Formation mechanism of silver nanoparticle 1D microstructures and their hierarchical assembly into 3D superstructures†
Lorenza
Suber
*a and
William. R.
Plunkett
b
aCNR-Istituto di Struttura della Materia, P.O. Box 10, 00016 Monterotondo St., Italy. E-mail: lorenza.suber@ism.cnr.it
bCenter for Advanced Materials Processing, Clarkson University, Potsdam, NY 13699-5814, USA
First published on 6th October 2009
Abstract
Flower-like silver nanoparticle superstructures are prepared by the reaction of silver nitrate and ascorbic acid in an acidic aqueous solution of a polynaphthalene system. The three-dimensional flower-like structure has a purely hierarchic arrangement, wherein each petal is composed of bundles of silver particle chains, each enclosed in a polymer sheath. The ordering arises from strong adsorption of silver ions onto the polymer and by the interplay of the redox properties of nitric and ascorbic acid. As a result, linear silver cyanide, formed on the polymer, probably due to intrinsic electric dipole fields, organizes the silver particle chains in dumbbell-like structures, resembling buds and flower-like structures. By dilution and heating of the mother liquors, it is also possible to obtain single petals, i.e. micrometer sized bundles of linearly aggregated silver nanoparticle chains, each enclosed in a polymer sheath.
The comprehension of the hierarchic assembly of silver nanoparticles, paves the way to a facile general method to prepare polymer–metal nanoparticle chains and flower-like superstructures.
The results of this study improve both the understanding of the formation mechanism of hierarchic structures at mild temperatures and our ability to tailor them to sizes and shapes appropriate for technological purposes.
1. Introduction
Noble metal nanoparticles have attracted considerable attention in the last few decades, due to their unique optical, electric, catalytic and magnetic properties.1a–c Many metals can now be processed into monodisperse particles with controllable composition and structure and can be produced in large quantities at low cost through solution-phase methods.2a–c Particle size and shape,3a–d as collective properties of assembled metal nanoparticles in 1-dimensional (1D), 2D or 3D structures, have been shown to greatly affect the behaviour of nanomaterials.4–6Fabrication of complex architectures with 3D or highly ordered nanostructures is much desired in current materials synthesis, holding promise for advanced applications in electronics and optoelectronics.7 It is still a great challenge, however, to develop simple and reliable methods for the synthesis of hierarchically self-assembled architectures with controlled morphologies, which strongly affect the nanomaterial properties.8a–f
The simplest route is probably self-assembly, in which ordered aggregates are formed in a spontaneous process.9Surfactant molecules having amphiphilic properties, usually formed by an ionic (cationic or anionic) head and a long carbon chain body, have the unique ability to self-organize at interfaces or in solution and can form thermodynamically stable supramolecular assemblies such as micelles, microemulsions, lyotropic liquid crystals and vesicles. Recently, a second class of aqueous lyotropic mesophases, termed chromonic liquid crystals, has come to be better recognized and understood.10a–c They are formed by water-soluble molecules that contain rigid polyaromatic cores. They do not show a clear separation of hydrophilic and hydrophobic parts, since the hydrophilic groups that impart water solubility are distributed all around the periphery of the hydrophobic aromatic rings. Consequently, they do not form micelles, nor do they show any appreciable surface activity. The driving force for self-association is a short-range intermolecular attraction involving both the σ- and π-bonds of the aromatic rings.11a–b These systems may also form relatively concentrated meso-phases, though the detailed nature of the molecular order within the aggregates has not been determined.
In a previous work, we used Daxad 19, a sodium salt of polynaphthalene sulfonate formaldehyde condensate, as a dispersing agent in the preparation of silver nanoparticles.12 The resulting formation of 2D structures, tabular hexagonal particles, platelets and strips, then induced us to further investigate its molecular organization. As a polyaromatic system with possible chromonic-like behaviour, Daxad could form lamellar structures and constitute casts for the 2D silver structures. The formation of the 2D meso-structures was explained by a polymer-assisted aggregation of anisometric silver particles.13
A growing number of reports are being published on dendritic and flower-like organic–inorganic structures, but there is little discussion about their formation mechanism.
It is difficult to find studies of the interplay between the different molecular components. Without this knowledge, the synthesis of complex structures is left to trial and error. Therefore, we have tried to study the formation mechanism of these perfect copies of natural flowers, composed of polymer and silver particles. A better understanding could help us to mimic Nature, while developing novel applications. For instance, the petals, formed by linearly aggregated silver nanoparticles inside a polymer sheath, show potential for applications in sub-wavelength optical guiding structures, i.e. in the transport of electromagnetic excitation along chains of non-contacting metal nanoparticles.14a–b Among the metals, 1D silver nanostructures are especially attractive because bulk silver shows the highest electrical and thermal conductivities and nanoscale silver exhibits strong surface plasmon resonance, dependent on its size and shape.15a–c
Herein we report the syntheses of micrometre-sized polymer–silver particle flower-like structures and bundles of polymer–silver particle chains by reduction of with ascorbic acid in an aqueous acidic solution of Daxad 19 (0.4–0.2 wt%), and discuss their formation mechanism, morphology and structure.
2. Experimental section
Materials
Silver nitrate and ascorbic acid, purchased from Aldrich, were of the highest purity grade. HNO3 69.7 wt% was purchased from Fischer. Daxad 19, henceforth referred to as Daxad, (sodium salt of polynaphthalene sulfonate formaldehyde condensate, Mw 8000) was obtained from the Hampshire Chemical Company. The method of preparation consists of reacting naphthalene with sulfuric acid to form naphthalene sulfonic acid. The material is then condensed with formaldehyde and the polymerised naphthalene sulfonic acid molecule is neutralized by sodium. Elemental analyses (wt%): C: 42.42; H: 3.44; S: 6.20.Analyses and instruments
Elemental analyses (C,H,N,S) were performed with a Perkin Elmer CHNS/O Elemental Analyser in the CNR-Laboratorio di Microanalisi, Area della Ricerca di Roma 1.FT-IR spectra were obtained with a FT-IR Perkin-Elmer 16F PC spectrometer. The samples were pressed in KBr pellets.
Powder X-ray diffraction (PXRD) measurements were carried out in the 2θ range 25–80° by means of an automated powder diffractometer using Cu Kα radiation.
Scanning electron microscopy (SEM) measurements were performed at 20 kV with a SEM-LEO1450VP unit, equipped with an INCA300 EDS microanalysis facility. A few drops of the sample suspension in water were filtered through a porous polycarbonate membrane and the filtrate on the membrane was left to dry in air. The membrane was fixed onto the SEM stub with some drops of silver paste and sputtered with gold to ensure electrical conductivity.
Transmission electron microscopy (TEM) and X-ray elemental analyses were performed with a JEOL 2010 TEM/STEM (scanning transmission electron microscope), equipped with an Oxford Instruments “Inca” EDS (energy dispersive spectrometer) system, at an accelerating voltage of 200 kV. A drop of a dilute sample suspension was placed on a carbon-coated grid and allowed to dry at room temperature.
Preparation of samples
| Sample | Morphology | Temperature/°C | Time/h | Ag NO3/mol L−1 | HNO3/mol L−1 | Daxad 19 (wt%) | Ascorbic acid/mol L−1 |
|---|---|---|---|---|---|---|---|
| 1 | polyhedral particles | 50 | 1 | 0.18 | 1.00 | 0.44 | 0.18 |
| 2 | flower-like superstructure | 50–100 | 20 | 0.18 | 1.00 | 0.44 | 0.18 |
| 3 | bundle superstructure | 60 | 16 | 0.10 | 0.57 | 0.24 | 0.10 |
3. Results and discussion
An easy chemical way to obtain nanostructured silver particles is by reduction, in aqueous solution, of Ag NO3, the most common silver salt, with ascorbic acid (C6H8O6) according to eqn (1).| 2Ag+ + C6H8O6 ⇄ 2Ag0 + C6H6O6 + 2H+ | (1) |
By mechanisms not yet completely understood, the silver atoms assemble, forming particles. In order to avoid particle agglomeration and maintain a good dispersion in water, a dispersing agent is usually employed. Daxad, a polymer formed by the condensation of naphthalene sulfonic acid with formaldehyde, is a good dispersing agent for silver particles, even in very strong acidic conditions where the reduction of Ag+ is slowed down due to the decrease of the double-deprotonated ascorbate anion, ascorbate2−. Being a stronger reducing agent than ascorbic acid, it is responsible for reduction in basic and neutral conditions.16 In this way, by increasing the time to reach the Ag0 supersaturation concentration, the particle formation process can be tailored.
However, the acidic conditions can represent a drawback for the reducing agent ascorbic acid. The nitrate group, at low pH, is a stronger oxidant than Ag+ and oxidizes ascorbic acid (eqns (2–4)).
| NO3− + 4H3O+ + 3e− ⇄ NO↑ + 6H2O E0 = 0.96 V | (2) |
| Ag+ + 1e− ⇄ Ag0E0 = 0.799 V | (3) |
| NO3− + H2O +2e− ⇄ NO2− + 2OH−E0 = 0 V | (4) |
In fact, after heating the grey particle dispersion (Fig. 1a) at 100 °C for 3–4 h, the solution first turns clear and orange (the colour of the Daxad solution) while the Ag0 is oxidized again to Ag+, and then turns cloudy.
 | ||
| Fig. 1 a) SEM micrograph of silver particles formed after 1 h reaction at 50 °C (Sample 1). The bar size is 1.1 µm. b) SEM micrograph of polymer–silver particle flower-like structures (Sample 2). The bar size is 10 µm. c): SEM micrograph of a single polymer–silver particle flower (Sample 2). The bar size is 1 µm. d): TEM micrograph of the top of a petal. The bar size is 100 nm. Inset: SAED pattern of silver particle flower-like structures. Rings are indexed according to the silver cubic structure (JCPDS-04-0783). | ||
After 15 h at 60 °C, the solution containing a beige precipitate, is cooled to room temperature. As shown in Figs. 1b–c, the precipitate consists of micrometer-sized flower-like structures.
The silver structure is formed by linearly aggregated silver particles (diameter 10–15 nm) enclosed in a polymer sheath (Fig. 1d). Selected-area electron diffraction (SAED) of silver particle flower-like structures (Fig.1d inset) showed rings indexed according to the silver cubic structure.
High-resolution TEM investigations of the silver particle structures revealed the presence of different particle shapes, among them icosahedra (Figs. 2–3). The icosahedron shape, resulting from 20 tetrahedra sharing an apex, is frequently observed for face-centred cubic metallic nanocrystals;3c it denotes multiple twinning, one of the most common defects in metal nanocrystals.17
 | ||
| Fig. 2 HR-TEM micrograph of silver particles showing twinning defects (Sample 2). The bar size is 2 nm. | ||
 | ||
| Fig. 3 HR-TEM micrograph of an icosahedral silver particle inside the polymer sheath (Sample 2). The bar size is 2 nm. | ||
Scheme 1 summarizes in 3 steps the formation of the flower-like polymer–silver particle structures.
 | ||
| Scheme 1 | ||
Silver particles are thermally unstable in the reaction solution. By increasing the temperature to 100 °C (step 2 in Scheme 1), they dissolve because the nitrate groups, in strong acidic conditions, oxidize Ag0 to Ag+ forming nitrogen oxides. The Ag+ ions probably remain on the Daxad, due to the presence of the co-ordinating sulfonic groups. Nitrogen oxide then reacts with dehydroascorbate, probably forming as an intermediate O-nitrosoascorbate. In turn, nitrosoascorbate decomposes into erythro-ascorbate and cyanides, perceptible by the characteristic smell (Scheme 2).18ab
 | ||
| Scheme 2 | ||
The FT-IR spectrum of Sample 2 (Fig. 4) shows an absorption at 2164 cm−1 assigned to the stretching vibration of the C≡N group of AgCN.19XRD peaks from AgCN are also present in the XRD spectrum of Sample 2 (see Fig. a of the ESI† ).
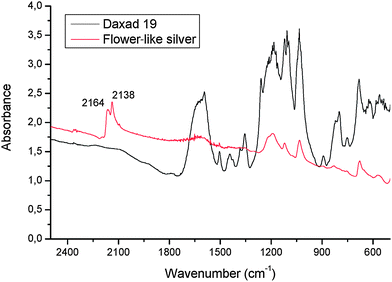 | ||
| Fig. 4 FT-IR spectra of Daxad and of polymer–silver particle flower-like structures (Sample 2) in a KBr pellet.19 | ||
The CN groups further increase the Ag+–polymer interactions, forming –Ag–C≡N–Ag–C≡N- linear chains parallel to the long direction of the polymer tubular sheath as shown, in the HR-TEM micrograph of Sample 2 in Fig. 5, by the orientation of the lattice fringes with a spacing of 0.301 nm corresponding to the 110 crystalline planes of AgCN. The preferential orientation is confirmed by the higher intensity of the [110] peak in the XRDspectrum of Sample 2 with respect to the AgCN powder spectrum (Fig. a of the ESI† ). Interestingly, Zhou et al.20 in the reduction of KAu(CN)2 by ascorbic acid with polyvinylpyrrolidone as a dispersant agent, have obtained similar linear polymer –particle structures, i.e. gold nanoparticle chains enclosed in polymer sheaths (Fig. 3 of ref. 20). The reason for the linear assembly, not investigated by the authors, could be explained by AuCN chains present on the polymer.
 | ||
| Fig. 5 HR-TEM micrograph showing the AgCN lattice fringes of 110 planes in Sample 2. | ||
Moreover, erythro-ascorbate is able to reduce Ag+ to Ag0 (Scheme 3).18b
 | ||
| Scheme 3 | ||
Silver particles then start to form linearly along the polymer, some forming contact areas of various configurations with neighbouring particles: ordinary boundaries or twin boundaries (one example is shown in Fig. 2, see also Figs. c–g of the ESI† ). In all cases, the assembly is driven by minimising the particle interface energy and reducing the exposed surface area. A further decrease of particle surface energy is obtained by interaction of the particle surface with the polymer chains that, folding around the particles, form the tubular polymer sheath.
As regards detection of possible flower-like water-soluble precursors, after a reaction time of 20 h, SEM and EDS investigations of the supernatant orange liquid (see Scheme 1, step 3) show a composite material consisting of silver and polymer. In Fig. 6, the elongated structures, probably due to the –Ag–C≡N–Ag–C≡N– linear chains that are growing on the polymer, are the precursors of the flower-like silver structures.
 | ||
| Fig. 6 SEM micrograph of the water-soluble precursor of the polymer–silver flower-like structures. The bar size is 16 µm. | ||
By further heating an aqueous solution of the supernatant liquor in fact, structures of bundles of tiny polymer rods containing linearly ordered silver nanoparticles precipitate (Sample 3). It is then sufficient to heat the diluted mother liquors of the flower-like structures (Sample 2) to obtain bundles of aligned silver particle chains enclosed in polymer sheaths (Fig. 7).
 | ||
| Fig. 7 SEM micrograph of polymer–silver bundles (Sample 3). Inset: a polymer–silver bundle showing its sub-structure. Both bars are 2.2 µm. | ||
As regards the self-assembly of the polymer–silver particles into flower-like structures, Fig. 1b shows that the “flowers” in Sample 2 form at each half of a dumbbell. The dumbbells are observed either alone or superimposed at their midpoints into more complex structures. The dumbbell shape may indicate that intrinsic electric dipoles are present, as reported for example by Kniep et al. for the morphogenesis of fluoroapatite–gelatin composites.21 In the present case, they might be due to the presence on the polymer of AgCN chains. In MCN linear compounds (M = metal elements of the groups I and II in the periodic table) in fact, electric dipoles are formed because of electron transfer from the metal atom to the CN group.22
Once the polymer becomes hydrophobic, because of the presence of the water-insoluble AgCN on its surface, the polymer–silver particle flower-like structures precipitate.
For the precipitation of calcium phosphate in colloidal aggregates to form meso-skeletons of interconnected inorganic needles, Mann suggested a dynamic interplay between the organic and the inorganic parts and attributed the filament formation to a compromise between the hydrophobic forces tending to fold the polymer and the hydrophilic ones instead drawing it towards the water solvent.23a–b In this case, the presence on the polymer surface of hydrophobic AgCN chains causes the precipitation of the dumbbell-like structures. The dumbbell-like structure probably results from the presence on the polymer of AgCN electric dipole fields. Each half dumbbell constitutes a bud (see for example Fig. 1b upper right) that, when open, reveals its perfect flower-like structure. Moreover, during the sedimentation process, two or more dumbbell structures may join at their mid-points forming multi-bud flower structures (Fig. 1b).
The perfection of the flower shape shown in Figs. 1b and c, is probably the result of mutual strong and fine chemical and structural interactions between the inorganic and organic agents modulated by the interplay of the redox properties of nitric and ascorbic acid towards silver (see Schemes 2 and 3).
Silver particles, rich in 111 facets with which the polymer probably better interacts, start to grow, tending to assume different shapes. The particles are driven to assemble linearly because of two contributions: i) the presence of AgCN chains aligned in the same direction and ii) an inter-particle aggregation mechanism tending to minimize surface energy through contact areas (Fig. 2) as observed by Giersig et al. in the formation of silver nanowires.24
Work is in progress to prepare, using as form-template MCN chains, 1D and 3D organic–inorganic superstructures with other metals (Cu, Au) and polymers (polyvinyl alcohol, chitosan, etc.).
Finally, we and other authors have recently observed permanent magnetism in Ag0thiol-capped nanoparticles.1c,25Cyano-bridged metal coordination polymers are known to show unusual magnetic behaviours.26a–b For these reasons, we have started to investigate the magnetic behaviour of the flower-like structures. Preliminary results, showing a hysteretic magnetic behaviour up to room temperature, are promising.
4. Conclusions
Morphological and structural analyses have shown the formation of perfect flower-like polymer–silver particle structures.The formation mechanism has been studied by analysing the reaction at different intermediate times. In 1 h reactions, by reduction of Ag+ ions by ascorbic acid, Ag0nanoparticles, covered by the polymer, are formed. After further heating, they dissolve because of the oxidation of Ag+ by nitrate. Ag+ ions probably, due to coordination to the polymer sulfonic groups, remain on the polymer.
Due to the interplay of the redox properties of ascorbic acid and nitric acid and to the strong coordination of Ag+ ions, AgCNlinear chains grow on the polymer. They, together with an oriented particle growth mechanism, contribute to the formation of silver particle chain structures. Probably due to the presence of the electric dipole fields of the AgCN linear structure, dumbbell-like structures are then formed, each half constituting a bud- or flower-like structure.
To our knowledge, this is the first example of the formation of micrometre-sized hierarchic polymer–nanoparticle structures resembling such perfect copies of natural flowers. It is also remarkable that they are easily obtained in water at mild temperatures.
This study has highlighted the important function of AgCN in the formation both of linear and of flower-like polymer–nanoparticle structures.
Acknowledgements
The authors wish to thank Dr. Patrizia Imperatori and Dr. Alessandra Mari for help with XRD measurements and in the preparation of the samples respectively.The work was supported by the NSF grant DMR-010244 and by a CNR project termed: Ricerca Spontanea a Tema Libero (RSTL.087.008).
Notes and references
- (a) Nanoscale Materials in Chemistry, ed. K. J. Klabunde, Wiley-Interscience, New York, 2001 Search PubMed; (b) A. Henglein, J. Phys. Chem., 1993, 97, 5457–5471 CrossRef CAS; (c) L. Suber, D. Fiorani, G. Scavia, P. Imperatori and W. R. Plunkett, Chem. Mater., 2007, 19, 1509–1517 CrossRef CAS and references therein.
- (a) L. Gou and C. Murphy, Chem. Mater., 2005, 17, 3668–3672 CrossRef CAS and references therein; (b) D. V. Goia, J. Mater. Chem., 2004, 14, 451–458 RSC and references therein; (c) M. Green, Chem. Commun., 2005, 3002–3011 RSC.
- (a) R. Jin, Y. W. Cao, C. A. Mirkin, K. L. Kelly, G. C. Schatz and J. G. Zheng, Science, 2001, 294, 1901–1903 CrossRef CAS; (b) C. J. Murphy, T. K. Sau, A. M. Gole, C. J. Orendorff, J. Gao, L. Gou, S. E. Hunyadi and T. Li, J. Phys. Chem. B, 2005, 109, 13857–13870 CrossRef CAS; (c) Y. Xiong, B. J. Wiley and Y. Xia, Angew. Chem., Int. Ed., 2007, 46, 7157–7159 CrossRef CAS; (d) Y. Sun and Y. Xia, Science, 2002, 298, 2176 CrossRef CAS and references therein.
- Y. Xia, P. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, Y. Yin, F. Kim and H. Yan, Adv. Mater., 2003, 15, 353–389 CrossRef CAS.
- L.-S. Zhong, J.-S. Hu, H-P. Liang, A.-M. Cao, W.-G. Song and L.-J. Wan, Adv. Mater., 2006, 18, 2426–2431 CrossRef CAS.
- A.-M. Cao, J.-S. Hu, H-P. Liang and L.-J. Wan, Angew. Chem., Int. Ed., 2005, 44, 4391–4395 CrossRef CAS.
- A. Biswas, H. Eilers, F. Hidden Jr., O. C. Aktas and C. V. S. Kiran, Appl. Phys. Lett., 2006, 88, 013103 CrossRef.
- (a) L. A. Estroff and A. D. Hamilton, Chem. Mater., 2001, 13, 3227–3235 CrossRef CAS; (b) H. Cölfen and S. Mann, Angew. Chem., Int. Ed., 2003, 42, 2350–2365 CrossRef; (c) J. Ba, A. Feldhoff, D. Fattakhova Rohlfing, M. Wark, M. Antonietti and M. Niederberger, Small, 2007, 3, 310–317 CrossRef CAS; (d) M. Antonietti, M. Niederberger and B. Smarsly, Dalton Trans., 2008, 18–24 RSC; (e) X. Sun and M. Hagner, Langmuir, 2007, 23, 9147–9150 CrossRef CAS; (f) W. Song, H. Jia, Q. Cong and B. Zhao, J. Colloid Interface Sci., 2007, 311, 456–460 CrossRef CAS.
- J. H. Fendler, Chem. Mater., 1996, 8, 1616–1624 CrossRef CAS.
- (a) J. Lyndon, Curr. Opinion Coll. Interface Sci, 1998, 3, 456–466 Search PubMed; (b) W. J. Harrison, D. L. Mateer and G. J. T. Tiddy, Faraday Discuss., 1996, 104, 139–154 RSC; (c) A. F. Kostko, B. H. Cipriano, O. L. Pinchuk, L. Ziserman, M. A. Anisimov, D. Danino and S. R. Raghaven, J. Phys. Chem. B, 2005, 109, 19126–19133 CrossRef CAS.
- (a) G. J. T. Tiddy, D. L. Mateer, A. P. Ormerod, W. J. Harrison and D. J. Edwards, Langmuir, 1995, 11, 390–393 CrossRef CAS; (b) C. A. Hunter and J. K. M. Sanders, J. Am. Chem. Soc., 1990, 112, 5525 CrossRef CAS.
- L. Suber, I. Sondi, E. Matijević and D. V. Goia, J. Colloid Interface Sci., 2005, 288, 489–495 CrossRef CAS.
- L. Suber, G. Campi, A. Pifferi, P. Andreozzi, C. La Mesa, H. Amenitsch, R. Cocco and W. R. Plunkett, J. Phys. Chem. C, 2009, 113, 11198–11203 CrossRef CAS.
- (a) S. A. Maier, M. L. Brongersma, P. G. Kik, S. Meltzer, A. A. G. Requicha and H. A. Atwater, Adv. Mater., 2001, 13, 1501–1505 CrossRef; (b) D. S. Citrin, Nano Lett., 2004, 4, 1561–1565 CrossRef CAS.
- (a) Y. Sun, Y. Yin, B. T. Mayers, T. Herricks and Y. Xia, Chem. Mater., 2002, 14, 4736–4745 CrossRef CAS; (b) M. E. Brennan, A. M. Whelan, J. M. Kelly and W. J. Blau, Synth. Met., 2005, 154, 205–208 CrossRef CAS; (c) M. Quinten, A. Leitner, J. R. Kren and F. R. Aussenegg, Opt. Lett., 1998, 23, 1331 CAS.
- A. I. Al-Ayash and M. T. Wilson, Biochem. J, 1979, 177, 641–648.
- Z. L. Wang, J. Phys. Chem. B, 2000, 104, 1153–1175 CrossRef CAS.
- (a) A. Kytzia, H. Korth, R. Sustmann, H. de Groot and M. Kirsch, Chem.–Eur. J., 2006, 12, 8786 CrossRef CAS; (b) T. Miyadera, Appl. Catal., B, 1998, 16, 155 CrossRef CAS.
- The absorption at 2138 cm−1 in Fig. 7 is due to an intercalation compound formed by the insertion of KBr, used for the IR pellet, between the AgCN chains as found in the case of CuCN by G. A. Bowmaker, B. J. Kennedy and J. C. Reid, Inorg. Chem., 1998, 37, 3968–3974 Search PubMed . This absorption in fact is not present in the IR spectrum of Sample 2 diluted in Nujol instead of KBr (Fig. b of the ESI†).
- Q. F. Zhou, J. C. Bao and Z. Xu, J. Mater. Chem., 2002, 12, 384–387 RSC.
- S. Busch, H. Dolhaine, A. DuChasne, S. Heinz, O. Hochrein, F. Laeri, O. Podebrad, U. Vietze, T. Weiland and R. Kniep, Eur. J. Inorg. Chem., 1999, 1643–1653 CrossRef.
- D. Lee, I. S. Lim, Y. S. Lee, D. Hagenbaum-Reignier and G. Jeung, J. Chem. Phys., 2007, 126, 244313–9 CrossRef.
- (a) S. Mann, Nature, 1988, 332, 119 CrossRef CAS; (b) S. Mann, Angew. Chem., Int. Ed., 2000, 39, 3392–3406 CrossRef CAS.
- M. Giersig, I. Pastoriza-Santos and L. M. Liz-Marzán, J. Mater. Chem., 2004, 14, 607–610 RSC.
- J. S. Garitaonandia, M. Insausti, E. Goikolea, M. Suzuki, J. D. Cashion, N. Kawamura, H. Ohsawa, Izaskun G. de Muro, K. Suzuki, F. Plazaola and T. Rojo, Nano Lett., 2008, 8, 661–667 CrossRef CAS.
- (a) B. Q. Ma, S. Gao and G. X. Xu, Angew. Chem., Int. Ed., 2001, 40, 434–437 CrossRef CAS; (b) Y. Guari, J. Larionova, K. Molvinger, B. Folch and C. Guérin, Chem. Commun., 2006, 2613–2615 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Additional XRD pattern, FT-IR spectrum, TEM and HR-TEM images. See DOI: 10.1039/b9nr00072k |
| This journal is © The Royal Society of Chemistry 2010 |
