Photoinduced cleaning of water-soluble dyes on patterned superhydrophilic/superhydrophobic substrates†
Xuemin
Zhang
a,
Junhu
Zhang
*a,
Zhiyu
Ren
b,
Xun
Zhang
a,
Tian
Tian
a,
Yunan
Wang
a,
Fengxia
Dong
a and
Bai
Yang
a
aState Key Lab of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, P. R. China. E-mail: zjh@jlu.edu.cn; Fax: +86-0431-85193423; Tel: +86-0431-85168478
bKey Laboratory of Functional Inorganic Material Chemistry, Ministry of Education, Laboratory of Physical Chemistry, School of Chemistry and Materials Science, Heilongjiang University, Harbin 150080, P. R. China
First published on 30th September 2009
Abstract
Herein we present a novel but simple method to fabricate photoinduced cleaning substrates with patterned superhydrophobic hierarchical silicon cone arrays (SCAs) and superhydrophilic TiO2nanorod clusters (TNCs). In our experiment, the photoinduced cleaning properties of the obtained substrate are investigated by repeatedly adsorbing and decomposing rhodamine B (RhB) molecules for at least six cycles. In addition, we demonstrate that the low-surface-energy coating on the superhydrophobic areas is stable, resulting in the high wettability contrast being well preserved during the renewal process. This straightforward method may open up new possibilities for the practical use of microchips with patterned superhydrophobic and superhydrophilic areas.
Introduction
To date, surfaces with patterned superhydrophobic and superhydrophilic areas have aroused extensive interest in both theoretical research and industrial applications. The most attractive property of such surfaces is their ability to selectively immobilise water droplets on hydrophilic areas. With this property, it is feasible to control liquid shape and flow.1–7 In addition, since surface hydrophilic groups on superhydrophilic areas can work as effective adsorption sites, functional materials dissolved or suspended in water droplets are easily deposited.8–10 Therefore, the obtained substrates are promising for important technological applications, including surface patterning,11,12 sensors,10water capture,1,13 and lab-on-a-chip devices.14–16Despite the advantages outlined above, problems still exist with this type of surface. For one thing the superhydrophilic areas on the surface are prone to adsorbing impurities in ambient environments,17,18 thus easily become contaminated. For another, after usage, the purposely deposited functional molecules are usually difficult to detach from the substrate. That is because materials tend to have favorable interactions with the superhydrophilic areas, and the superhydrophobic areas around will also prevent water streams from rinsing the superhydrophilic areas completely. Residual molecules will turn into impurities in subsequent experiments. Therefore, substrates with patterned superhydrophobicity and superhydrophilicity can only be used a limited number of times, which is not only time/cost-wasting, but also unfavorable for devices that need repeated use. Formerly, benefiting from the combination of photocatalytic19,20 and photoinduced superhydrophilic21,22 properties, photosensitive semiconductors such as TiO2 and ZnO were widely used as unique self-cleaning materials. Fujishima and co-workers have reported the fabrication of patterned superhydrophobic and superhydrophilic areas on these types of materials.23,24 For these substrates, organic impurities adsorbed on the superhydrophilic areas can be decomposed when exposed to UV light. But low-surface-energy molecules on the superhydrophobic areas will also be degraded to some extent, leading to the loss of wettability contrast between the original superhydrophobic and superhydrophilic areas.
Herein, a novel but simple method to fabricate photoinduced cleaning substrates with patterned superhydrophobic hierarchical silicon cone arrays (SCAs) and superhydrophilic TiO2nanorod clusters (TNCs) is reported. In our experiment, the cleaning properties of the obtained substrate are investigated by repeatedly adsorbing and decomposing rhodamine B (RhB) molecules for at least six cycles. In addition, we show that the low-surface-energy coating on the superhydrophobic areas is stable, resulting in the high wettability contrast being well preserved during the renewal process. To the best of our knowledge, this is the first report concerning the concept of fabricating photoinduced cleaning substrates with patterned extreme wettability. This straightforward method may open up new possibilities for the practical use of microchips with patterned superhydrophobic and superhydrophilic areas.
Experimental
Deposition of patterned superhydrophilic TNCs on superhydrophobic SCAs
The fabrication steps employed are outlined in Fig. 1. Firstly superhydrophobic SCAs modified with trichloro(1H,1H,2H,2H-perfluorooctyl) silane (PFS, from Aldrich) were fabricated using reactive ion etching with two-dimensional silica colloidal spheres of 1115 nm as masks 25 (see the ESI† ). Photoresist (BP212-37, positive photoresist, Kempur (Beijing) Microelectronics, Inc.) was then spin-coated on the surface of the obtained superhydrophobic SCAs (with spinning speed 4000 rpm for 1 min and the resulting resist film thickness being 2 µm) and patterned using a conventional photolithography method. After development, the exposed PFS monolayer was removed by O2 plasma etching, while those PFS molecules embedded in the photoresist were well protected. A hydrothermal process26 was then adopted by immersing the substrate in an aqueous titanium trichloride (0.15 M) solution supersaturated with NaCl at 160 °C for 2 h to deposit TNCs on the surface of photoresist film and exposed SCAs. Finally residual photoresist was removed along with the TNCs deposited above by ultrasonication in ethanol for ∼1 min and then rinsed with deionized water and dried in a nitrogen stream, leading to the formation of patterned TNCs surrounded by SCAs modified with a PFS monolayer.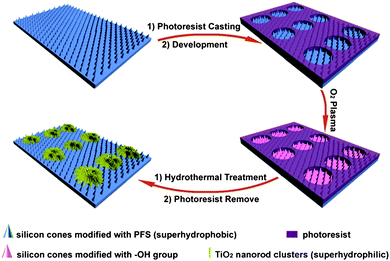 | ||
| Fig. 1 Schematic illustration of the procedure for the fabrication of substrates with patterned superhydrophilic TNCs and superhydrophobic SCAs. | ||
Adsorption and photodegradation of organic dyes
The adsorption process was performed by simply immersion the obtained substrate in a 0.5 mM RhB (reagent grade) water solution or 1 mM fluorescent isothiocyanate (FITC, reagent grade) water solution for a certain duration. Decomposition of adsorbed RhB molecules was achieved by putting the substrates under UV light irradiation (wavelength ranging from 300–400 nm with a power of 1000 W) at a distance of 15 cm for 10 min.Characterization
Scanning electron microscope (SEM) micrographs were taken with a JEOL JSM 6700F field-emission scanning electron microscope with primary electron energy of 3 kV, and the samples were sputtered with a layer of Pt (ca. 2 nm thick) prior to imaging to improve conductivity. Water droplets of 3 µL were used for the contact angle measurements. All of the measurements were performed at room temperature using a drop-shape analysis system (DSA 10 MK2, KRÜSS). At least five measurements were averaged for all of the data reported here. Fluorescent photographs were taken using an Olympus fluorescence microscope (BX51). A HR4000 UV–visible spectrometer (from Ocean Optics) was used to measure the absorbance of RhB from 400–750 nm. The chemical compositions were determined by X-ray photoelectron spectroscopy (XPS, VG ESCALAB MKII) with an Al Kα X-ray source (1486.6 eV).Results and discussion
Fig. 2 shows the corresponding SEM images of each step. Fig. 2A shows the obtained hierarchical SCAs modified with a PFS monolayer, with average height of 1431 nm. From the inset, nanoscale tips can be found on the surface of microscale cones. These two length scale roughness and low surface energy of the modified PFS monolayer render the substrate perfectly superhydrophobic.25 After photoresist casting and development, SCAs are selectively embedded in the photoresist film (Fig. 2B). The subsequent O2 plasma etching treatment will replace the exposed PFS monolayer with –OH groups. Therefore, SCAs not embedded in the photoresist will convert from superhydrophobic to superhydrophilic, facilitating the subsequent hydrothermal deposition of TNCs. The reason why we chose a hydrothermal process is not only its simplicity but also the unique morphology of the obtained TNCs. SEM images of well patterned TiO2 areas surrounded by PFS-modified SCAs are shown in Fig. 2C. TiO2 papillae can only be found on the surface of O2-plasma-treated SCAs. From the magnified view, each of these papillae is composed of TiO2nanorods with diameters ranging from 30 nm to 60 nm. This relatively high surface area morphology is helpful in the subsequent functional molecule adsorption. The obtained substrate with patterned surfaces has an apparent static contact angle of 139° (which is considered to monotonically decrease with the increase in superhydrophilic area), whereas the contact angle of areas with TNCs is ∼0° (with a wetting time less than 0.5 s, see the ESI† ) and without TNCs is ∼154° (sliding angle ∼3°), indicating the obtained substrate is patterned with superhydrophilic TNCs and superhydrophobic SCAs (as shown in Figs. 2D and 2E). | ||
| Fig. 2 (A) SEM images of obtained hierarchical silicon cone arrays (SCAs). Inset is the corresponding SEM image with a tilt angle of 60°. (B) SCAs selectively embedded in the photoresist film. The diameter of the exposed area is ∼30 µm. Inset is a magnified view of the area labeled by the red rectangle. (C) SEM image of patterned TiO2nanorod clusters surrounded by PFS-modified SCAs. Inset is the magnified view of a single TiO2 papilla. (D) and (E) show water-droplet photographs of areas with and without TiO2nanorod clusters. Scale bars in the insets are 1 µm (A), 1 µm (B), and 500 nm (C) respectively. | ||
In addition, it is noteworthy that two templates contribute to the fabrication of patterned TNCs with good confinement during the hydrothermal process. One is the patterned photoresist film (as a physical template), and the other is the high wettability contrast between the exposed SCAs and those embedded in the photoresist (as a chemical template). It is believed that both of these two templates will prevent TiCl3water solution from coming into contact with the embedded superhydrophobic areas. In our experiments, it was found that both of the templates were indispensable, and this conclusion was drawn by two contrastive experiments. The first contrastive experiment was carried out with only the wettability contrast used as a chemical template (the photoresist was removed by ultrasonication in ethanol before TiO2deposition). In that case, the amount of TNCs deposited on the original superhydrophilic and superhydrophobic areas was almost the same, and no patterns could be observed. It is considered that without the protection of the photoresist film, the superhydrophobicity of the PFS-modified SCAs is not stable enough to survive the rigorous reaction conditions during the TNC deposition process, resulting in TNCs depositing on the whole substrate with a uniform distribution. When the photoresist film works as a physical template only (both SCAs exposed or embedded in the photoresist are superhydrophilic), it was found that most of TNCs deposit on the originally exposed SCAs, however, TiO2nanorods could also be observed in areas embedded in the photoresist (see Fig. S1 of the ESI† ). This may be due to the TiCl3water solution permeating into the inter-space between the photoresist and the superhydrophilic SCAs in the TNC deposition process. Consequently, both templates are prerequisites to fabricate patterned TNCs with good confinement, which is determinant to the wettability contrast (between superhydrophilic TNCs and superhydrophobic SCAs) being well preserved during the final photoinduced cleaning process.
In addition, the obtained pattern of TNCs can be simply adjusted during the photolithography process, ranging from separated circles and triangles to continuous strips and networks (as shown in Fig. 3). Separated superhydrophilic arrays can locate non-interacting water droplets thus may find usefulness as microreactors, whereas the continuous superhydrophilic strips and networks can served as channels for liquid transportation. So this method promises the ability of fabricating complex and function-integrated microchips, and exhibiting extreme wettability of varying patterns. Rapid creation of functional molecule arrays is expected to be easily achieved by immersing the substrate in a water solution. Fluorescence micrographs of the obtained substrates after immersion in RhB and FITC solutions are shown in Fig. 3. High fluorescence intensity contrast between the modified TNCs and SCAs indicates that all the deposited TiO2nanorods are well confined. Furthermore, the fluorescence properties of the organic dye-stained substrates are well preserved even after rinsing with a water stream repeatedly, indicating that the adsorbed fluorescent molecules are stable enough and not easily detached from the TNCs.
 | ||
| Fig. 3 Fluorescent photographs of the obtained superhydrophilic TNCs with various patterns on the surface of superhydrophobic SCAs, Cycles (A), triangles (B), strips (C) and networks (D), where the substrate are stained with RhB (A and D) and FITC (B and C), respectively. Scale bars = 50 µm. | ||
Experimentally, we show the photoinduced cleaning properties of the obtained substrates lie in two aspects. On the one hand, renewability of the superhydrophilic TNCs: organic impurities adsorbed on the surface of TNCs can be easily degraded through UV light irradiation. In our experiment, RhB is adopted as a test pollutant. Fig. 4A shows the temporal evolution of the spectral changes of the adsorbed RhB molecules on TNCs under UV light irradiation. It is noted that an apparent decrease in absorbance takes place with a concomitant wavelength shift of the band to shorter wavelengths, from 554 nm (max absorbance of RhB) to ∼500 nm after exposure to UV light for 10 min. This gradual peak wavelength blue-shift is attributed to the de-ethylation of RhB molecules as disclosed by previously reports,27–29 while the decrease of absorbance peak indicates that the RhB molecules have decomposed. After 10 min exposure, the color of the RhB-modified TNCs changes from fresh red (color of RhB) to the original white color (color of deposited TNCs), which also implies that its chromophoric structure is destroyed. Fig. 4B shows the concentration change of RhB on TNCs against irradiation duration. For comparison, photodegration of RhB on bare silicon wafers is also performed. After 10 min irradiation, approximate 92% of the original RhB is photodegraded, whereas RhB molecules are relatively stable in the absence of TiO2. In addition, due to their photostimulated superhydrophilic properties, the superhydrophilicity of TNCs can be recovered during the renewal process. On the other hand, another critical issue we should take into consideration is the stability of the modified PFS monolayer during the renewal process, which is investigated using XPS analysis. To make it easier for investigation, a blank sample without adsorbed RhB molecules was used. From Fig. 5A, the numerical value of the surface atomic ratios of F/Ti and C/Ti on the blank sample (atoms of C and F are all from PFS molecules, and the content of Ti on each substrate is assumed as a constant) are mainly unchanged after at least 120 min UV light irradiation, indicating the PFS molecules modified on superhydrophobic SCAs will not be degraded during the renewal process. Insets show water droplets on the surface of UV-light-irradiated samples. Before contact angle measurements, the samples were irradiated for 0, 60 and 120 min, respectively, nevertheless, a decrease in the apparent static contact angles is not observed, which can serve as further evidence to prove our conclusion.
 | ||
| Fig. 4 (A) UV–vis spectral changes of RhB as a function of irradiation duration. (B) RhB concentration changes as a function of irradiation duration under conditions with TNCs and on bare silicon wafers. | ||
 | ||
| Fig. 5 (A) XPS analysis of PFS-modified substrates after varying UV light irradiation duration. Insets are water droplets on the obtained substrates. Contact angles are ∼139°, ∼140° and ∼137°, respectively. (B) Cycling runs of repeatedly adsorption and photo-degradation of RhB molecules using the same substrate. | ||
The reuse of the as-prepared substrate is demonstrated by repeatedly adsorbing and decomposing RhB molecules for several cycles (shown as Fig. 5B). Ci,0 and Ci,1 represent the concentration of the adsorbed RhB molecules on the substrate before and after photo-degradation during the cycle i, where i is the cycle number. In order to compare the amount of RhB molecules adsorbed in following cycles, C1,0 (the modified RhB concentration of the first cycle) is used as the reference (100%). In our experiment, the numerical values of Ci,0/C1,0 and Ci,1/Ci,0 remain relatively steady in at least six cycles, implying the adsorptive value and the ability of photoinduced cleaning of the obtained substrates are well maintained.
Conclusions
In conclusion, we have succeeded in fabricating photoinduced cleaning substrates with patterned superhydrophobic SCAs and superhydrophilic TNCs. The cleaning properties are demonstrated by repeatedly adsorbing RhB molecules on the superhydrophilic TNCs and then decomposing them through UV light irradiation for six cycles. In addition, this method is compatible with conventional photolithography, and different organic dyes can be adsorbed on the obtained superhydrophilic TNCs. Combining all of these advantages with the non-toxic, biocompatible and environmentally-friendly properties of TiO2, this method affords opportunities for the fabrication of complex, integrated and reusable lab-on-a-chip devices.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 20921003, 20534040 and 20874039) and the National Basic Research Program of China (2007CB936402).References
- L. Zhai, M. C. Berg, F. C. Cebeci, Y. Kim, J. M. Milwid, M. F. Rubner and R. E. Cohen, Nano Lett., 2006, 6, 1213 CrossRef CAS.
- V. Jokinen, L. Sainiemi and S. Franssila, Adv. Mater., 2008, 20, 3453 CrossRef CAS.
- H. S. Lim, J. T. Han, D. Kwak, M. Jin and K. Cho, J. Am. Chem. Soc., 2006, 128, 14458 CrossRef CAS.
- K. Tadanaga, J. Morinaga, A. Matsuda and T. Minami, Chem. Mater., 2000, 12, 590 CrossRef CAS.
- K. Teshima, H. Sugimura, A. Takano, Y. Inoue and O. Takai, Chem. Vap. Deposition, 2005, 11, 347 CrossRef CAS.
- S. J. Pastine, D. Okawa, B. Kessler, M. Rolandi, M. Llorente, A. Zettl and J. M. J. Fréchet, J. Am. Chem. Soc., 2008, 130, 4238 CrossRef CAS.
- S.-J. Choi, K. Y. Suh and H. H. Lee, J. Am. Chem. Soc., 2008, 130, 6312 CrossRef CAS.
- H.-C. Kim, C. R. Kreller, K. A. Tran, V. Sisodiya, S. Angelos, G. Wallraff, S. Swanson and R. D. Miller, Chem. Mater., 2004, 16, 4267 CrossRef CAS.
- P. Maury, M. Escalante, D. N. Reinhoudt and J. Huskens, Adv. Mater., 2005, 17, 2718 CrossRef CAS.
- Z.-Z. Gu, A. Fujishima and O. Sato, Angew. Chem., Int. Ed., 2002, 41, 2067 CrossRef CAS.
- G. Lu, W. Li, J. Yao, G. Zhang, B. Yang and J. Shen, Adv. Mater., 2002, 14, 1049 CrossRef CAS.
- W. Li, Y. Nie, J. Zhang, D. Zhu, X. Li, H. Sun, K. Yu and B. Yang, Macromol. Chem. Phys., 2008, 209, 247 CrossRef CAS.
- A. R. Parker and C. R. Lawrence, Nature, 2001, 414, 33 CrossRef.
- H. Notsu, W. Kubo, I. Shitanda and T. Tatsuma, J. Mater. Chem., 2005, 15, 1523 RSC.
- H. Gau, S. Herminghaus, P. Lenz and R. Lipowsky, Science, 1999, 283, 46 CrossRef CAS.
- J. S. Mohammed, M. A. DeCoster and M. J. McShane, Biomacromolecules, 2004, 5, 1745 CrossRef CAS.
- S. Takeda, M. Fukawa, Y. Hayashi and K. Matsumoto, Thin Solid Films, 1999, 339, 220 CrossRef CAS.
- B. Yan, J. Tao, C. Pang, Z. Zheng, Z. Shen, C. H. A. Huan and T. Yu, Langmuir, 2008, 24, 10569 CrossRef CAS.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69 CrossRef CAS.
- R. Wang, K. Hashimoto, A. Fujishima, M. Chikuni, E. Kojima, A. Kitamura, M. Shimohigoshi and T. Watanabe, Nature, 1997, 388, 431 CrossRef CAS.
- X. Feng, L. Feng, M. Jin, J. Zhai, L. Jiang and D. Zhu, J. Am. Chem. Soc., 2004, 126, 62 CrossRef CAS.
- X. Zhang, M. Jin, Z. Liu, D. A. Tryk, S. Nishimoto, T. Murakami and A. Fujishima, J. Phys. Chem. C, 2007, 111, 14521 CrossRef CAS.
- X.-T. Zhang, O. Sato and A. Fujishima, Langmuir, 2004, 20, 6065 CrossRef CAS.
- X. Zhang, J. Zhang, Z. Ren, X. Li, X. Zhang, D. Zhu, T. Wang, T. Tian and B. Yang, Langmuir, 2009, 25, 7375 CrossRef CAS.
- X. Feng, J. Zhai and L. Jiang, Angew. Chem., Int. Ed., 2005, 44, 5115 CrossRef CAS.
- Y. Ma and J. Yao, Chemosphere, 1999, 38, 2407 CrossRef CAS.
- H. Fu, C. Pan, W. Yao and Y. Zhu, J. Phys. Chem. B, 2005, 109, 22432 CrossRef CAS.
- T. Watanabe, T. Takizawa and K. Honda, J. Phys. Chem., 1977, 81, 1845 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and SEM image of poorly patterned TNCs, and a movie that illustrates the superhydrophilicity of TNCs. See DOI: 10.1039/b9nr00055k |
| This journal is © The Royal Society of Chemistry 2010 |
