Solution phase chemical synthesis of nano aluminium particles stabilized in poly(vinylpyrrolidone) and poly(methylmethacrylate) matrices†
Sekher Reddy
Ghanta
a and
Krishnamurthi
Muralidharan
*ab
aAdvanced Centre of Research in High Energy Materials (ACRHEM), University of Hyderabad, Hyderabad 500046, AP, India
bSchool of Chemistry, University of Hyderabad, Hyderabad 500046, AP, India. E-mail: kmsc@uohyd.ernet.in; Fax: +91 40 2301 2460; Tel: +91 40 23134819
First published on 14th April 2010
Abstract
The reduction of aluminium trichloride by lithium aluminium hydride in the presence of poly(vinylpyrrolidone) or poly(methylmethacrylate) in mesitylene yielded nano aluminium particles in the matrices of respective polymers. Solution phase synthesis methodology was used successfully to produce composites of various Al/polymer ratios. The composites were charecterized by powder XRD patterns and 27Al-NMR with MAS spectroscopic study. The method was useful to produce up to 10 g of nano aluminium that were pure and stable.
Introduction
Interest for metal nano particles1–3 is driven by their novel applications in various fields. Particularly, several research efforts have been directed to synthesis, characterization and stabilization of reactive metals such as aluminium and zirconium in their submicron sizes. These metal nano particles have high enthalpy of combustion. They also release extremely large amounts of energy per volume of reactants over conventional energetic materials.4–6 Hence, they received greater attention for many uses as propellants, explosives and in pyrotechnics.7–9Propellant is a composite of a high energy material10,11 as fuel and an oxidizer mixed with a polymeric binder. Since high energy is released in the oxidation process to alumina, aluminium nano particles (Al-NPs) are used as fuel in solid rocket propellant formulations.12 They are also proven to enhance the energy release from propellant composites by increasing burning rate.13,14 It is well understood that the particle burn rate increases with a decrease in particle diameter as a result of high specific surface area. Recently,15 it has been shown that the burning rate could be enhanced by a factor of 5–10 by adding Al–NPs in a propellant formulation. Aumann et al.7 have reported that the activation energy for oxidation of Al–NPs with the average diameter of 24–65 nm was much lesser than that for bulk aluminium samples. Hence, the present researches are focused on Al–NPs.
Producing Al–NPs continues to be a technical challenge. Most of the physical methods involve nano sizing of bulk material by mechanical attrition, vapor condensation and templated electrochemical deposition.16–18Albeit physical methods are established to produce Al–NPs, only few reports are on the solution phase chemical synthesis.19–21
In propellant science and technology, oxide formation on the surface of the Al–NPs prior to combustion and its quick agglomeration while burning are major problems.22–24 Recent results25 showed that the protected Al–NPs have an increased stability to oxidation in air and water during storage period. In order to protect, they were either coated with another metals26 or passivated by perfluorinated and non fluorinated alcohols, acids and 5-(hexadecyloxy)isophthalic acid.27,28
Alternative method to inhibit the surface oxidation of Al–NPs could be the stabilization of particles inside a polymer matrix.29 To the best of our knowledge, there is no report on the chemical synthesis of Al–NPs stabilized in a polymer matrix. Herein, we report our straight forward strategy towards the solution phase chemical synthesis of Al–NPs stabilized in the matrices of poly(vinylpyrrolidone) (PVP) and poly(methylmethacrylate) (PMMA) (Chart 1).
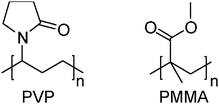 | ||
| Chart 1 PVP and PMMA | ||
Experimental section
Materials and synthetic procedures
Lithium aluminium hydride, aluminium trichloride, poly(vinylpyrrolidone) (PVP) (Mol. Wt. 10000) and poly(methylmethacrylate) (PMMA) (Mol. Wt. 120000) were purchased from Aldrich and were used without further purification. Mesitylene was washed in a separating funnel with concentrated H2SO4 until colourless, and then washed successively with deionized water, 5% NaOH solution and deionized water. Then it was dried by stirring overnight with CaH2, distilled and stored over type 4 Å molecular sieves.A typical procedure that was followed with an objective to obtain 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 theoretical weight ratio of Al/PVP is explained as follows (Scheme 1). Aluminium chloride (1.33 g, 10 mmol) and PVP (1.08 g) were suspended in deoxygenated mesitylene (10 ml) in a 100 ml two neck round bottom flask. Three equivalents of lithium aluminium hydride (1.14 g, 30 mmol) was added to it under nitrogen atmosphere. A reflux condenser equipped with nitrogen inlet and outlet on the top, was fixed on the flask. The mixture was stirred vigorously using magnetic stirrer at 165 °C for 24 h. On completion of reaction the solvent was evaporated under vacuum to obtain Al/PVP composite. The lithium chloride that was formed as side product in the reaction and other impurities (unreacted AlCl3 and LiAlH4) were removed by washing the composite with dry acetone.
1 theoretical weight ratio of Al/PVP is explained as follows (Scheme 1). Aluminium chloride (1.33 g, 10 mmol) and PVP (1.08 g) were suspended in deoxygenated mesitylene (10 ml) in a 100 ml two neck round bottom flask. Three equivalents of lithium aluminium hydride (1.14 g, 30 mmol) was added to it under nitrogen atmosphere. A reflux condenser equipped with nitrogen inlet and outlet on the top, was fixed on the flask. The mixture was stirred vigorously using magnetic stirrer at 165 °C for 24 h. On completion of reaction the solvent was evaporated under vacuum to obtain Al/PVP composite. The lithium chloride that was formed as side product in the reaction and other impurities (unreacted AlCl3 and LiAlH4) were removed by washing the composite with dry acetone.
 | ||
| Scheme 1 | ||
The reactions were carried out to obtain desired Al/PMMA composites (aluminium to PMMA weight ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, 1
0.25, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) using similar procedure described above. However, Al/PMMA composites were washed by dried methanol to remove impurities.
1.5) using similar procedure described above. However, Al/PMMA composites were washed by dried methanol to remove impurities.
These reactions yielded Al–NPs embedded in the polymer matrix as white to grey powders depending upon the aluminium to polymer ratios. Most of these reactions were carried out to form about 1 g of nano aluminium. Using the same methodology, scale up possibility was established by producing 10 g of Al–NPs with either PVP or PMMA.
Instruments and sample preparations
The pure samples obtained after removal of lithium chloride from Al/PVP and Al/PMMA composites were used for X-ray powder diffraction (XRD), scanning electron microscopy (SEM), field emission SEM (FESEM), energy dispersive X-ray (EDX) and 27Al NMR spectroscopy with magic angle spin (MAS) analyses. XRD patterns were recorded using Cu-Kα λ radiation (35 kV, 25 mA) on a Philips PW1830 X-ray diffractometer. The XRD samples were prepared by smear mount of Al/polymer composites on 2 × 5 cm glass plate. Thermo gravimetric and differential thermal (TG-DTA) analyses were conducted using Netzsch STA 409PC instrument with heating rate 10 °C min−1 from room temperature to 900 °C under dynamic nitrogen atmosphere. The Fourier transform infrared (FT-IR) spectra (KBr pellet) were recorded on a Jasco 5300 spectrophotometer.The isotropic solid state 27Al (100% abundant, I = ½) NMR spectra were recorded by MAS speed of 4 kHz in a zirconia rotor with the following acquisition parameters using a Bruker Avance 400 spectrometer (27Al resonance frequency of 104.34 MHz): FID was recorded at 4 K data point with scanning width of 312 kHz. The number of accumulations was 512 with pulse width 3.9 μs and 0.5 s recycle delay. Relatively large pulse width is due to the presence of polymers (RF is reflected more). For each analysis, about 100 mg of powdered samples of either Al/PVP or Al/PMMA materials were filled in the rotor under nitrogen atmosphere and caped with KEL-F capes.
The SEM analyses of Al–NPs were performed using Philips XL-30 ESEM operating at 20 kV. The FESEM analyses were performed on a Hitachi S–4500 SE/N instrument operating at 20 kV. The samples of Al/PVP and Al/PMMA in mesitylene were stirred separately in a vial for 2–3 min to obtain clear suspensions. These suspensions were dispersed on a glass surface for SEM analysis. Specimens for the analysis in FESEM were prepared by dusting Al/PVP or Al/PMMA composites on carbon tape. The EDX analyses were carried out on the pellets made out of the composites as well on the SEM samples.
Results and discussion
Characterization
The EDX analysis conducted in SEM on the Al/PVP (Fig. 1) and Al/PMMA composites showed a strong peak corresponding to the aluminium particles. Presence of peak corresponding to trace amounts of chlorine in EDX spectrum may also be due to the presence of LiCl that was formed as side product from these reactions. Presence of peak corresponding to the oxygen could be attributed to oxygens in the polymers.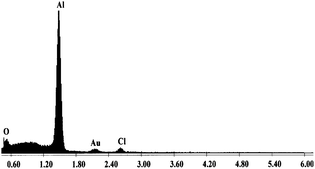 | ||
| Fig. 1 EDX spectrum of Al/PVP composite. | ||
The XRD pattern (Fig. 2) of the pure composites obtained in these reactions were compared with JCPDS-ICDD (# 04-0787), and was found to be in agreement with reported XRD pattern of Al-NPs. There were no other peaks related to aluminium oxide observed in XRD patterns. The Al2O3, if present, might be in amounts below the detection limit of XRD.
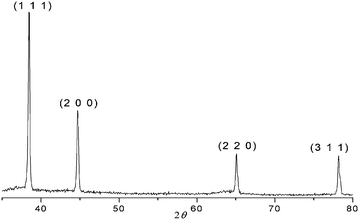 | ||
| Fig. 2 Powder XRD spectrum of Al/PVP composite. | ||
In order to check the stability of the Al-NPs, XRD patterns were recorded for the samples of Al/PVP and Al/PMMA composites stored in a glass vial for eight months. It was observed that the sharp well defined peaks corresponding to the pure aluminium in XRD patterns were unaltered and contained no reflections for aluminium oxide. Absence of any considerable change in the line width of powder X-ray diffraction spectra of the Al/polymer composites even after eight months indicated that there was no considerable change in the size of the Al-NPs.
In recent years18,30,31 high resolution solid state 27Al NMR spectroscopy with magic angle spin (MAS) has evolved as a potential tool to study the state of aluminium. It was recognized that the chemical shifts correlate with aluminium coordination number for both the organo aluminium compounds as well as for the inorganic compounds of aluminium.30 For example, 27Al signal of aluminium with 6-coordinate, 5-coordiante, 4-coordinate oxygen environment appeared from −10 to 15 ppm, 25 to 35 ppm, 65 to 85 ppm respectively, while that of aluminium metal appeared at 1640 ppm.18,31 The solid state 27Al NMR with MAS spectra obtained for the freshly prepared Al/PVP and Al/PMMA samples showed a strong signal at 1645.5 ppm (Fig. 3B). A weak signal in the spectrum around 14 ppm was due to unreacted AlCl3. The absence of any signal corresponding to other forms of aluminium essentially reveals the purity of Al/polymer composites. Hence, 27Al NMR with MAS could be considered as an useful method to study the purity of Al-NPs. The 27Al NMR spectrum of AlCl3 which was used as starting material in synthesis is also shown in Fig. 3A for comparison.
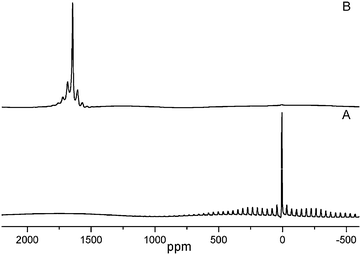 | ||
| Fig. 3 27Al MAS NMR Spectra of (a) AlCl3, (b) Al/PVP composite. | ||
Composition analysis
The reactions were carried out with an aim to obtain various aluminium to polymer weight ratios with an assumption of near completion of reduction reaction. However, the actual weight ratios in the composites were calculated from Thermo gravimetric analyses (TGA). The TGA results showed that the Al/polymer ratio depended on percentage yield of aluminium in the reduction reaction and the solvent used to purify the composites. The solvent for purification was selected based on the solubility of respective polymers in it.The TG-DTA curves obtained for the Al/PMMA composite before and after purification are shown in the Fig. 4 and 5. The data obtained from TGA of various samples of Al/PVP and Al/PMMA composites are shown in Table 1. The weight loss during TGA corresponds to the amount of polymer in the composites. The experiments were conducted until there was no further weight loss after all organic part was removed by the decomposition. Hence, the residual weight (percentage of weight remained) obtained from TGA of the composites indicate the quantity of aluminium present in it after decomposing the polymers. The calculated ratio of residual weight to weight lost implied the ratio of aluminium to polymer in the composites (column 5 in the table).
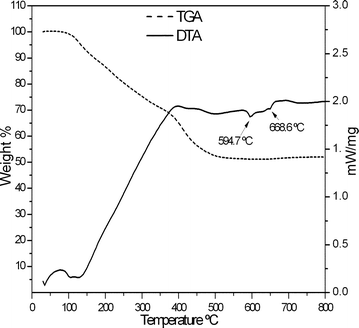 | ||
| Fig. 4 TG-DTA curves of Al/PMMA composite before purification. | ||
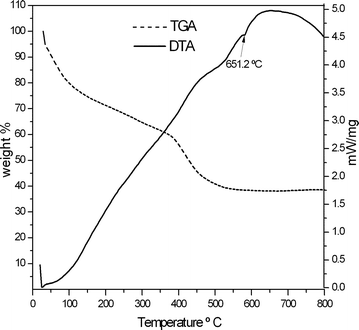 | ||
| Fig. 5 TG-DTA curves of Al/PMMA composite after purification. | ||
The increased aluminium percentage in the Al/PVP composite compared to theoretical objective could be explained by the partial loss of PVP during washing in acetone. Since PMMA is not soluble in methanol, the decrease in the aluminium percentage in Al/PMMA composite with respect to theoretical value could be attributed only to the percentage yield of aluminium in reduction reaction.
The presence and absence of endothermic peak in DTA curves corresponding to the melting point of lithium chloride before and after purification of composites demonstrated that the composites were cleanly separated from lithium chloride.
Microscopic investigations
Scanning electron microscopic (SEM) and field emission SEM (FESEM) (Fig. 6, 7) investigations of the composites revealed the presence of nano aluminium particles of various shapes and sizes. SEM micrographs (Fig. 6A) of Al in PMMA showed dendritic growth of aluminium particles with particle size less than 300 nm. Furthermore, it was observed by FESEM that few of the Al-NPs are trigonal prismatic (Fig. 6B).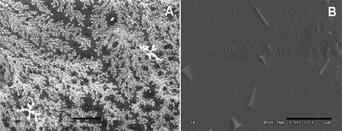 | ||
Fig. 6 (A) SEM image (scale bar 10 μm), (B) FESEM image (scale bar 1 μm) obtained for the Al in PMMA (Al : PMMA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
 | ||
Fig. 7 (A) SEM image (scale bar 1 μm), (B) FESEM image (scale bar 1 μm) obtained for the Al in PVP (Al : PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
Similarly, SEM micrographs (Fig. 7A) of Al in PVP showed the particles of size ranging from 47 to 158 nm. Closer observation of dilute suspension of Al/PVP in FESEM showed more number of cubical particles (Fig. 7B).
Stabilization of nano particles
The FT-IR spectra of the PVP and PMMA containing Al-NPs in their matrices were compared with that of the pure PVP and PMMA respectively. The carbonyl stretching frequency of pyrrolidone in the PVP and that of ester group in PMMA which appeared at 1600 cm−1 was unaltered after the reaction. This shows that the lone pair of electrons present in the oxygen and nitrogen atoms of the polymers are responsible for stabilization of nano particles. These electrons appear to be coordinating with atoms on the surface of the Al-NPs leaving aluminium atoms inside the bulk unaffected. Similar phenomenon of stabilization of metal nano particles by a lone pair of electrons present in the nitrogen atoms of poly(methylphenylphosphazene) and poly(vinylpyrrolidone) has been established in gold,3 silver32 and copper.33Since the aluminium atoms on the surface of the n Al-NPs are bound by coordination bonds with oxygens in PMMA and oxygens and nitrogens from the pyrrolidone rings of the PVP, the movement of nano particles could be arrested or slowed down. Hence, the physics of burning of Al-NPs stabilized in these polymer matrices is expected to be different from free Al-NPs. Thus the polymers are expected to play dual roles as to prevent aggregation of nano particles and protecting it from the oxidation prior to burning.
Conclusion
Our research proved that the solution phase synthetic method can be used to obtain Al-NPs stabilized in poly(vinylpyrrolidone) (PVP) and poly(methylmethacrylate) (PMMA) matrices in one step. This method could be scaled to produce up to 10 g of Al-NPs stabilized in these polymers with different aluminium to polymer weight ratios. Powder XRD, 27Al NMR and DTA studies proved that the Al/polymer composites were entirely free from impurities such as lithium chloride and aluminium oxide. The XRD patterns tested after eight months proved that the Al-NPs obtained through this solution phase synthetic procedure are stable from atmospheric oxidation.Acknowledgements
The authors gratefully acknowledge the support of the Defence Research and Development Organization (DRDO) India, in the form of grant to ACRHEM and School of Chemistry, University of Hyderabad for infrastructure.References
- K. Pelzer, O. Vidoni, K. Philippot, B. Chaudret and V. Collière, Adv. Funct. Mater., 2003, 13(2), 118–126 CrossRef CAS
.
- D. Jose and B. R. Jagirdar, J. Phys. Chem. C, 2008, 112(27), 10089–10094 CrossRef CAS
.
- C. H. Walker, J. V. St. John and P. Wisian-Neilson, J. Am. Chem. Soc., 2001, 123(16), 3846–3847 CrossRef CAS
.
- C. E. Aumann, G. L. Skofronick and J. A. Martin, J. Vac. Sci. Technol., B, 1995, 13(3), 1178–1183 CrossRef CAS
.
- J. T. DeSena and K. K. Kuo, J. Propul. Power, 1999, 15(6), 794–800 CrossRef CAS
.
- Y. Yang, Z. Sun, S. Wang and D. D. Dlott, J. Phys. Chem. B, 2003, 107(19), 4485–4493 CrossRef CAS
.
-
(a) Y. F. Ivanov, M. N. Osmonoliev, V. S. Sedoi, V. A. Arkhipov, S. S. Bondarchuk, A. B. Vorozhtsov, A. G. Korotkikh and V. T. Kuznetsov, Propellants, Explos., Pyrotech., 2003, 28(6), 319–333 CrossRef CAS
; (b) D. E. G. Jones, R. Turcotte, R. C. Fouchard, Q. S. M. Kwok, A.-M. Turcotte and A. Abdel-Qader, Propellants, Explos., Pyrotech., 2003, 28(3), 120–131 CrossRef CAS
.
- L. Meda, G. Marra, L. Galfetti, F. Severini and L. De. Luca, Mater. Sci. Eng., C, 2007, 27, 1393–1396 CrossRef CAS
.
- P. Brousseau and C. J. Anderson, Propellants, Explos., Pyrotech., 2002, 27(5), 300–306 CrossRef CAS
.
-
(a)
T. M. Klapötke, Ed., High Energy High Density Materials, Structure and Bonding, Springer, Heidelberg, Germany, 2007, 125, 286 p, ISBN: 978-3-540-72201-4 Search PubMed
; (b) R. P. Singh, R. D. Verma, D. T. Meshri and J. M. Shreeve, Angew. Chem., Int. Ed., 2006, 45(22), 3584–3601 CrossRef CAS
.
- K. Muralidharan, B. A. Omotowa, B. Twamley, C. Piekarski and J. M. Shreeve, Chem. Commun., 2005, 5193–5195 RSC
.
- L. L. Wang, Z. A. Munir and Y. M. Maximov, J. Mater. Sci., 1993, 28(14), 3693–3708 CrossRef CAS
.
- K. Park, D. Lee, A. Rai, D. Muherjee and M. R. Zachariah, J. Phys. Chem. B, 2005, 109(15), 7290–7299 CrossRef CAS
.
- V. I. Levitas, Combust. Flame, 2009, 156(2), 543–546 CrossRef CAS
.
-
(a) R. W. Armstrong, B. Baschung, D. W. Booth and M. Samirant, Nano Lett., 2003, 3(2), 253–255 CrossRef CAS
; (b) G. V. Ivanov and F. Tepper, 4th Int. Symp. Spec. top. Chem. Propul., 1997, 636 Search PubMed
.
- J. Eckert, J. C. Holzer, C. C. Ahn, Z. Fu and W. L. Johnson, Nanostruct. Mater., 1993, 2(4), 407–413 CrossRef CAS
.
-
G. A. Pozarnsky, U. S. Patent 6676727 and 6689190, 2004
.
- M. B. Pomfret, D. J. Brown, A. Epshteyn, A. P. Purdy and J. C. Owrutsky, Chem. Mater., 2008, 20(19), 5945–5947 CrossRef CAS
.
- J. A. Haber and W. E. Buhro, J. Am. Chem. Soc., 1998, 120(42), 10847–10855 CrossRef CAS
.
- C. Mahendiran, R. Ganesan and A. Gedanken, Eur. J. Inorg. Chem., 2009, 2050–2053 CrossRef CAS
.
- A. P. Purdy, J. B. Miller, R. M. Stroud and K. A. Pettigrew, Mater Res. Soc. Symp. Proc., 2008, 1056, 1056HH03–18
.
- E. L. Dreizin, Combust. Flame, 1999, 117(4), 841–850 CrossRef CAS
.
- N. Eisenreich, H. Fietzek, M. del. M. Juez-Lorenzo, V. Kolarik, A. Koleczko and V. Weiser, Propellants, Explosives, Pyrotechnic, 2004, 29(3), 139–145 Search PubMed
.
- A. Rai, K. Park, L. Zhou and M. R. Zachariah, Combust. Theory Modell., 2006, 10(5), 843–859 Search PubMed
.
- A. Gromov, A. Ilyin, U. Förter-Barth and U. Teipel, Propellants, Explos., Pyrotech., 2006, 31(5), 401–409 CrossRef CAS
.
- T. J. Foley, C. E. Johnson and K. T. Higa, Chem. Mater., 2005, 17(16), 4086–4091 CrossRef CAS
.
- R. J. Jouet, A. D. Warren, D. M. Rosenberg, V. J. Bellitto, K. Park and M. R. Zachariah, Chem. Mater., 2005, 17(11), 2987–2996 CrossRef CAS
.
- M. J. Meziani, C. E. Bunker, F. Lu, H. Li, W. Wang, E. A. Guliants, R. A. Quinn and Ya-Ping Sun, ACS Appl. Mater. Interfaces, 2009, 1(3), 703–709 Search PubMed
.
- G. V. Ramesh, S. Porel and T. P. Radhakrishnan, Chem. Soc. Rev., 2009, 38, 2646–2656 RSC
.
-
(a) A. G. Potapov, V. V. Terskikh, G. D. Bukatov and V. A. Zakharov, J. Mol. Catal. A: Chem., 1997, 122, 61–65 CrossRef CAS
; (b) A. G. Potapov, V. V. Terskikh, V. A. Zakharov and G. D. Bukatov, J. Mol. Catal. A: Chem., 1999, 145, 147–152 CrossRef CAS
; (c) A. G. Potapov, V. V. Terskikh, G. D. Bukatov and V. A. Zakharov, J. Mol. Catal. A: Chem., 2000, 158, 457–460 CrossRef CAS
.
- M. E. Smith, Appl. Magn. Reson., 1993, 4, 1–64 CrossRef CAS
.
- P. K. Khanna, N. Singh, D. Kulkarni, S. Deshmukh, S. Charan and P. V. Adhyapak, Mater. Lett., 2007, 61(16), 3366–3370 CrossRef CAS
.
- I. Haas, S. Shanmugam and A. Gedanken, J. Phys. Chem. B, 2006, 110, 16947–16952 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary figures. See DOI: 10.1039/b9nr00337a |
| This journal is © The Royal Society of Chemistry 2010 |
