A chemical combination reaction within single-walled carbon nanotubes
Ning
Wang
and
Lunhui
Guan
*
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, YangQiao West Road 155#, Fuzhou, Fujian 350002, P.R. China. E-mail: guanlh@fjirsm.ac.cn; Fax: +86-591-8379-2835; Tel: +86-591-8379-2835
First published on 24th March 2010
Abstract
We presented an approach for realizing a chemical combination reaction of iodine and potassium inside the 1 nm channel of single-walled carbon nanotubes.
Single-walled carbon nanotubes (SWNTs), which are graphitic tubular structures, have attracted enormous attention for their unusual electronic and structural properties.1 Engineering the electronic properties of SWNTs is of critical importance for their potential applications in transistors, diode or other nanodevices. SWNTs typically have a 1 nm internal diameter, so they can be regarded as the smallest test tubes. A lot of efforts have been made to introduce foreign materials into the inner place of SWNTs, since the materials confined in this nano space behave totally different structures and reactivity compared with that under the macroscopic condition. Theoretical and experimental results both proved that the inner phase of SWNTs had important effect on the structures and chemical reactions.2 For example, Sloan et al. firstly synthesized a one-dimensional discrete KI crystal that is three atomic layers thick within a SWNT by molten media method.3 Our previous results indicated that the structures of iodine inside SWNTs are polymorphic and strongly depended on the diameters of the SWNTs.4 The chemical compounds which could be intercalated inside SWNTs were limited. Only those with a low surface tension could be introduced into SWNTs.5 In order to synthesize other materials encapsulated in SWNTs (materials@SWNTs), an alternative way is to realize chemical reactions inside SWNTs. Previous results on chemical reactions inside SWNTs mainly focused on the decomposition or coalescence of one species.6 If one can introduce one precursor into the inner cavity of SWNTs and then deliver a second precursor, a chemical reaction may happen inside the nano-channel of the SWNTs. With this in mind, we realized a truly chemical combination reaction of iodine and potassium within SWNTs for the first time. The reaction scheme is illustrated in Scheme 1. Briefly, iodine was intercalated into the intact SWNTs as first precursor by molten media method, and then the so-obtained material was doped with potassium as second precursor, eventually forming KI within SWNTs.
 | ||
| Scheme 1 A schematic illustration of the combination reaction of iodine and potassium within SWNTs. | ||
SWNTs with ultrahigh purity (99.5%) and average diameter of 1.4 nm were produced by our recent methods.7 The SWNTs were first filled with iodine by heating SWNTs and iodine powder in an evacuated glass tube at 150 °C in 1 × 10−3 Pa for 48 h. The excess iodine remaining on the SWNT surface was removed by washing several times with ethanol until the solvent became colorless. The obtained material was denoted as I@SWNTs. Then the I@SWNTs was degassed at 473 K 1 × 10−3 Pa vacuum and subsequently exposed to potassium vapor at 473 K over 50 h. The samples were characterized by high resolution transmission electron microscopy (HR-TEM) and Raman spectra.
HR-TEM observation provided direct evidence of the fine structures of materials confined in SWNTs. As reported by our previous results, the structures of the iodine inside SWNTs are strongly depended on the diameters of the host SWNTs.4 As for the arc-discharged SWNTs with mean diameters of 1.4 nm, the iodine inside them has helical structures, mainly double or triple chains. The typical filling ratio was around 30%. Fig. 1a and b show HR-TEM images and schematic illustrations of a double helical chain inside a SWNT with the diameter of ∼1.4 nm and a triple chain inside a SWNT with the diameter of ∼1.5 nm. The HR-TEM images also indicated that even after doping with iodine, there were still some empty places inside the SWNTs which could accommodate other small atoms. Furthermore, because of these empty places, the iodine chains were flexible. They moved frequently inside the SWNTs during the observation. In that case, it is difficult to observe the individual iodine atoms during the exposure time, typically 1 s. After doping with potassium, a prominent change of structure occurred for the I@SWNTs. We seldom found the flexible polymorphic helical structures inside the SWNTs any more. The most common materials we observed were crystals, which have fixed structures. The filling ratio remains around 30%. Detailed analysis revealed that the crystals inside the SWNTs were exactly similar to the KI crystals reported by Sloan et al.8Fig. 1c shows a HR-TEM image of the newly formed ‘all surface’ 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 co-ordinated (2 × 2) KI crystal encapsulated inside a SWNT with the diameter of 1.4 nm. The black spots represent immobile iodine atoms which can be easily imaged. The KI crystal has a preferred orientation, with the rock salt 〈001〉 direction parallel to the tubule axes. The spots are arranged at average intervals of ∼0.35 nm, which was ascribed to the {2 0 0} d-spacing of KI. Meanwhile along the SWNT's axes, the spacing increases to ∼0.4 nm due to the confinement effect from the outer SWNTs. As for the host SWNTs with diameter of 1.5 nm, after doping with potassium, the triple iodine chains changed to three layers of KI crystal (Fig. 1d). An apparent contraction along the KI 〈001〉 direction were also observed in the three layer crystal. It should be noted that the combination reaction could not be realized if the SWNTs were doped with potassium as first precursor, and then doped with iodine. The phenomenon is easily understood, if the inner place of the SWNTs were first occupied by the small atoms (K), it is difficult to proceed with delivery of larger atoms (I) to the SWNT interior.
4 co-ordinated (2 × 2) KI crystal encapsulated inside a SWNT with the diameter of 1.4 nm. The black spots represent immobile iodine atoms which can be easily imaged. The KI crystal has a preferred orientation, with the rock salt 〈001〉 direction parallel to the tubule axes. The spots are arranged at average intervals of ∼0.35 nm, which was ascribed to the {2 0 0} d-spacing of KI. Meanwhile along the SWNT's axes, the spacing increases to ∼0.4 nm due to the confinement effect from the outer SWNTs. As for the host SWNTs with diameter of 1.5 nm, after doping with potassium, the triple iodine chains changed to three layers of KI crystal (Fig. 1d). An apparent contraction along the KI 〈001〉 direction were also observed in the three layer crystal. It should be noted that the combination reaction could not be realized if the SWNTs were doped with potassium as first precursor, and then doped with iodine. The phenomenon is easily understood, if the inner place of the SWNTs were first occupied by the small atoms (K), it is difficult to proceed with delivery of larger atoms (I) to the SWNT interior.
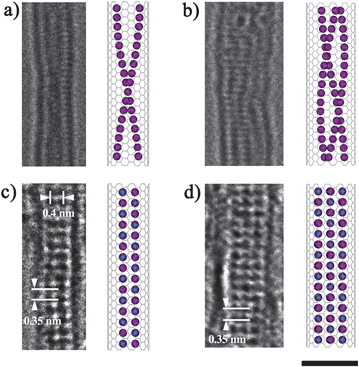 | ||
| Fig. 1 HR-TEM images and schematic illustration of (a) a double helical iodine chain inside a 1.4 nm SWNT; (b) a triple iodine chain inside a 1.5 nm SWNT; (c) a two layer KI inside a 1.4 nm SWNT and (d) a three layer KI inside a 1.5 nm SWNT. Scale bar = 2 nm. | ||
The chemical reaction inside SWNTs was also proven by Raman spectra, which probes the change of the electronic structure of SWNTs.9 Many theoretical calculations predicted that one-dimensional nanowire or chain is suitable guests for modifying the electronic structure of SWNTs. Fig. 2 shows Raman spectra of pristine SWNTs, I@SWNTs and I@SWNTs after doping with potassium (KI@SWNTs) excited at 785 nm NIR diode lasers. The Raman spectra of the I@SWNTs after doping with potassium were as same as that of the KI@SWNTs reported by previous results.10 Typical Raman spectra of SWNTs composed two characteristic peaks: the tangential band (G+ band located at 1591 cm−1 and G− band located at between 1450 and 1560 cm−1) of the intact SWNT, associated with carbon atom vibrations along the nanotube axis; and the radial-breathing mode (RBM) centered at ∼160 cm−1 which was scaled with the diameters of the SWNTs. The G+ band is quite sensitive to doping: p-doping induces an upshift, while n-doping induces a downshift. As for I@SWNT, the frequency of the G+ band upshifted by 11 cm−1 compared with the pristine SWNT, resulting from electron transfer from the host SWNT to the guest iodine. The new peak around 300 cm−1 was ascribed to the iodine chains inside the SWNTs.11 After doping with potassium, the G+ band moved back to 1591 cm−1. The recovery of the G+ band can be explained by forming KI from iodine and potassium inside SWNTs. As reported with previous results, the G+ band upshifted slightly while the G− band quenched strongly after direct doping with KI.10 Compared with the intact SWNTs, there were no significant RBM shifts but changes in their relative intensities of the I@SWNTs after doping with potassium. The recovery of the RBM mode was also ascribed to the reaction of iodine and potassium inside SWNTs.
 | ||
| Fig. 2 Raman spectra of the pristine SWNT, I@SWNT and I@SWNTs after doping with potassium (KI@SWNTs). | ||
In summary, the chemical combination reaction of iodine and potassium inside the nano channel of the SWNTs was realized by introducing iodine and subsequently potassium inside the SWNTs. The reaction was demonstrated by HR-TEM observation and Raman spectra. The method should be applicable for other chemical reactions, eventually forming polymorphic materials inside SWNTs.
Financial support for this study was provided by the Fujian Institute of Research on the Structure of Matter (Grant No. SZD09003), the Chinese Academy of Sciences, the National Key Project on Basic Research (Grant No. 2009CB939801), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, Ministry of Human Resources and Social Security of China.
Notes and references
- R. H. Baughman, A. A. Zakhidov and W. A. de Heer, Science, 2002, 297, 787 CrossRef CAS.
- (a) M. D. Halls and K. Raghavachari, Nano Lett., 2005, 5, 1861 CrossRef CAS; (b) X. L. Pan, Z. L. Fan, W. Chen, Y. J. Ding, H. Y. Luo and X. H. Bao, Nat. Mater., 2007, 6, 507 CrossRef CAS; (c) L. H. Guan, K. Suenaga, T. Okazaki, Z. Shi, Z. Gu and S. Iijima, J. Am. Chem. Soc., 2007, 129, 8954 CrossRef; (d) K. Koga, G. T. Gao, H. Tanaka and X. C. Zeng, Nature, 2001, 412, 802 CrossRef CAS.
- R. R. Meyer, J. Sloan, R. E. Dunin-Borkowski, A. I. Kirkland, M. C. Novotny, S. R. Bailey, J. L. Hutchison and M. L. H. Green, Science, 2000, 289, 1324 CrossRef CAS.
- L. H. Guan, K. Suenaga, Z. J. Shi, Z. N. Gu and S. Iijima, Nano Lett., 2007, 7, 1532 CrossRef CAS.
- M. Monthioux, Carbon, 2002, 40, 1809 CrossRef CAS.
- (a) T. Okazaki, K. Suenaga, K. Hirahara, S. Bandow, S. Iijima and I. E. Shinohara, J. Am. Chem. Soc., 2001, 123, 9673 CrossRef CAS; (b) L. Guan, K. Suenaga, S. Okubo, T. Okazaki and S. Lijima, J. Am. Chem. Soc., 2008, 130, 2162 CrossRef CAS; (c) L. H. Guan, K. Suenaga and S. Iijima, Nano Lett., 2008, 8, 459 CrossRef CAS.
- C. X. Wu, J. X. Li, G. F. Dong and L. H. Guan, J. Phys. Chem. C, 2009, 113, 3612 CrossRef CAS.
- J. Sloan, A. I. Kirkland, J. L. Hutchison and M. L. H. Green, Acc. Chem. Res., 2002, 35, 1054 CrossRef CAS.
- M. S. Dresselhaus, G. Dresselhaus, R. Saito and A. Jorio, Phys. Rep., 2005, 409, 47 CrossRef.
- A. Ilie, J. S. Bendall, D. Roy, E. Philp and M. L. H. Green, J. Phys. Chem. B, 2006, 110, 13848 CrossRef CAS.
- Z. Y. Wang, L. Wang, Z. J. Shi, J. Lu, Z. N. Gu and Z. X. Gao, Chem. Commun., 2008, 3429 RSC.
| This journal is © The Royal Society of Chemistry 2010 |
