DNA-based nanostructures for molecular sensing
Jong Bum
Lee
a,
Michael John
Campolongo
b,
Jason Samuel
Kahn
a,
Young Hoon
Roh
a,
Mark Richard
Hartman
a and
Dan
Luo
*ab
aDepartment of Biological & Environmental Engineering, Cornell University, 226 Riley Robb, Ithaca, New York 14853. E-mail: dan.luo@cornell.edu; Fax: +607-255-4080; Tel: +607-255-8193
bDepartment of Biomedical Engineering, Cornell University, Ithaca, New York 14853
First published on 20th October 2009
Abstract
Nanotechnology has opened up new avenues towards ultra-sensitive, highly selective detection of biological molecules and toxic agents, as well as for therapeutic targeting and screening. Though the goals may seem singular, there is no universal method to identify or detect a molecular target. Each system is application-specific and must not only identify the target, but also transduce this interaction into a meaningful signal rapidly, reliably, and inexpensively. This review focuses on the current capabilities and future directions of DNA-based nanostructures in sensing and detection.
1. Introduction
Nanotechnology has opened up new avenues towards ultra-sensitive, highly selective detection of biological molecules and toxic agents, as well as for therapeutic targeting and screening. Though the goals may seem singular, there is no universal method to identify or detect a molecular target. Each system is application-specific and must not only identify the target, but also transduce this interaction into a meaningful signal rapidly, reliably, and inexpensively. This review focuses on the current capabilities and future directions of DNA-based nanostructures in sensing and detection. The DNA molecule has long been known to define our genetic makeup, but is now also giving rise to new possibilities and directions in molecular sensing based on its high specificity and controllability. Coupled with the vast array of enzymes that serve as a ‘molecular toolkit’,1 rationally designed DNA-based nanostructures can be integrated into highly versatile, multiplexed molecular detection schemes.The most straightforward use of DNA involves the ability of single-stranded DNA (ssDNA) to hybridize to complementary sequences. Within the realm of sensing, this hybridization event is converted into an optical, electrical, or coupled response by labelingoligonucleotides with fluorescent dyes and/or by immobilizing them onto a surface. Though these signaling mechanisms may lead to effective transduction, amplification is often needed to enhance the signal, especially when only small quantities of target are present. For example, the polymerase chain reaction (PCR ) has often been used to increase the concentration of an oligonucleotide target. The ease of producing large quantities of identical DNA molecules, as well as the ability to add active chemical groups, has allowed the production of DNA-based biosensors with sensitivities down to the zeptomolar range.2
DNA is a desirable molecule for sensing not only because of its specificity and controllability, but because of its robustness and ability to function under a wide range of temperatures and conditions.3 Furthermore, through rational sequence design of multiple DNA strands, DNA can organize into complex structures.4 Continued advances in DNA sequence design and the expanding view that DNA can be used as structural building materials at the nanoscale allows for a high level of control of DNA structures for signal amplification, as well as multiplexed detection. DNA with branch points allows for not only multiple fluorescent labels per target, but different color ratios that may correspond to different target sequences. In addition, though functional control of their final structure is often determined through evolutionary mechanisms, aptamers and DNA enzymes (DNAzymes) provide a direct molecular bridge linking protein sensing and catalytic reactions, respectively, with DNA-based biosensors .5,6
Despite the numerous strategies for molecular detection, the optimal solution depends entirely on the goals of the system, whether it be reaching the highest possible sensitivity, minimization of cost, or providing the most mobile platform for rapid and easy-to-read results. This review aims to provide comprehensive insight into the versatile mechanisms that define current work in DNA-based biosensors and the interplay of ideas between the vastly different approaches.
2. Sensing based on linear DNA
2.1. Nanoparticle-based strategies
Oligonucleotide–nanoparticle conjugates and their application towards molecular diagnostics and detection have been thoroughly investigated over the past decade.7–10 The functionality of these systems is based on a capture-mediated approach in which the nanoparticle-immobilized ssDNA hybridizes with a target. The high sensitivity and biocompatibility of such detection techniques have made these hybrid systems particularly attractive.Considerable attention has been directed towards oligonucleotides covalently tethered to gold nanoparticles (AuNPs) due to their higher sensitivity and selectivity, lower cost, and greater ease of detection compared to conventional probes used in molecular diagnostics.11 The assembly of gold nanoparticles (AuNPs) with the aid of DNA linkers was independently reported by both Mirkin and Alivisatos in 1996.12,13 One year later, Mirkin used DNA-modified AuNPs to develop a highly selective, colorimetric DNA detection technique based on DNA hybridization, resulting in aggregation of the nanoparticles (Fig. 1a).14 Since then, colorimetric methods have been used for the detection of proteins,15mercuric ions,16cysteine,17 single-base imperfections in target DNA sequences,18 and in studies of binding affinity and selectivity of DNA-binding molecules based on screening for temperature-dependent aggregate stability.19,20Colorimetric techniques have been able to detect targets with sensitivity down to the zeptomole scale without target or signal amplification,2 yet are simple enough to alleviate the need for expensive and complex instrumentation.
 | ||
| Fig. 1 a) Scheme demonstrating the reversible aggregation of DNA-modified gold nanoparticles that occurs in the presence of a complementary target sequence.14 b) Schematic of the bio-barcode assay based on the capture of a target sequence between ‘barcoded’ nanoparticles and magnetic microparticles. The captured nanoparticles are separated and the target is identified by scanometric analysis of collected barcode DNA.11 | ||
Many other strategies have been employed in DNA–nanoparticle-based molecular detection, including methods that rely on light scattering,21 bioelectronic assays ,22–24 real-time detection methods,25,26 and Raman spectroscopic methods.27 Niemeyer and coworkers have used DNA as an intermediate linker for the generation of protein–nanoparticle conjugates for immunological assays .28,29 His group further investigated the reversibility of protein–DNA–nanoparticle conjugates, demonstrating that protein bioactivity is maintained after dissociation from the nanoparticle conjugates.30 In another work, Alivisatos and coworkers developed a molecular ruler by covalently linking double-stranded DNA (dsDNA) to AuNPs, which enabled real-time, label-free measurements of protein–DNA interactions.31 Mirkin and coworkers developed the well-known nanoparticle-based bio-barcode assay , which has been adapted for multiplexed detection of proteins and nucleic acids (Fig. 1b).11,15,32–37 This method is based on the ability of target DNA to bridge gold nanoparticles and magnetic microparticles, each of which are modified by ssDNA that are complementary to opposite ends of the target. Magnetic microparticles are collected by applying a magnetic field, leaving behind unbound nanoparticle probes. ‘Barcode’ DNA is isolated from the collected nanoparticles and is subsequently used in a scanometric amplification assay for detection.38
A variety of strategies that incorporate quantum dots have also been reported.39 Nie and coworkers developed a multiplexed encoding scheme for DNA detection by embedding different color ratios of quantum dots within microbeads.40 In a variation of this strategy, controlled ratios of quantum dots were coated onto the surface of paramagnetic microbeads.41 The beads were then labeled with capture oligonucleotides that were complementary to target sequences, and the magnetic beads were captured onto a magnetic array for quantification. A simple approach for multiplexed detection using a minimal number of quantum dots was demonstrated through dual-hybridization of target DNA with capture probes, both of which were labeled with a unique quantum dot (QD) to enable identification.42 Libchaber reported quantum dots encapsulated by phospholipid micelles that were used for in vitro and in vivo imaging, but also functioned as fluorescent DNA probes.43 Alivisatos and coworkers reported DNA–quantum dot conjugates that enabled the rapid detection of both single-nucleotide polymorphisms and single-base deletions.44 Wang reported a DNA nanosensor based on fluorescence resonance energy transfer between quantum dots and Cy5-labeled DNA reporter probes.45 This method provided a signal based on individual quantum dots that was distinguishable from the background despite few targets and a large number of excess probes.
In addition to spherical nanoparticles, carbon nanotubes have also been investigated for their unique electronic properties that can be exploited by surface immobilization of DNA molecules. The inclusion of different modifications allows for functionalization of the sides or ends of the CNTs with DNA.46–49 Though much attention has recently been focused on controlled synthesis of single-walled carbon nanotubes (SWCNTs) due to their more well-defined electro-optical properties, multi-walled carbon nanotubes (MWCNTs) have also been used successfully in biosensing devices. These devices utilize conjugated DNA to capture either a DNA target or DNA-coated nanoparticles.47,50DNA has recently been used to guide the self-assembly of CNT systems,51 but these nanotubes can also function as electrochemical sensors. Through fixation to a conducting surface such as gold, small changes in conductivity, caused by events such as hybridization, occur in surface-immobilized DNA allowing a measure of the target concentration in the surrounding medium.46 Conjugation of DNA to the ends of the CNT allows the tube to be tethered from either end between two electrodes. This potentially provides a more direct and sensitive measure of changes in conductivity with hybridization as there is increased surface area for target DNA-coated NPs to bind to the surface.47–49
2.2. Enzymatic strategies
Substantial increases in the sensitivity of immunoassays has been achieved by using ssDNA-conjugated antibodies. Cantor and coworkers first developed the immuno-polymerase chain reaction (immuno-PCR ) technique by combining antibody/antigen specificity with PCR -driven amplification of the DNA marker signal (Fig. 2a).52 Immuno-PCR improved upon the commonly-used enzyme-linked immunosorbent assay (ELISA) by a nearly 1000-fold enhancement of the detection limit. This method is capable of detecting multiple targets simultaneously by coupling specifically designed ssDNA to each of the analyte-specific monoclonal antibodies.53 Three analytes, human thyroid stimulating hormone (hTSH), human chorionic gonadotropin (hCG), and β-galactosidase, were detected simultaneously at sensitivities exceeding the limit of ELISA by about three orders of magnitude. In addition, real-time PCR detection has been applied to immuno-PCR ,54,55 demonstrating both an enhancement in sensitivity and fewer errors.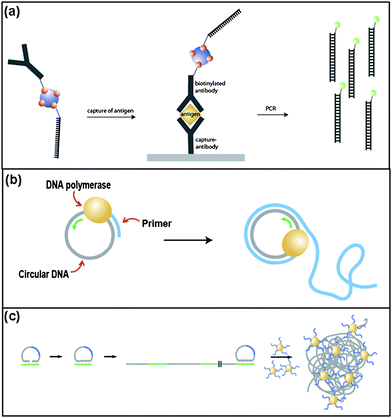 | ||
| Fig. 2 a) Schematic of the immunoassay based on immuno-PCR .53 b) DNA-templated polymerization by RCA. c) Sensitive molecular sensing using volume-amplified magnetic nanobeads.61 | ||
Rolling circle amplification (RCA) is another enzymatic method for signal amplification based on continuous polymerization of a circular template to produce a repeated sequence.56 During this reaction, a short primer sequence hybridizes with the circular template, allowing for a DNA polymerase to begin copying the template. The polymerase traverses the circular template, displacing the copied strand and continuing the polymerization. This results in a long single-stranded product containing multiple complementary copies of the template (Fig. 2b). Due to its robustness and simplicity, the use of RCA in the detection of target molecules has been utilized in techniques such as in situ genotyping and nanoparticle-based amplifications. The formation of circular DNA in a target-driven in situ circularization reaction allows for genotyping.57 Fluorophore-labeled probes with sequences complementary to the target sequence allow for detection of this amplified product. In addition, RCA has also been utilized in DNA-based immunoassays similar to immuno-PCR ; however, it does not require the use of a thermal cycling instrument.58
RCA was used to generate elongated ssDNA from primers immobilized on gold nanoparticle surfaces.59 Afterwards, these RCA products could be recognized by complementary sequences, facilitating signal enhancement. In a similar application of RCA, single nucleotide polymorphisms (SNPs) in amplified human genomic DNA samples were analyzed by observing the formation of aggregates by scanning electron microscopy (SEM) after the addition of gold nanoparticles conjugated with complementary ssDNA.60 Due to the excellent contrast of gold nanoparticles against the background, this method dramatically enhanced the signal-to-noise ratio. In another example of RCA coupled with nanoparticles, magnetic nanoparticles were hybridized to coiled RCA products, resulting in an aggregated complex. These aggregated nanoparticles possessed a dramatically different magnetization spectrum compared to the free magnetic nanoparticles in solution (Fig. 2c).61,62 This method can detect several types of targets by employing different sizes of probe-tagged magnetic nanoparticles.
3. Sensing based on branched DNAnanostructures
3.1. Branched DNA molecules
Branched DNA molecules, which are assembled from rationally-designed, single-stranded oligomers, are a unique class of materials that have inspired the synthesis of highly-complex DNA-based nanostructures. These structures expand upon the utility of linear DNA by allowing for the precise modification of multiple termini. Seeman and coworkers pioneered the first stable, synthetic branched DNAnanostructures by designing asymmetrical, partially-complementary oligonucleotides (Fig. 3a).3,63–68 Since then, this work has inspired an assortment of novel branched DNA architectures, including various shapes (such as cubes,69 octahedrons,70 hexagons71), supramolecular assemblies,72 and dendrimeric structures (Fig. 3b).73–75 The ability to design complex ‘active’ architectures has been a more recent development, leading to structures such as DNA walkers,76–78 tweezers,79 and even a DNA motor.80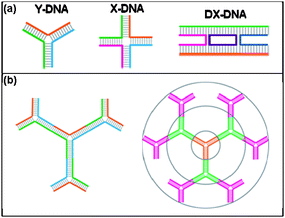 | ||
| Fig. 3 a) Schematic of various branched DNA molecules, including double crossover (DX).3 b) Schematic illustration of dendrimer-like DNA (DL-DNA) and controllable and directional growth of higher generation DL-DNA.75 | ||
Luo and coworkers developed a multiplexed detection strategy based on fluorescently-labeled DNA nanobarcodes,81,82 which were inspired by the synthesis of dendrimer-like DNA.75 Y-shaped DNA structures were designed such that two arms carried a combination of fluorescent dyes or a fluorescent dye and molecular probe; the other free arm functioned as a rationally designed point for ligation (Fig. 4a). Mixing these Y-DNA structures together with the addition of DNA ligase produced the desired nanobarcode (Fig. 4b). Multicolor fluorescence-intensity-encoded nanobarcodes could be fabricated by precisely controlling the dye type and number, allowing for the possibility of tens of thousands of distinct color ratios that could each correspond to a molecular probe (Fig. 4c). DNA nanobarcodes enabled the multiplexed detection of several different pathogenic DNA molecules simultaneously using fluorescence microscopy, dot blotting, and flow cytometry with attomole sensitivity and rapid detection.83,84
 | ||
| Fig. 4 a) Schematic of the of synthesis of an anisotropically-labeled Y-DNA-based nanobarcode building block. b) Construction of a typical DL-DNA-based nanobarcode. c) Schematic illustration of barcode decoding. The nanobarcodes 4G1R, 2G1R, 1G1R, 1G2R and 1G4R were decoded based on the ratio of fluorescence intensity.81 d) Fluorescent nanotags based on branched DNA templates and intercalating dyes.85 | ||
Branched DNAnanostructures were also applied as fluorescent nanotags by intercalation of dyes between base pairs (Fig. 4d).85,86 Here, DNAnanostructures served as carriers for the assembly of multiple intercalating dyes within a small and well-defined region. The compact structure of the nanotag allowed for efficient energy transfer to remote acceptor groups, resulting in bright multichromophore assemblies. More recently, a more compact 3D DNA tetrahedron nanostructure has been synthesized.86 Using a similar concept, branched DNA was used to amplify the signal for sensitive detection of DNA targets by in situ hybridization.87 Based on the branched DNA signal amplification method, multi-labeled fluorescent dyes on one branched DNA molecule can detect one target DNA or RNA molecule per cell with a high fluorescence signal.
Recently, Luo and coworkers demonstrated a target-driven polymerization strategy based on branched DNA.88 Anisotropic, branched, and crosslinkable (ABC) monomers were assembled from X- and Y-shaped DNA subunits (Fig. 5a). These subunits were labeled with various moieties including two QDs, one photo-responsive polyethylene glycol monoacrylate (PEGA) group, and one single-stranded oligonucleotide probe that was complementary to a specific pathogen DNA. These ABC monomers formed dimers only in the presence of a targeted pathogen DNA molecule because the pathogen DNA served as a complementary linker DNA for the two DNAnanostructures. Upon exposure to short-wavelength UV illumination, these dimers polymerized into aggregates that were assayed by fluorescence microscopy and identified by their unique color codes (Fig. 5b). However, polymerization did not occur unless the target was present. This approach may lead to applications of target-driven polymerization, enabling rapid signal amplification to detect pathogens with high specificity and sensitivity.
 | ||
| Fig. 5 a) Schematic of the assembly of an ABC monomer. Multiple moieties are conjugated on Y-DNA donors. The functionalized Y-DNA are then connected to corresponding end sequences of acceptor X-DNA to form the ABC monomer. b) Target-driven polymerization using ABC monomers.88 | ||
3.2. Complex assemblies from DNA tiles
DNA tiles are capable of self-assembly into extended two-dimensional patterns. For these purposes, Seeman and coworkers developed rigid DNA motifs, such as double-crossover (DX),89 triple-crossover (TX),90 and paranemic-crossover (PX) DNA.91 The DNA tile subunit is typically based on a variation of the crossover DNA motif, in which several double-stranded helices are linked by multiple crossover junctions; this DNA conformation offers enhanced rigidity that facilitates long-range order. The first two-dimensional DNA lattice was reported approximately ten years ago.92 Since then, a broad assortment of DNA tile arrays have been reported including a millimetre-scale “nano-chessboard” DNA array,93 as well as nanoparticle-labeled tiled arrays that curl up into tubules.94 In one notable development, Rothemund introduced the “DNA origami” strategy, in which a long ssDNA strand is shaped by many short ssDNA staple strands.95 This simple approach produced high yields of two-dimensional patterns in various sizes and shapes. The DNA origami approach was also recently extended to three dimensions to construct a DNA “box” which could open in the presence to a DNA target strand.96 This DNA box could effectively act as a logic gate for DNA computing or as a vehicle for controlled release of “nanocargo” (Fig. 6). | ||
| Fig. 6 Controllable opening of a nanoscale DNA box. The DNA box was composed of six DNA origami faces. The box was then locked by DNA hybridization and opened by the addition of a target sequence. The DNA box lid was labeled with Cy5 and Cy3 fluorophores (red and green spheres) which demonstrated the opening of the box via fluorescence measurements before (black curve) and after (red curve) the opening of the box.96 | ||
Given the important role of DNA in modern molecular biology, it is no surprise that DNA tiled arrays have been proposed as appealing platforms for a variety of biosensing applications. For example, cross-shaped DX-DNA building blocks were shown to self-assemble into 2D nanogrid lattices.97 In a follow-up report, these DNA tiled arrays were applied to multiplexed detection by using detection probes with differently-labeled DNA tile arrays.98 In this system, each DNA tile array contained fluorescent dyes that corresponded to particular targets, and each array was also labeled with detection probes. The fluorescently-labeled detection probes were partially hybridized with the array but were also complementary to the ssDNA target. Therefore, when the ssDNA targets were added, the detection probes preferentially hybridized to the targets and carried their fluorescent signal away from the array. Using fluorescence microscopy, a color change was observed resulting from the presence of various targets (Fig. 7). In addition, this system could also detect small molecules or proteins through the use of fluorescent DNA aptamer probes.
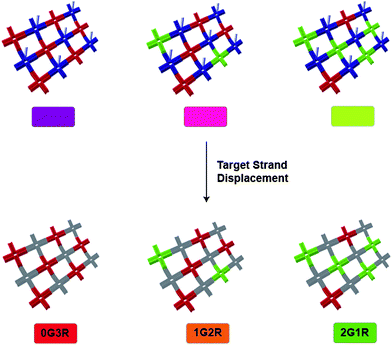 | ||
| Fig. 7 Self-assembled DNA tile arrays for multiplexed sensing. Left: DNA tiles are labeled with red dye (Cy5), with green dye (Rhodamine Red-X), or with ssDNA that is complementary to a detection probe. The detection probe is labeled with blue dye (Alexa Fluor 488). The detection probe preferentially binds to the target in solution and carries the blue dye away from the DNA tile array.98 | ||
As an alternative to fluorescent detection, atomic force microscopy (AFM) has also been used with DNA scaffolds in a label-free detection approach.99,100 In these reports, DNA origami rectangles were prepared with free sticky-end probes located within the design. The hybridization of the target oligonucleotides (DNA or RNA) to the sticky-end site resulted in a nanoscale bump that was detected by AFM, and the amount of rigidity measured at the hybridization site revealed information about the length of the target oligonucleotides. An important feature in this design is the index spot, which allows for multiplexed detection by enabling a correspondence between location and target identity.
DNA tile arrays can also be used as templates for the precise arrangement of proteins, which could be useful for monitoring protein–protein, protein–DNA, or protein–inorganic interactions. For example, DNA origami parallelograms were designed to include aptamer sequences for the binding of specific proteins.101 In a modification of this approach, the distance-dependent binding effects between aptamers and thrombin were studied.102 Evenly-spaced DNA aptamers have also been demonstrated based on a linear DNA tile array.103 As an alternative to non-covalent binding, a DNA-peptide linkage can be achieved through direct chemical conjugation; two-dimensional peptide arrays have been constructed from DNA tile arrays by click-chemistry104 and by coupling via4-(N-maleimidomethyl)cyclohexanecarboxylic acid N-hydroxysuccinimide ester (SMCC).105 One advantage of using DNA tile arrays to template proteins, compared with conventional microarray systems, is the ability to sense and study protein binding with a precision that approaches the single-molecule level.
The self-assembly of DNA tile arrays is a delicate process that is sensitive to a variety of factors. This sensitivity can be utilized for molecular sensing when the self-assembly process is triggered by the presence of a certain target. For example, a ssDNA target served as the template for assembling DNA tiles into an array (Fig. 8).106 This two-dimensional array encoded a pattern, detectable with AFM, which corresponded to the original ssDNA target. In a similar approach, a ssDNA target was generated by RCA.107 This sequence was then used to assemble DNA tiles into a specific pattern viaself-assembly.
 | ||
| Fig. 8 DNA tile assembly around a target strand (red). Tiles without a hairpin loop encode a “0” (tile with no black dot), while tiles with a hairpin loop encode a “1” (tile with dot); the pattern 01101 is shown here.106 | ||
In addition to sensing, DNA-tile-mediated assembly has also been utilized for DNA-based computation. For instance, a DNA origami rectangle acted as a seed that triggered the formation a large tiled pattern out of DNA tile building blocks.108 Here, a specific target acted as “input” and the resulting DNA tile array acted as “output”, and the design of the seed determined the structure of the resulting pattern. In another report, a fundamental computation (the XOR operation) was demonstrated via the one-dimensional algorithmic self-assembly of DNA triple-crossover (TX-DNA) molecules.109 The TX-DNA input tiles were attached to the output tiles by sticky-end hybridization, and the output tiles were ligated together to produce a “solution strand”. This solution strand corresponded to the target sequence, so that the target could be determined through gel electrophoresis. As evidenced in these examples, there is an unclear boundary between sensing and computing; in either case, a specific target (input) results in a specific assembly (output). Thus, computing can be regarded as an elaborate type of sensing.
4. Sensing based on unique DNA motifs: aptamers, DNAzymes, and nanomachines
The high conformational flexibility of nucleic acids allows some sequences to self-hybridize into unique tertiary structures. Aptamers are short nucleotide sequences, usually 15–40 nucleotides in length, that can selectively bind to other molecules with high affinity.110 They are produced through SELEX (systematic evolution of ligands by exponential enrichment),111,112 which is an iterative, in vitro process based on identification of a tight binding sequence from a randomly generated library. During the past two decades, aptamers have been generated for a variety of known proteins and other molecules, including carbohydrates, lipids, and nucleotides.The high selectivity and affinity of aptamers for their targets has made them quite useful for biomedical applications.113 In particular, there has been much interest in the integration of aptamers into nanoarchitectures for cancer targeting and treatment (Fig. 9a).6,10,114,115 For example, Farokhzad and coworkers used QD–aptamer conjugates for simultaneous imaging and sensing of drug delivery to cancer cells.115 In this approach, RNA aptamers specific to prostate specific membrane antigen were used for cellular targeting. Doxorubicin, a DNA and RNA duplex intercalating drug, was embedded in the duplex of the RNA and also served to quench the QD fluorescence. Release of the drug activated the fluorescence of the QDs, and could be monitored by confocal fluorescence microscopy. Many others have reported aptamer-based diagnostics that rely on ligand-induced conformational changes to produce fluorescence signals.116–119
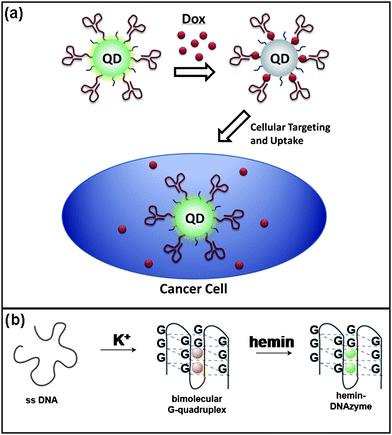 | ||
| Fig. 9 a) Targeting of cancer cells with aptamer-labeled quantum dots. Delivery of a fluorescence quenching drug is indicated by an increase in fluorescence within the cancer cell.115 b) K+ selectively promotes the formation of the G-quadruplex, which is activated by complexing with a hemin compound.131 | ||
In addition to tightly-binding aptamers, catalytic DNA structures (DNA enzymes, or DNAzymes) have also been generated and implemented in detection strategies. The use of these DNAzymes offers several advantages to traditional protein enzymes based on their thermal stability, ability to directly hybridize to or interact with both analytes and sensing molecules, and large-scale synthesis through PCR .120–122 Functionally active DNA structures have demonstrated sensing application through not only catalyzing cleavage of specific DNA sites in the presence of a target, importantly metal ions or DNA strands, but the production of fluorescent signal and/or color changes from molecular precursors.
A main focus of DNAzyme sensing revolves around the use of a particularly useful four-stranded DNA molecule termed the G-quadruplex.123–126 Organized around hemin , an iron-based compound, this structure exhibits peroxidase-like activity, oxidizing ABTS to form ABTS+ with an accompanying color change (Fig. 9b). Thus, in drawing together this aspect of the G-quadruplex with other molecules' or ions' abilities to enhance or decrease its activity through structural changes, accurate sensing of these effectors can be accomplished. Dong and coworkers have successfully used various iterations of this system for detection of many targets, such as thrombin,127DNA,128cocaine,129cysteine,130 and potassium131 and silver ions.130 Willner and coworkers expanded on the use of G-quadruplex for colorimetric or chemiluminescent detection by creating rationally designed hairpin loops132 and aptamer-DNAzyme conjugates that restricted formation of the functional structure until the target caused reorganization of the system by interrupting the natural hybridization.133
Though the G-quadruplex is commonly used as a stable, efficient mechanism for production of a colorimetric signal, it is by no means the only DNAzyme available. Other less complex structures catalyze the specific cleave of adjacent DNA sequences to yield colorimetric signals by separation of donor–acceptor dyes attached to the cleaved sequences134 or by the breakdown of nanoparticle aggregates.135 Lu and coworkers focused on the use of DNA-coated gold nanoparticles for lead sensing by hybridizing a Pb-activated DNAzyme to the linking DNA that acted as the tethering strands connecting the nanoparticles into higher-order aggregates (Fig. 10a).136 These gold aggregates displayed a blue color, but in the presence of lead, the activated DNAzyme cleaved the linking sequences to release gold nanoparticles that displayed their traditional red color. Thus, in relying on the inherent color changes in different nanoparticle aggregation states, lead was sensitively and selectively detected between 100 nM and 4 µM.
 | ||
| Fig. 10 a) Schematic drawing of molecular sensing by hybridizing a Pb-activated DNAzyme.136 b) Schematic drawing of a DNA nanomachine for pH sensing in the ‘open’ state at high pH and ‘closed’ state at low pH.142 | ||
RCA has been used to generate aptamers137 and DNAzymes for the detection of proteins and small molecules.138,139 Linear DNA chains containing periodic repeats of aptamer sequences for the protein thrombin and lysozyme were created by RCA. Protein–aptamer binding onto a DNA aptamer-encoded template resulted in periodic arrays of the nanostructures. The successful assembly of proteins onto the ssDNA scaffold was easily visualized by fluorescence microscopy or AFM. In addition, by modifying the DNA sequence, multiple proteins can be attached onto a single scaffold for multiplexed molecular detection. Repeated DNAzyme sequences can be produced using a similar approach. These DNAzyme chains produced chemiluminescence by generating light intensities with H2O2/luminal as the substrates that stimulated the light emission.
Though less representative of DNAzymes because they do not express catalytic functional activity, DNA ‘nanomachines’ can be designed in a manner that allows conformational changes depending on environment. Through the selective attachment of fluorescent signals to the DNA, one can create DNA structures that will present either an increase or decrease in signal strength depending on whether the structure causes quenching of the markers.79,140–142 This method is especially useful, for example, when tracking pH changes in vitro,143–146 and more recently, in vivo.142 The technique is based on fluorescence resonance energy transfer (FRET). The i-tetraplex,147 a complex of oligonucleotides based on cytosine-rich sequences that forms under acidic conditions, controls the level of FRET within the structure. Modi and colleagues demonstrated that pH levels within a cell undergoing endocytosis could be mapped, both spatially and temporally, within the pH range of 5.5–6.8 (Fig. 10b).142 Though other fluorescent signal carriers have been used in the past,148–150DNA allows the unique capabilities of simultaneously tracking multiple targets within the cell using different fluorescent markers.
5. Conclusions
DNA has many unique properties that make it a promising material for molecular sensing viaDNA-based nanostructures. A wide range of molecular sensing methods is already available through modulation of the unique optical, plasmonic, magnetic, and electrical properties of these systems. Several types of DNA-based nanostructures for molecular sensing coexist in the literature, including: (1) linear DNA-mediated nanostructures with diverse nanomaterials such as gold nanoparticles, quantum dots, magnetic nanoparticles, and carbon nanotubes; (2) rational nanostructures from branched DNA such as fluorescence nanobarcodes, ABC monomers, fluorescence DNA nanotags, and DNA tile arrays; (3) functional tertiary structures of DNA.DNA-based nanostructures have been precisely controlled to respond to external chemical and biological stimuli. One of the main advantages in rationally designing DNA is that the resultant products can simultaneously accomplish several functions through controlled, anisotropic functionalization. For example, DNA-based nanostructures provide affinity sites that are spatially well-defined, and also possess predefined chemical and physical properties via functional nanoparticles. Thus, these hybrid materials offer promising routes toward achieving versatile and sensitive molecular sensing. In addition, the versatility of DNA-based nanostructures allows simultaneous detection of multiple targets with high sensitivity using a variety of amplification strategies. DNA-based biosensing systems may represent a subset of available devices and ongoing research, but it is clear that DNA, through its functionality, specificity, and robustness, provides clear solutions to tackling these important issues. Future work on DNA-based nanostructures and their application to in situ sensing may allow further characterization and understanding of complex biological systems as well as improved techniques for detection in regard to specificity and sensitivity.
References
- D. Luo, Mater. Today, 2003, 6, 38–43 CrossRef CAS.
- J. J. Storhoff, A. D. Lucas, V. Garimella, Y. P. Bao and U. R. Müller, Nat. Biotechnol., 2004, 22, 883–887 CrossRef CAS.
- U. Feldkamp and C. M. Niemeyer, Angew. Chem., Int. Ed., 2006, 45, 1856–1876 CrossRef CAS.
- N. C. Seeman, Nature, 2003, 421, 427–431 CrossRef.
- M. Famulok, J. S. Hartig and G. Mayer, Chem. Rev., 2007, 107, 3715–3743 CrossRef CAS.
- R. A. Potyrailo, R. C. Conrad, A. D. Ellington and G. M. Hieftje, Anal. Chem., 1998, 70, 3419–3425 CrossRef CAS.
- C. S. Thaxton, D. G. Georganopoulou and C. A. Mirkin, Clin. Chim. Acta, 2006, 363, 120–126 CrossRef CAS.
- C. M. Niemeyer, Angew. Chem., Int. Ed., 2001, 40, 4128–4158 CrossRef CAS.
- E. Katz and I. Willner, Angew. Chem., Int. Ed., 2004, 43, 6042–6108 CrossRef CAS.
- Z. Wang and Y. Lu, J. Mater. Chem., 2009, 19, 1788–1798 RSC.
- S. I. Stoeva, J.-S. Lee, C. S. Thaxton and C. A. Mirkin, Angew. Chem., Int. Ed., 2006, 45, 3303–3306 CrossRef CAS.
- C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature, 1996, 382, 607–609 CrossRef CAS.
- A. P. Alivisatos, K. P. Johnsson, X. Peng, T. E. Wilson, C. J. Loweth, M. P. Bruchez and P. G. Schultz, Nature, 1996, 382, 609–611 CrossRef CAS.
- R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. A. Mirkin, Science, 1997, 277, 1078–1081 CrossRef CAS.
- J.-M. Nam, C. S. Thaxton and C. A. Mirkin, Science, 2003, 301, 1884–1886 CrossRef CAS.
- J. S. Lee, M. S. Han and C. A. Mirkin, Angew. Chem., Int. Ed., 2007, 46, 4093–4096 CrossRef CAS.
- J. S. Lee, P. A. Ulmann, M. S. Han and C. A. Mirkin, Nano Lett., 2008, 8, 529–533 CrossRef CAS.
- J. J. Storhoff, R. Elghanian, R. C. Mucic, C. A. Mirkin and R. L. Letsinger, J. Am. Chem. Soc., 1998, 120, 1959–1964 CrossRef CAS.
- M. S. Han, A. K. Lytton-Jean, B. Oh, J. Heo and C. A. Mirkin, Angew. Chem., Int. Ed., 2006, 45, 1807–1810 CrossRef CAS.
- S. J. Hurst, M. S. Han, A. K. R. Lytton-Jean and C. A. Mirkin, Anal. Chem., 2007, 79, 7201–7205 CrossRef.
- T. A. Taton, G. Lu and C. A. Mirkin, J. Am. Chem. Soc., 2001, 123, 5164–5165 CrossRef CAS.
- J. Wang, Small, 2005, 1, 1036–1043 CrossRef CAS.
- S.-J. Park, T. A. Taton and C. A. Mirkin, Science, 2002, 295, 651–1506 CrossRef.
- T. G. Drummond, M. G. Hill and J. K. Barton, Nat. Biotechnol., 2003, 21, 1192–1199 CrossRef CAS.
- R. C. Bailey, J.-M. Nam, C. A. Mirkin and J. T. Hupp, J. Am. Chem. Soc., 2003, 125, 13541–13547 CrossRef CAS.
- X. Xu, M. S. Han and C. A. Mirkin, Angew. Chem., 2007, 119, 3538–3540 CrossRef.
- Y. C. Cao, R. Jin and C. A. Mirkin, Science, 2002, 297, 1536–1540 CrossRef CAS.
- C. M. Niemeyer and B. Ceyhan, Angew. Chem., Int. Ed., 2001, 40, 3685–3688 CrossRef CAS.
- P. Hazarika, B. Ceyhan and C. M. Niemeyer, Small, 2005, 1, 844–848 CrossRef CAS.
- P. Hazarika, F. Kukolka and C. M. Niemeyer, Angew. Chem., Int. Ed., 2006, 45, 6827–6830 CrossRef CAS.
- G. L. Liu, Y. D. Yin, S. Kunchakarra, B. Mukherjee, D. Gerion, S. D. Jett, D. G. Bear, J. W. Gray, A. P. Alivisatos, L. P. Lee and F. Q. F. Chen, Nat. Nanotechnol., 2006, 1, 47–52 CrossRef CAS.
- J.-M. Nam, S. J. Park and C. A. Mirkin, J. Am. Chem. Soc., 2002, 124, 3820–3821 CrossRef CAS.
- S. I. Stoeva, J. S. Lee, J. E. Smith, S. T. Rosen and C. A. Mirkin, J. Am. Chem. Soc., 2006, 128, 8378–8379 CrossRef CAS.
- H. D. Hill and C. A. Mirkin, Nat. Protoc., 2006, 1, 324–336 Search PubMed.
- E. D. Goluch, J.-M. Nam, D. G. Georganopoulou, T. N. Chiesl, K. A. Shaikh, K. S. Ryu, A. E. Barron, C. A. Mirkin and C. Liu, Lab Chip, 2006, 6, 1293–1299 RSC.
- J.-M. Nam, S. I. Stoeva and C. A. Mirkin, J. Am. Chem. Soc., 2004, 126, 5932–5933 CrossRef CAS.
- C. S. Thaxton, H. D. Hill, D. G. Georganopoulou, S. I. Stoeva and C. A. Mirkin, Anal. Chem., 2005, 77, 8174–8178 CrossRef CAS.
- T. A. Taton, C. A. Mirkin and R. L. Letsinger, Science, 2000, 289, 1757–1760 CrossRef CAS.
- P. Alivisatos, Nat. Biotechnol., 2004, 22, 47–52 CrossRef.
- M. Han, X. Gao, J. Z. Su and S. Nie, Nat. Biotechnol., 2001, 19, 631–635 CrossRef CAS.
- P. S. Eastman, W. Ruan, M. Doctolero, R. Nuttall, G. de Feo, J. S. Park, J. S. F. Chu, P. Cooke, J. W. Gray and S. Li, Nano Lett., 2006, 6, 1059–1064 CrossRef CAS.
- Y.-P. Ho, M. C. Kung, S. Yang and T.-H. Wang, Nano Lett., 2005, 5, 1693–1697 CrossRef CAS.
- B. Dubertret, P. Skourides, D. J. Norris, V. Noireaux, A. H. Brivanlou and A. Libchaber, Science, 2002, 298, 1759–1762 CrossRef CAS.
- D. Gerion, F. Chen, B. Kannan, A. Fu, W. J. Parak, D. J. Chen, A. Majumdar and A. P. Alivisatos, Anal. Chem., 2003, 75, 4766–4772 CrossRef CAS.
- C.-Y. Zhang, H.-C. Yeh, M. T. Kuroki and T.-H. Wang, Nat. Mater., 2005, 4, 826–831 CAS.
- P. He, Y. Xu and Y. Fang, Microchim. Acta, 2006, 152, 175–186 CrossRef.
- J. Wang, Electroanalysis, 2005, 17, 7–14 CrossRef CAS.
- J. Wang, G. Liu and M. R. Jan, J. Am. Chem. Soc., 2004, 126, 3010–3011 CrossRef CAS.
- S. Daniel, T. P. Rao, K. S. Rao, S. U. Rani, G. R. K. Naidu, H.-Y. Lee and T. Kawai, Sens. Actuators, B, 2007, 122, 672–682 CrossRef.
- M. Guo, J. Chen, D. Liu, L. Nie and S. Yao, Bioelectrochemistry, 2004, 62, 29–35 CrossRef CAS.
- C. Dwyer, M. Guthold, M. Falvo, S. Washburn, R. Superfine and D. Erie, Nanotechnology, 2002, 13, 601–604 CrossRef CAS.
- T. Sano, C. L. Smith and C. R. Cantor, Science, 1992, 258, 120–122 CrossRef CAS.
- E. R. Hendrickson, T. M. H. Truby, R. D. Joerger, W. R. Majarian and R. C. Ebersole, Nucleic Acids Res., 1995, 23, 522–529 CrossRef CAS.
- M. Adler, R. Wacker and C. M. Niemeyer, Biochem. Biophys. Res. Commun., 2003, 308, 240–250 CrossRef CAS.
- A. McKie, D. Samuel, B. Cohen and N. A. Saunders, J. Immunol. Methods, 2002, 261, 167–175 CrossRef CAS.
- S. Beyer, P. Nickels and F. C. Simmel, Nano Lett., 2005, 5, 719–722 CrossRef CAS.
- C. Larsson, J. Koch, A. Nygren, G. Janssen, A. K. Raap, U. Landegren and M. Nilsson, Nat. Methods, 2004, 1, 227–232 CrossRef CAS.
- B. Schweitzer, S. Roberts, B. Grimwade, W. Shao, M. Wang, Q. Fu, Q. Shu, I. Laroche, Z. Zhou and V. T. Tchernev, Nat. Biotechnol., 2002, 20, 359–365 CrossRef CAS.
- W. Zhao, Y. Gao, S. A. Kandadai, M. A. Brook and Y. Li, Angew. Chem., Int. Ed., 2006, 45, 2409–2413 CrossRef CAS.
- B. Nie, M. R. Shortreed and L. M. Smith, Anal. Chem., 2006, 78, 1528–1534 CrossRef CAS.
- M. Strömberg, J. Göransson, K. Gunnarsson, M. Nilsson, P. Svedlindh and M. Strømme, Nano Lett., 2008, 8, 816–821 CrossRef.
- M. Strömberg, T. Zardán Gómez de la Torre, J. Göransson, K. Gunnarsson, M. Nilsson, P. Svedlindh and M. Strømme, Anal. Chem., 2009, 81, 3398–3406 CrossRef.
- R.-I. Ma, N. R. Kallenbach, R. D. Sheardy, M. L. Petrillo and N. C. Seeman, Nucleic Acids Res., 1986, 14, 9745–9753 CrossRef CAS.
- N. R. Kallenbach, R.-I. Ma and N. C. Seeman, Nature, 1983, 305, 829–831 CrossRef CAS.
- N. C. Seeman and N. R. Kallenbach, Biophys. J., 1983, 44, 201–209 CrossRef CAS.
- F. A. Aldaye, A. L. Palmer and H. F. Sleiman, Science, 2008, 321, 1795–1799 CrossRef CAS.
- C. Lin, Y. Liu and H. Yan, Biochemistry, 2009, 48, 1663–1674 CrossRef CAS.
- J. J. Storhoff and C. A. Mirkin, Chem. Rev., 1999, 99, 1849–1862 CrossRef CAS.
- J. H. Chen and N. C. Seeman, Nature, 1991, 350, 631–633 CrossRef CAS.
- Y. W. Zhang and N. C. Seeman, J. Am. Chem. Soc., 1994, 116, 1661–1669 CrossRef CAS.
- F. A. Aldaye and H. F. Sleiman, Angew. Chem., Int. Ed., 2006, 45, 2204–2209 CrossRef CAS.
- Y. He, T. Ye, M. Su, C. Zhang, A. E. Ribbe, W. Jiang and C. Mao, Nature, 2008, 452, 198–201 CrossRef CAS.
- J. Wang, M. Jiang, T. W. Nilsen and R. C. Getts, J. Am. Chem. Soc., 1998, 120, 8281–8282 CrossRef CAS.
- T. W. Nilsen, J. Grayzel and W. Prensky, J. Theor. Biol., 1997, 187, 273–284 CrossRef CAS.
- Y. Li, Y. D. Tseng, S. Y. Kwon, L. d'Espaux, J. S. Bunch, P. L. McEuen and D. Luo, Nat. Mater., 2004, 3, 38–42 CrossRef CAS.
- P. Yin, H. Yan, X. G. Daniell, A. J. Turberfield and J. H. Reif, Angew. Chem., Int. Ed., 2004, 43, 4906–4911 CrossRef.
- J.-S. Shin and N. A. Pierce, J. Am. Chem. Soc., 2004, 126, 10834–10835 CrossRef CAS.
- T. Omabegho, R. Sha and N. C. Seeman, Science, 2009, 324, 67–71 CrossRef CAS.
- B. Yurke, A. J. Turberfield, A. P. Mills Jr., F. C. Simmel and J. L. Neumann, Nature, 2000, 406, 605–608 CrossRef CAS.
- S. Venkataraman, R. M. Dirks, P. W. K. Rothemund, E. Winfree and N. A. Pierce, Nat. Nanotechnol., 2007, 2, 490–494 CrossRef.
- Y. Li, Y. T. H. Cu and D. Luo, Nat. Biotechnol., 2005, 23, 885–889 CrossRef CAS.
- S. H. Um, J. B. Lee, S. Y. Kwon, Y. Li and D. Luo, Nat. Protoc., 2006, 1, 995–1000 Search PubMed.
- S. M. Stavis, J. B. Edel, Y. Li, K. T. Samiee, D. Luo and H. G. Craighead, Nanotechnology, 2005, 16, S314–S323 CrossRef.
- S. M. Stavis, J. B. Edel, Y. Li, K. T. Samiee, D. Luo and H. G. Craighead, J. Appl. Phys., 2005, 98, 044903 CrossRef.
- A. L. Benvin, Y. Creeger, G. W. Fisher, B. Ballou, A. S. Waggoner and B. A. Armitage, J. Am. Chem. Soc., 2007, 129, 2025–2034 CrossRef CAS.
- H. Özhalıcı-Ünal and B. A. Armitage, ACS Nano, 2009, 3, 425–433 CrossRef.
- A. N. Player, L.-P. Shen, D. Kenny, V. P. Antao and J. A. Kolberg, J. Histochem. Cytochem., 2001, 49, 603–611 CAS.
- J. B. Lee, Y. H. Roh, S. H. Um, H. Funabashi, W. Cheng, J. J. Cha, P. Kiatwuthinon, D. A. Muller and D. Luo, Nat. Nanotechnol., 2009, 4, 430–436 CrossRef CAS.
- T. J. Fu and N. C. Seeman, Biochemistry, 1993, 32, 3211–3220 CrossRef CAS.
- T. H. LaBean, H. Yan, J. Kopatsch, F. R. Liu, E. Winfree, J. H. Reif and N. C. Seeman, J. Am. Chem. Soc., 2000, 122, 1848–1860 CrossRef CAS.
- Z. Y. Shen, H. Yan, T. Wang and N. C. Seeman, J. Am. Chem. Soc., 2004, 126, 1666–1674 CAS.
- E. Winfree, F. Liu, L. A. Wenzler and N. C. Seeman, Nature, 1998, 394, 539–544 CrossRef CAS.
- Y. He, Y. Tian, Y. Chen, Z. Deng, A. E. Ribbe and C. Mao, Angew. Chem., Int. Ed., 2005, 44, 6694–6696 CrossRef CAS.
- J. Sharma, R. Chhabra, A. Cheng, J. Brownell, Y. Liu and H. Yan, Science, 2009, 323, 112–116 CrossRef CAS.
- P. W. K. Rothemund, Nature, 2006, 440, 297–302 CrossRef CAS.
- E. S. Andersen, M. Dong, M. M. Nielsen, K. Jahn, R. Subramani, W. Mamdouh, M. M. Golas, B. Sander, H. Stark, C. L. P. Oliveira, J. S. Pedersen, V. Birkedal, F. Besenbacher, K. V. Gothelf and J. Kjems, Nature, 2009, 459, 73–76 CrossRef CAS.
- H. Yan, S. H. Park, G. Finkelstein, J. H. Reif and T. H. LaBean, Science, 2003, 301, 1882–1884 CrossRef CAS.
- C. Lin, Y. Liu and H. Yan, Nano Lett., 2007, 7, 507–512 CrossRef CAS.
- Y. Ke, J. Nangreave, H. Yan, S. Lindsay and Y. Liu, Chem. Commun., 2008, 5622–5624 RSC.
- Y. Ke, S. Lindsay, Y. Chang, Y. Liu and H. Yan, Science, 2008, 319, 180–183 CrossRef CAS.
- R. Chhabra, J. Sharma, Y. Ke, Y. Liu, S. Rinker, S. Lindsay and H. Yan, J. Am. Chem. Soc., 2007, 129, 10304–10305 CrossRef CAS.
- S. Rinker, Y. Ke, Y. Liu, R. Chhabra and H. Yan, Nat. Nanotechnol., 2008, 3, 418–422 CrossRef CAS.
- Y. Liu, C. Lin, H. Li and H. Yan, Angew. Chem., Int. Ed., 2005, 44, 4333–4338 CrossRef CAS.
- J. Chao, W.-Y. Huang, J. Wang, S.-J. Xiao, Y.-C. Tang and J.-N. Liu, Biomacromolecules, 2009, 10, 877–883 CrossRef CAS.
- B. A. Williams, K. Lund, Y. Liu, H. Yan and J. C. Chaput, Angew. Chem., Int. Ed., 2007, 46, 3051–3054 CrossRef CAS.
- H. Yan, T. H. LaBean, L. Feng and J. H. Reif, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 8103–8108 CrossRef CAS.
- D. Lubrich, J. Bath and A. J. Turberfield, Tetrahedron, 2008, 64, 8530–8534 CrossRef CAS.
- R. D. Barish, R. Schulman, P. W. K. Rothemund and E. Winfree, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 6054–6059 CrossRef CAS.
- C. Mao, T. H. LaBean, J. H. Reif and N. C. Seeman, Nature, 2000, 407, 493–496 CrossRef CAS.
- Y. Fichou and C. Ferec, Trends Biotechnol., 2006, 24, 563–570 CrossRef CAS.
- C. Tuerk and L. Gold, Science, 1990, 249, 505–510 CrossRef CAS.
- D. W. Drolet, R. D. Jenison, D. E. Smith, D. Pratt and B. J. Hicke, Comb. Chem. High Throughput Screening, 1999, 2, 271–278 CAS.
- S. M. Nimjee, C. P. Rusconi and B. A. Sullenger, Annu. Rev. Med., 2005, 56, 555–583 CrossRef CAS.
- O. C. Farokhzad, J. J. Cheng, B. A. Teply, I. Sherifi, S. Jon, P. W. Kantoff, J. P. Richie and R. Langer, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 6315–6320 CrossRef CAS.
- V. Bagalkot, L. Zhang, E. Levy-Nissenbaum, S. Jon, P. W. Kantoff, R. Langer and O. C. Farokhzad, Nano Lett., 2007, 7, 3065–3070 CrossRef CAS.
- M. Levy, S. F. Cater and A. D. Ellington, ChemBioChem, 2005, 6, 2163–2165 CrossRef CAS.
- X. Fang, A. Sen, M. Vicens and W. Tan, ChemBioChem, 2003, 4, 829–834 CrossRef CAS.
- J. J. Li, X. Fang and W. H. Tan, Biochem. Biophys. Res. Commun., 2002, 292, 31–40 CrossRef CAS.
- R. Nutiu and Y. Li, J. Am. Chem. Soc., 2003, 125, 4771–4778 CrossRef.
- I. Willner, B. Shlyahovsky, M. Zayats and B. Willner, Chem. Soc. Rev., 2008, 37, 1153–1165 RSC.
- Y. Lu and J. Liu, Curr. Opin. Biotechnol., 2006, 17, 580–588 CrossRef CAS.
- Y. Lu and J. Liu, Acc. Chem. Res., 2007, 40, 315–323 CrossRef CAS.
- P. Travascio, Y. Li and D. Sen, Chem. Biol., 1998, 5, 505–517 CrossRef.
- P. Travascio, A. Bennet, D. Wang and D. Sen, Chem. Biol., 1999, 6, 779–787 CrossRef.
- P. Travascio, P. K. Witting, A. G. Mauk and D. Sen, J. Am. Chem. Soc., 2001, 123, 1337–1348 CrossRef.
- T. Li, L. Shi, E. Wang and S. Dong, Chem.–Eur. J., 2009, 15, 1036–1042 CrossRef CAS.
- T. Li, E. Wang and S. Dong, Chem. Commun., 2008, 5520–5522 RSC.
- T. Li, S. Dong and E. Wang, Chem. Commun., 2007, 4209–4211 RSC.
- T. Li, E. Wang and S. Dong, PLoS One, 2009, 4, e5126 CrossRef.
- T. Li, L. Shi, E. Wang and S. Dong, Chem.–Eur. J., 2009, 15, 3347–3350 CrossRef CAS.
- T. Li, E. Wang and S. Dong, Chem. Commun., 2009, 580–582 RSC.
- Y. Xiao, V. Pavlov, T. Niazov, A. Dishon, M. Kotler and I. Willner, J. Am. Chem. Soc., 2004, 126, 7430–7431 CrossRef CAS.
- D. Li, B. Shlyahovsky, J. Elbaz and I. Willner, J. Am. Chem. Soc., 2007, 129, 5804–5805 CrossRef CAS.
- S. Sando, T. Sasaki, K. Kanatani and Y. Aoyama, J. Am. Chem. Soc., 2003, 125, 15720–15721 CrossRef CAS.
- J. Liu and Y. Lu, J. Fluoresc., 2004, 14, 343–354 CrossRef CAS.
- J. Liu and Y. Lu, J. Am. Chem. Soc., 2003, 125, 6642–6643 CrossRef CAS.
- Z. Cheglakov, Y. Weizmann, A. B. Braunschweig, O. I. Wilner and I. Willner, Angew. Chem., Int. Ed., 2008, 47, 126–130 CrossRef CAS.
- M. M. Ali and Y. Li, Angew. Chem., Int. Ed., 2009, 48, 3512–3515 CrossRef CAS.
- Z. Cheglakov, Y. Weizmann, B. Basnar and I. Willner, Org. Biomol. Chem., 2007, 5, 223–225 RSC.
- J. Bath and A. J. Turberfield, Nat. Nanotechnol., 2007, 2, 275–284 CrossRef CAS.
- W. Shih, Nat. Mater., 2008, 7, 98–100 CrossRef CAS.
- S. Modi, M. G. Swetha, D. Goswami, G. D. Gupta, S. Mayor and Y. Krishnan, Nat. Nanotechnol., 2009, 4, 325–330 CrossRef CAS.
- D. S. Liu and S. Balasubramanian, Angew. Chem., Int. Ed., 2003, 42, 5734–5736 CrossRef CAS.
- T. Liedl and F. C. Simmel, Nano Lett., 2005, 5, 1894–1898 CrossRef CAS.
- D. Liu, A. Bruckbauer, C. Abell, S. Balasubramanian, D.-J. Kang, D. Klenerman and D. Zhou, J. Am. Chem. Soc., 2006, 128, 2067–2071 CrossRef CAS.
- Y. Mao, D. Liu, S. Wang, S. Luo, W. Wang, Y. Yang, Q. Ouyang and L. Jiang, Nucleic Acids Res., 2007, 35, e33 CrossRef.
- K. Gehring, J.-L. Leroy and M. Guéron, Nature, 1993, 363, 561–565 CrossRef CAS.
- G. Miesenböck, D. De Angelis and J. Rothman, Nature, 1998, 394, 192–195 CrossRef CAS.
- R. L. Grant and D. Acosta, In Vitro Cell. Dev. Biol.: Anim., 1997, 33, 256–260 Search PubMed.
- M. K. Koo, C. H. Oh, A. L. Holme and S. Pervaiz, Cytometry, Part A, 2007, 71a, 87–93 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
