIR excitation wavelength is specific only to the Ln3+-doped nanoparticle and will not excite any other fluorophores in the vicinity. NIR light has better penetration into biological tissues than visible or UV light, and does not cause damage to the specimen under study.9
One useful application employs Ln3+-doped nanoparticles as luminescent biolabels, which can readily (up)convert the NIR excitation to a higher energy wavelength capable of transferring energy radiatively or non-radiatively to a nearby absorbing species, which might be fluorescent or a photosensitizing agent, for example. Fluorescent Resonance Energy Transfer (FRET) from upconverting nanoparticles to various acceptor species including gold nanoparticles and organic dyes (i.e. TAMRA (tetramethyl-6-carboxyrhodamine), FITC (fluorescein isothiocyanate), and TRITC (5(6)-tetramethyl-rhodamine isothiocyanate)) has recently been shown.10,11 Furthermore, radiative energy transfer of the upconverted radiation to photosensitizers such as 9,10-anthracenedipropionic acid (ADPA) and zinc phthalocyanine to generate singlet oxygen species for use in photodynamic therapy applications has also been reported.12,13 Radiative energy transfer is usually very inefficient unless the concentration of the acceptor species is high and for many purposes non-radiative transfer processes (Förster and/or Dexter transfer) are of more interest.14,15 The efficiency of such energy transfer can be very high for appropriately oriented acceptor species in close proximity to the energy donor.16,17
In the literature, non-radiative energy transfer is usually referred to as “FRET”. However, since emission from lanthanides is not fluorescence, radiationless energy transfer from a lanthanide donor to an appropriate acceptor has been called “LRET” (which might stand for lanthanide resonance energy transfer or luminescence resonance energy transfer).18 In this paper, we study the feasibility of using NIR light excitation of NaYF4:Er3+, Yb3+ upconverting nanoparticles to sensitize a light harvesting protein, R-Phycoerythrin.
2. Materials and methods
2.1 Sample preparation
The NaYF4:Er3+, Yb3+ nanoparticles (2 mol% Er3+, 18 mol% Yb3+, respectively) were synthesized via a solvothermal process.19 In a typical experiment, 3.6 mmol of NaCl (99.99%, Aldrich), 1.44 mmol of YCl3·6H2O (99.99%, Aldrich), 0.036 mmol of ErCl3·6H2O (99.995%, Aldrich), and 0.324 mmol of YbCl3·6H2O (99.998%, Aldrich) were dissolved in a 27 mL solution of ethylene glycol (99+%, Aldrich) containing 0.45 g of branched polyethylenimine (Mw ≈ 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Aldrich) and stirred for approximately 60 min. Subsequently, a solution of 17 mL ethylene glycol with 7.2 mmol NH4F (99.99+%, Aldrich) was added to the initial solution containing the chlorides and stirred for another 30 min. The resulting clear solution was then placed in a 250 mL Teflon lined autoclave (Berghof/America) and heated with stirring for 24 h at 200 °C. The resulting nanoparticles were isolated via centrifugation and washed twice with distilled water and ethanol. The nanoparticles were then dried under vacuum for 24 h. A 1 wt% colloidal dispersion was prepared for all spectroscopic measurements.
000, Aldrich) and stirred for approximately 60 min. Subsequently, a solution of 17 mL ethylene glycol with 7.2 mmol NH4F (99.99+%, Aldrich) was added to the initial solution containing the chlorides and stirred for another 30 min. The resulting clear solution was then placed in a 250 mL Teflon lined autoclave (Berghof/America) and heated with stirring for 24 h at 200 °C. The resulting nanoparticles were isolated via centrifugation and washed twice with distilled water and ethanol. The nanoparticles were then dried under vacuum for 24 h. A 1 wt% colloidal dispersion was prepared for all spectroscopic measurements.
2.2 Biotinylation of NaYF4:Er3+, Yb3+ nanoparticles
The bioconjugation of biotin to the NaYF4:Er3+, Yb3+ nanoparticles was achieved as follows. First 20 mg of the as-synthesized upconverting nanoparticles and 0.1 mmol of biotin (≥99.0%, Fluka) in 2.5 mL of MES buffer (pH = 6, ≥99%, Sigma) were ultrasonicated for approximately 10 min. Subsequently, 0.1 mmol of the cross-linking reagent N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDAC, Fluka) and 0.2 mmol of the secondary reagent N-hydroxysuccinimide (NHS, 98%, Aldrich) were added and mixed for 3 h under moderate stirring. Finally, the nanoparticles were washed 3× with a water/ethanol mixture to remove any excess reagents (EDAC, NHS, and/or biotin) and the biotinylated nanoparticles were isolated via centrifugation. The biotinylated upconverting nanoparticles were subsequently dispersed in 20 mL of MES (2-(N-morpholino)ethanesulfonic acid) buffer.2.3 Luminescence resonance energy transfer (LRET) experiments
The binding of the ExtrAvidin–R-Phycoerythrin (Sigma) labels to the biotinylated upconverting nanoparticles was achieved by taking a 2 mL aliquot of the above biotinylated nanoparticle solution and diluting it further with 8 mL of MES buffer. The resulting 10 mL solution was in turn separated into five equal 2 mL aliquots where the appropriate amount of ExtrAvidin–R-Phycoerythrin (20, 40, 60, 80, and 100 µL) was added to each and left to incubate for 3 h with stirring at room temperature.2.4 X-Ray powder diffraction (XRPD) analysis
XRPD patterns were measured using a Scintag XDS-2000 Diffractometer equipped with a Si(Li) Peltier-cooled solid state detector, CuKα source at a generator power of 45 kV and 40 mA, divergent beam (2 mm and 4 mm) and receiving beam slits (0.5 mm and 0.2 mm). Scan range was set from 20–80° 2θ with a step size of 0.02° and a count time of 2 s. The sample was measured using a quartz “zero background” disk.2.5 Transmission electron microscopy (TEM) and selected area electron diffraction (SAED)
TEM measurements of the colloidal dispersion of nanoparticles were performed with a Philips CM200 microscope operating at 200 kV equipped with a charge-coupled device (CCD) camera (Gatan). A minute amount of sample was dispersed in an appropriate amount of toluene to yield an approximate 0.1 wt% solution. A drop of the resulting solution was evaporated on a formvar/carbon film supported on a 300 mesh copper grid (3 mm in diameter).2.6 Upconversion luminescence spectroscopy
Visible emissions from the NaYF4:Er3+, Yb3+ nanoparticles were obtained upon excitation with 980 nm using a Coherent 6-pin fiber-coupled F6 series 980 nm laser diode (maximum power of 800 mW at 1260 mA), coupled to a 100 µm (core) fiber. For the spectroscopic studies, the colloidal solution was placed in a Hellma, QS quartz cuvette (1 cm path length). The upconverted visible emissions were collected at π/2 with respect to the incident beam and then dispersed by a 1 m Jarrell-Ash Czerny-Turner double monochromator with an optical resolution of ∼0.15 nm. The visible emissions from the sample exiting the monochromator were detected by a thermoelectrically cooled Hamamatsu R943-02 photomultiplier tube. A preamplifier, model SR440 Standard Research Systems, processed the photomultiplier signals and a gated photon-counter model SR400 Standard Research Systems data acquisition system was used as an interface between the computer and the spectroscopic hardware. The signal was recorded under computer control using the Standard Research Systems SR465 software data acquisition/analyzer system.3. Results
Upconverting NaYF4:Er3+, Yb3+ nanoparticles (2 mol%, 18 mol%, respectively) were prepared via solvothermal synthesis using the metal chlorides and ammonium fluoride as the starting materials in the presence of ethylene glycol and using polyethylenimine (PEI) as a capping ligand.19 The TEM image of the as-prepared nanoparticles is presented in Fig. 1A and shows that they are nearly spherical in shape with an approximate diameter of 18 nm. The PEI capped nanoparticles have a cubic structure (α-NaYF4 phase) and readily disperse in water due to the terminal –NH2 groups of the capping ligand. The high crystallinity of the nanoparticles was confirmed from both high resolution TEM (HRTEM) and selected area electron diffraction (SAED). The HRTEM image shows the lattice fringes of the NaYF4:Er3+, Yb3+ nanoparticles (Fig. 1B) and the spacing between the fringes was determined to be approximately 3.2 Å corresponding to the (111) plane. Furthermore, the SAED and XRD can be indexed to the (111), (200), (220), and (311) planes of α-NaYF4 confirming their cubic structure (see Fig. 1C and D).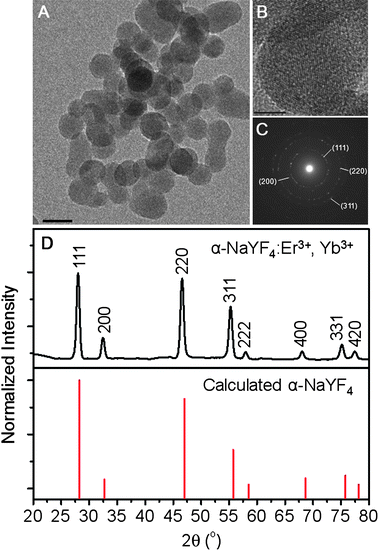 | ||
| Fig. 1 (A) TEM image of the NaYF4:Er3+, Yb3+ nanoparticles (scale bar = 20 nm). (B) High resolution TEM image showing lattice fringes (scale bar = 20 nm). (C) Selected area electron diffraction (SAED) pattern showing the cubic structure of the NaYF4:Er3+, Yb3+ nanoparticles. (D) (Top) X-Ray diffraction (XRD) pattern of NaYF4:Er3+, Yb3+ nanoparticles. (Bottom) Calculated line pattern for α-NaYF4 shown for comparison (JCPDS: 6-0342). | ||
The NaYF4:Er3+, Yb3+ nanoparticles are capable of (up)converting the NIR excitation light (980 nm) to green and red light (Fig. 2). Emission in the green region (500 to 575 nm) is observed and attributed to the transitions from the 2H11/2 and 4S3/2 excited states of the Er3+ ion to the 4I15/2 ground state (denoted as 2H11/2, 4S3/2 → 4I15/2). Similarly, emission in the red region (625 to 700 nm) is also observed from the 4F9/2 Er3+ excited state to the ground state (denoted as 4F9/2 → 4I15/2).
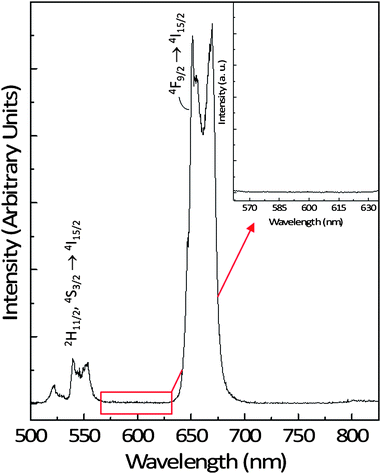 | ||
| Fig. 2 Upconversion emission spectrum of a 1 wt% colloidal dispersion of the NaYF4:Er3+, Yb3+ nanoparticles in water following excitation with 980 nm showing the green and red emissions. Inset: region showing the absence of emission in the upconversion spectrum of NaYF4:Er3+, Yb3+ nanoparticles between the 2H11/2, 4S3/2 (green) and 4F9/2 (red) emission where R-Phycoerythrin emission is observed. | ||
The upconversion of NIR light to higher energies in Er3+/Yb3+ co-doped nanoparticles is well documented (see for example ref. 20 and 21). The green and red emitting states of Er3+ are populated as a result of successive energy transfers from excited Yb3+ ions in the 2F5/2 excited state, initially populating the 4I11/2 intermediate state of Er3+ followed by the subsequent population of the 4F7/2 excited state (shown schematically in Fig. 3 as steps 1 and 2). The emitting states (2H11/2, 4S3/2, and 4F9/2) are in turn populated via non-radiative decay (see Fig. 3). It should also be noted that the red emitting state 4F9/2 state can also be populated directly. Once the ion is excited to the intermediate state (4I11/2), it can decay non-radiatively to the lower lying level (4I13/2). From there, a transfer of energy from an excited Yb3+ ion will populate the 4F9/2 state directly (step 3 in Fig. 3). We emphasize here that Er3+–Er3+ processes are also present in the upconversion process but not shown for brevity. The Yb3+ ion is added to the matrix as a sensitizer to enhance the efficiency of the upconversion process. This is because the Yb3+ ion has only one excited state (2F5/2), which coincides well with the 980 nm pump wavelength and has an approximately ten-fold higher absorption coefficient at 980 nm than the Er3+ ion, which also has a level (4I11/2) at the corresponding energy22 (see Fig. 3).
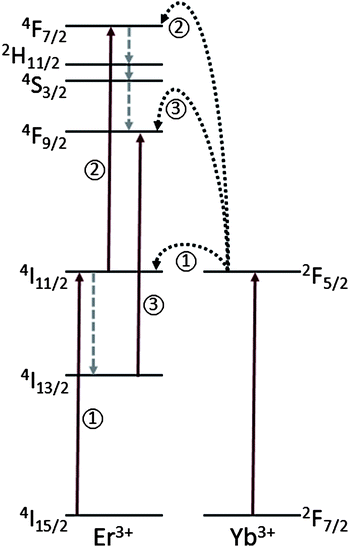 | ||
| Fig. 3 The energy level diagrams of the Er3+/Yb3+ dopant ions as well as the mechanisms responsible for the upconversion process following excitation with 980 nm. The arrows pointing upwards represent energy absorption, dotted arrows represent multiphonon relaxation (non-radiative decay), and the curved arrows represent energy transfer. Note: only relevant energy levels of Er3+ are shown for simplicity. | ||
Ln3+-doped upconverting nanoparticles are ideal labels for LRET-based bioassays in principle since they can be excited with NIR light reaching an excited state suitable for sensitizing an acceptor label. The ability to excite a luminescent nanoparticle in the NIR is valuable as that excitation wavelength will not be absorbed by any impurities nor will it excite any fluorophores present in the sample. Sample autofluorescence is a major issue limiting the sensitivity and dynamic range of bioassays, and any technique which minimizes this is potentially important. The only possible source of autofluorescence, when using an upconverting energy donor as a biolabel, is that excited by the upconverted visible/UV radiation upon reabsorption by the sample, and this can be held to a very low level with appropriate design. As such, high upconversion efficiencies are not required. The Er3+ ion is a particularly useful dopant for upconversion in LRET applications since its primary emission corresponds to wavelengths in the green spectral region where several important acceptor labels can be excited.
However, more interesting is its favorable emission spectrum, which has a total absence of signal in the region of 575–625 nm where many of the organic labels emit (Fig. 2, inset). Thus, an Er3+ (and Yb3+) doped nanoparticle can be feasibly coupled with a conventional organic label or a fluorescent protein and be used as an LRET donor–acceptor pair.
In this study, we have chosen R-Phycoerythrin, a fluorescent phycobiliprotein derived from cyanobacteria and eukaryotic algae23,24 and coupled it with an Er3+/Yb3+ co-doped upconverting nanoparticle to excite a fluorescent species in proximity via LRET. R-Phycoerythrin is a highly suitable energy acceptor for Er3+ due to its high molar extinction coefficient and near-unity quantum yield. It also possesses a rather wide absorption band (∼425 to 600 nm) with peak absorption near 550 nm, matching well with the Er3+ transitions from the 2H11/2 and 4S3/2 excited states centered at about 545 nm.
In order for the upconverting nanoparticle to excite the fluorescent protein, the two species (donor and acceptor) must be in close enough proximity given the R−6 dependence of LRET. Typically LRET will only be efficient if the donor and acceptor labels are within less than c 10 nm of each other. This steep distance dependence means that much of the lanthanide ions in large nanoparticles will effectively be out of range for LRET to surface-bound species, and in practice particle diameters of the order of 10–20 nm offer a reasonable compromise for LRET applications. Within the nanoparticle some degrees of energy delocalization are expected as resonant transfers between Er3+ ions will transport energy to the surface, but this is not expected to be a major factor as dopant concentrations are fairly low for efficient upconverting labels. Binding of R-Phycoerythrin to the nanoparticle surface was accomplished by exploiting the selective recognition of biotin to avidin as well as the strong binding of the pair. Thus, ExtrAvidin labeled R-Phycoerythrin was coupled to an upconverting nanoparticle covalently conjugated with biotin to study LRET between these two labels (Fig. 4).
 | ||
| Fig. 4 Schematic representation of the upconversion nanoparticle–R-Phycoerythrin system. | ||
The conjugation of biotin to the NaYF4:Er3+, Yb3+ nanoparticles was achieved using a two-step process (see Scheme 1) with N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDAC) and N-hydroxysuccinimide (NHS).25 In the initial step, an o-acylisourea active intermediate is formed via the reaction of EDAC and the carboxylic acid terminal group of the biotin. This is subsequently followed by the reaction of the active intermediate with NHS to yield an NHS ester intermediate. The purpose of this step is two-fold; the first being an increase in the stability of the ester intermediate as the o-acylisourea intermediate is highly subject to hydrolysis in aqueous media. Secondly, the addition of NHS is known to increase the reaction yield by up to 20-fold.26 Finally, the NHS intermediate reacts with the primary amine of the polyethylenimine (PEI) capping ligand coordinating the NaYF4:Er3+, Yb3+ nanoparticle forming the amide bond and resulting in the biotinylated nanoparticles.
 | ||
| Scheme 1 Mechanism for the conjugation of biotin to the upconverting NaYF4:Er3+, Yb3+ nanoparticles. | ||
To determine if NIR-excited NaYF4:Er3+, Yb3+ nanoparticles can sensitize the fluorescent protein, different amounts of ExtrAvidin-labeled R-Phycoerythrin were added to an aqueous suspension of biotinylated nanoparticles. A schematic representation of the upconversion nanoparticle–R-Phycoerythrin system connected via the biotin–ExtrAvidin linkage is shown in Fig. 4. Following upconversion of the 980 nm pump radiation, some of the energy is transferred non-radiatively to the R-Phycoerythrin via LRET. The fluorescent protein subsequently emits light with a peak wavelength of 578 nm. As the quantity of ExtrAvidin is increased, more biotinylated nanoparticles are bound and thus the sensitized emission of R-Phycoerythrin increases (Fig. 5A). It should be noted that there is some deviation from linearity (r2 = 0.97) in the sensitized emission spectrum of R-Phycoerythrin as the amount of ExtrAvidin increased (Fig. 5B). However, of greater significance is the fact that the ExtrAvidin being detected only ranges between 4 and 20 µg attesting to the high discrimination potential when using upconverting nanoparticles.
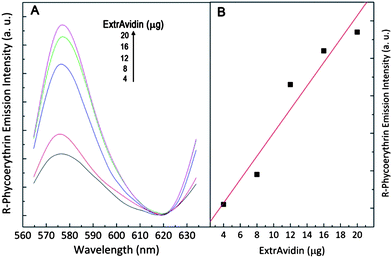 | ||
| Fig. 5 (A) R-Phycoerythrin emission at 578 nm following sensitized via LRET from upconverting NaYF4:Er3+, Yb3+ nanoparticles excited with 980 nm. (B) Graph showing the sensitized R-Phycoerythrin emission as a function of ExtrAvidin. | ||
To determine the level of radiative energy transfer (versus non-radiative) of the biotinylated upconverting nanoparticles to the ExtrAvidin-labeled R-Phycoerythrin, a control experiment was carried out where equal amounts of R-Phycoerythrin, however, lacking the ExtrAvidin moiety, were added to the biotinylated upconverting nanoparticles. Very little signal was detected, consistent with a very low level of reabsorption of upconverted emission by the fluorescent label. Thus, these results suggest that upconverting nanoparticles can be used as donor labels in LRET bioassays for example. Current experiments are underway to ascertain the biosensing ability of these nanoparticles.
4. Conclusions
In conclusion, we have shown that water dispersible PEI-capped NaYF4:Er3+, Yb3+ upconverting nanoparticles prepared via solvothermal synthesis can be used as luminescent donor labels in LRET-based applications. The nanoparticles convert 980 nm radiation to higher energies via an efficient multiphoton excitation process. Following biotinylation, these nanoparticles were bound to R-Phycoerythrin labeled with ExtrAvidin. The excited state responsible for the green emission can transfer energy non-radiatively via LRET to the R-Phycoerythrin label in close proximity and emission was observed at 578 nm from the protein. Control experiments revealed minimal non-specific binding.Acknowledgements
The authors thank the Natural Sciences and Engineering Research Council (NSERC) of Canada and the Gouvernement du Québec, Ministère du Développement économique, de l'Innovation et de l'Exportation for funding. FV and CGM also thank the Royal Society (UK) for funding.References
- X. Gao, Y. Cui, R. M. Levenson, L. W. K. Chung and S. Nie, Nat. Biotechnol., 2004, 22, 969 CrossRef CAS.
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538 CrossRef CAS.
- R. H. Page, K. I. Schaffers, P. A. Waide, J. B. Tassano, S. A. Payne, W. F. Krupke and W. K. Bischel, J. Opt. Soc. Am. B, 1998, 15, 996 Search PubMed.
- F. Auzel, Chem. Rev., 2004, 104, 139 CrossRef CAS.
- D. K. Chatterjee, A. J. Rufaihah and Y. Zhang, Biomaterials, 2008, 29, 937 CrossRef CAS.
- E. Downing, L. Hesselink, J. Ralston and R. Macfarlane, Science, 1996, 273, 1185 CrossRef CAS.
- S. F. Lim, R. Riehn, W. S. Ryu, N. Khanarian, C.-K. Tung, D. Tank and R. H. Austin, Nano Lett., 2006, 6, 169 CrossRef CAS.
- L. Wang and Y. Li, Chem. Commun., 2006, 2557 RSC.
- K. König, J. Microsc. (Oxford, U. K.), 2000, 200, 83 Search PubMed.
- Z. Li, Y. Zhang and S. Jiang, Adv. Mater., 2008, 20, 4765 CrossRef CAS.
- L. Wang, R. Yan, Z. Huo, L. Wang, J. Zeng, J. Bao, X. Wang, Q. Peng and Y. Li, Angew. Chem., 2005, 44, 6054 CrossRef CAS.
- D. K. Chatterjee and Z. Yong, Nanomedicine, 2008, 3, 73 CrossRef CAS.
- P. Zhang, W. Steelant, M. Kumar and M. Scholfield, J. Am. Chem. Soc., 2007, 129, 4526 CrossRef CAS.
- D. L. Dexter, J. Chem. Phys., 1953, 21, 836 CrossRef CAS.
- T. Förster, Ann. Phys., 1948, 2, 55 CAS.
- F. Kukolka, B. K. Müller, S. Paternoster, A. Arndt, C. M. Niemeyer, C. Bräuchle and D. C. Lamb, Small, 2006, 2, 1083 CrossRef CAS.
- I. L. Medintz and H. Mattoussi, Phys. Chem. Chem. Phys., 2009, 11, 17 RSC.
- P. R. Selvin, T. M. Rana and J. E. Hearst, J. Am. Chem. Soc., 1994, 116, 6029 CrossRef CAS.
- F. Wang and X. Liu, J. Am. Chem. Soc., 2008, 130, 5642 CrossRef CAS.
- F. Vetrone, R. Naccache, V. Mahalingam, C. G. Morgan and J. A. Capobianco, Adv. Funct. Mater., 2009, 19, 2924 CrossRef CAS.
- F. Wang and X. Liu, Chem. Soc. Rev., 2009, 4, 976 RSC.
- B. G. Wybourne, Spectroscopic Properties of Rare Earths, Wiley- Interscience, New York, 1965 Search PubMed.
- A. N. Glazer, J. Appl. Phycol., 1994, 6, 105 Search PubMed.
- R. MacColl, L. E. Eisele, E. C. Williams and S. S. Bowser, J. Biol. Chem., 1996, 271, 17157 CrossRef CAS.
- G. T. Hermanson, Bioconjugate Techniques, Academic Press, San Diego, CA, 2nd edn, 2008 Search PubMed.
- J. V. Staros, R. W. Wright and D. M. Swingle, Anal. Biochem., 1986, 156, 220 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
