Recognition and transmembrane delivery of bioconjugated Fe2O3@Au nanoparticles with living cells†
Linlin
Sun
a,
Jine
Wang
ab and
Zhenxin
Wang
*a
aState Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022, China. E-mail: wangzx@ciac.jl.cn; Fax: +86-431-85262243
bGraduate School of the Chinese Academy of Sciences, Beijing, 100039, China
First published on 1st October 2009
Abstract
Here, we describe the synthesis of peptide- and/or protein-functionalized Fe2O3 core–Au shell (Fe2O3@Au) nanoparticles for imaging and targeting of living cells. When functionalized with the transmembrane peptide RRRRRRRR (R8), the Fe2O3@Au nanoparticles (R8-Fe2O3@Au) are able to serve as cellular trafficking agents with excellent biocompatibility. The internalization mechanism and delivery efficiency of the R8-Fe2O3@Au nanoparticles have been characterized with dark-field microscopy and fluorescence confocal scanning laser microcopy. Experimental result suggests that the R8-Fe2O3@Au nanoparticles are internalized initially by binding with the membrane-associated proteoglycans on cell surfaces, especially heparan sulfate proteoglycans (HSPGs), following an energy-dependent endocytosis process to enter into living cells. After conjugation with the epidermal growth factor receptorantibody (anti-EGFR), these nanoparticles can also be used for the recognition of cell membrane antigens to specifically label tumor cells.
1. Introduction
During the last decade, because of their unique optical and electronic properties, nanomaterials have proved promising in a wide range of bioanalytical and biomedical applications, especially as highly sensitive and selective sensors and separators.1–9 As a typical example, biomolecule-modified nanoparticles have been used as effective targeting and delivering systems for ultrasensitive detection and imaging methods for bio-reorganizing events and revealing the functions of cells and tissues.1,4–7,9–17 Based on the development of monometal magnetic nanoparticles, core–shell superparamagnetic particles have attracted a broader interest because of their combined advantages of the heteromaterials.18–24 Among them, offering tunable plasmonic properties, facile thiol modification, and enhanced chemical stability, gold-coated iron oxide nanoparticles are known to have a wide range of promising applications.20–24 While it should be noticed that a considerable number of studies have been undertaken on the synthesis and coating process of gold-coated iron oxide nanoparticles, attention devoted to the applications of this kind of heteromaterial in cell biology is still limited.For their intrinsic relevance for biological applications, peptides have been recognized as suitable candidates for the stabilization and functionalization of inorganic nanoparticles.25–27 In particular, peptides with the sequence CALNN can conjugate with the gold nanoparticle surface via Au–S covalent interactions, making gold nanoparticles stable under physiological conditions.25 Our previous study also shows that the peptide CALNNGGRRRRRRRR-modified gold nanoparticles present an efficient intracellular targeting ability.28 Here, in order to implement multiplex cellular analysis, bioconjugated Fe2O3@Au nanoparticles have been synthesized. The stability and functionality of the Fe2O3@Au nanoparticles are achieved by surface modification with the biotinylated peptide, CALNNGK(biotin)G, and standard biotin–streptavidin chemistry. After further functionalized with biotinylated transmembrane peptide R8 (biotin-R8) or biotinylated anti-EGFRantibody (biotin-anti-EGFR), the bioconjugated Fe2O3@Au particles were employed as intracellular transfer agents or cellular recognition labels, respectively. Furthermore, the strong resonance light scattering of the nanoparticles allows easy tracking of the cellular uptake or recognition processes by dark-field microscopy. In a broader sense, the kind of bioconjugated Fe2O3@Au nanoparticles reported here are thus a valuable optical probe that can generate important qualitative/quantitative information via optical imaging methods such as dark-field microscopy.
2. Experimental
Materials
Peptides, CALNNGK(biotin)G and biotin-R8 (purity 98%, measured by HPLC) were purchased from Scilight Biotechnology Ltd. Co. (Beijing, China). Streptavidin and heparin were purchased from Shenggong, Ltd. (Shanghai, China). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco Co. (USA). All other reagents were analytical grade and purchased from Sigma-Aldrich Co. (USA). Milli-Q water (18.2 MΩ cm) was used in all experiments.Synthesis and functionalization of Fe2O3@Au nanoparticles
The Fe2O3@Au nanoparticles were synthesized according to the protocol developed by Lyon.20 Briefly, it started with Fe3O4 seed formation via the reduction of FeCl2 and FeCl3 (Fe2+/Fe3+ = 0.5) in 1.5 M NaOH solution with vigorous stirring. Then the Fe3O4 precipitate was washed with water until the solution pH value changed to 6. The magnetic Fe2O3 core was formed by oxidizing Fe3O4 seed using 0.01 M HNO3 solution with stirring at 100 °C. And gold was sequentially deposited onto the Fe2O3 cores via reduction of AuCl4− in the presence of citrate and hydroxylamine. The sizes of the Fe2O3 seeds and Fe2O3@Au nanoparticles were measured using a JEOL 2000FX transmission electron microscope (TEM) operated at an accelerating voltage of 200 kV. The relative amounts of metals in the Fe2O3@Au nanoparticles were determined by energy dispersive spectroscopy (EDS, 20 kV, SUTW Sapphire detector).
Peptide functionalized Fe2O3@Au nanoparticles were prepared by adding an aqueous CALNNGK(biotin)G solution to the solution of Fe2O3@Au nanoparticles to give a final concentration of total peptides of 1.38 mM as previous reports.25 After 1 h reaction at room temperature, excess peptides were removed by centrifugation (5000 rpm, 3×) and re-dispersed in water. For preparation of streptavidin-functionalized Fe2O3@Au (streptavidin-Fe2O3@Au) nanoparticles: the peptide-functionalized nanoparticles were subsequently incubated with streptavidin (10![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000× excess) for 1 h, then, purified by centrifugation (5000 rpm, 3×). For preparation of R8-Fe2O3@Au nanoparticles, biotin-R8, biotin-fluorescein (1 : 1) conjugated Fe2O3@Au (F-R8-Fe2O3@Au) nanoparticles, and biotin-fluorescein conjugated Fe2O3@Au (F-Fe2O3@Au) nanoparticles for the transmembrane functionality study, and biotin-anti-EGFR conjugated Fe2O3@Au (anti-EGFR-Fe2O3@Au) nanoparticles for the cellular surface antigen recognition study: the streptavidin-Fe2O3@Au nanoparticles were reacted with 10
000× excess) for 1 h, then, purified by centrifugation (5000 rpm, 3×). For preparation of R8-Fe2O3@Au nanoparticles, biotin-R8, biotin-fluorescein (1 : 1) conjugated Fe2O3@Au (F-R8-Fe2O3@Au) nanoparticles, and biotin-fluorescein conjugated Fe2O3@Au (F-Fe2O3@Au) nanoparticles for the transmembrane functionality study, and biotin-anti-EGFR conjugated Fe2O3@Au (anti-EGFR-Fe2O3@Au) nanoparticles for the cellular surface antigen recognition study: the streptavidin-Fe2O3@Au nanoparticles were reacted with 10![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000× excess peptidebiotin-R8, biotin-fluorescein, and biotin-anti-EGFR for 1 h, respectively; following purification by centrifugation (5000 rpm, 3×). After purification, all of the materials were re-dispersed in Milli-Q water and stored at 4 °C. Combined with TEM, UV–visible spectroscopy and inductively coupled plasma-mass spectrometry (ICP-MS) measurements, the molar extinction coefficient for the R8-Fe2O3@Au particles was found to be ca. 5.26 × 109 M−1 cm−1 by a previously reported method.4
000× excess peptidebiotin-R8, biotin-fluorescein, and biotin-anti-EGFR for 1 h, respectively; following purification by centrifugation (5000 rpm, 3×). After purification, all of the materials were re-dispersed in Milli-Q water and stored at 4 °C. Combined with TEM, UV–visible spectroscopy and inductively coupled plasma-mass spectrometry (ICP-MS) measurements, the molar extinction coefficient for the R8-Fe2O3@Au particles was found to be ca. 5.26 × 109 M−1 cm−1 by a previously reported method.4
Incubation of functionalized Fe2O3@Au nanoparticles with HeLa cells
HeLa (human cervical cancer) cells were grown in 5% CO2 at 37 °C in DMEM supplemented with 10% FBS. Before treatment with nanoparticles, cells were grown for 24 h in a fresh medium at a density of 1 × 105 cells cm−2 on the cover glass in 48-well plates. Then the cells were washed with phosphate-buffered saline (PBS) (3×) for the following studies.For the incubation concentration study, the cells were incubated with R8-Fe2O3@Au nanoparticles with the desired concentrations (0.07, 0.11, 0.15 and 0.19 nM) in 150 µL fresh medium with 10% FBS for 24 h. Then the cells were washed with PBS (3×), and imaged using a XSP-11CE optical microscope (Changfang Optical Instruments Ltd., China) equipped with a S5 IS digital camera (Canon Inc., Japan).
Intracellular tracking of R8-Fe2O3@Au nanoparticles with HeLa cells
HeLa cells were incubated with R8-Fe2O3@Au nanoparticles (0.19 nM) in 150 µL fresh medium with 10% FBS for 0, 0.5, 1, 2, 4, 8 and 24 h. After washing with PBS (3×), the light scattering intensity of the intracellularnanoparticles was collected using a microscope in dark-field mode. The light scattering intensity of each cell was analyzed by Image J software (National Institutes of Health, USA) which involved segmenting each cell image, analyzing the average intensity and subtracting the background signal. As a control sample, the light scattering intensity of non-treated cells was also collected by the same procedure. For each sample, about 50 cells in arbitrarily chosen areas in the culture plate were analyzed, and the average signal and standard deviation were calculated.Cell viability and proliferation determination
After treatment with R8-Fe2O3@Au nanoparticles of the desired concentration (0, 0.02, 0.07, 0.11, 0.15, 0.19 and 0.22 nM, respectively) for 24 h, the viability of HeLa cells was examined by Trypan Blue staining. Briefly, 0.1 mL of the cell suspension (1 × 107 cells mL−1) was added to 0.1 mL Trypan Blue solution (0.4% w/v), followed by incubation for 10 min at room temperature. Then, the number of viable and dead cells was counted on a hemacytometer under a microscope. For the proliferation assay , HeLa cells at a density of 1 × 105 cells cm−2 were incubated in the medium containing R8-Fe2O3@Au nanoparticles of the desired concentration (0, 0.02, 0.07, 0.11, 0.15, 0.19 and 0.22 nM, respectively) for 24 h, then the proliferation rates related to the control sample were calculated on the basis of the number of HeLa cells. The control samples were HeLa cells which were cultured under same experimental condition without any nanoparticles.Internalization mechanism of R8-Fe2O3@Au nanoparticles with HeLa cells
For the incubation temperature analysis, the cells were incubated with R8-Fe2O3@Au nanoparticles (0.19 nM) in DMEM with 10% FBS for 4 h at either 4 °C or 37 °C, and then the cells were washed and analyzed as described in the intracellular tracking study.For the HSPG competition study, the cells were treated with varying concentrations of heparin (0, 2, 5, 15, 30 and 50 µg mL−1) for 30 min in serum-free media. Then R8-Fe2O3@Au nanoparticles (0.19 nM) were added into the heparin-containing medium and co-cultured for 4 h, followed by washing, and analyzed as described in the intracellular tracking study.
Delivery efficiency of R8-Fe2O3@Au nanoparticles
For the delivery efficiency study, HeLa cells were incubated with 0.19 nM F-Fe2O3@Au or F-R8-Fe2O3@Au nanoparticles in 150 µL fresh medium with 10% FBS for 8 h, followed by washing with PBS (×3), trypsinized and analyzed by a TCS SP2 confocal scanning laser microscope (Leica Co., Germany) equipped with a 20× objective without fixing the cells.For studying the quantification and percentage of uptaken nanoparticles, HeLa cells (1 × 105 cells cm−2) were treated with R8-Fe2O3@Au, F-R8-Fe2O3@Au or F-Fe2O3@Au nanoparticles (0.19 nM) for varying times (0, 0.5, 2, 4 and 8 h), respectively. Then the cells were washed with PBS (×3), trypsinized with 0.02% EDTA and analyzed by an XseriesIIICP-MS instrument (Thermo Scientific, USA).
Cell surface antigen targeting of anti-EGFR-Fe2O3@Au nanoparticles
HeLa and 293 cells (1 × 105 cells cm−2) were incubated with anti-EGFR-Fe2O3@Au nanoparticles (0.3 nM) in PBS for 2 h at 37 °C. Then the cells were washed with PBS (×3), trypsinized with 0.02% EDTA, and analyzed by dark-field microscopy. For the EGFR blocking study, HeLa cells were first incubated with anti-EGFR (0.3 mg mL−1, 100 µL) for 1 h. After washing with PBS (×3), cells were incubated with anti-EGFR-Fe2O3@Au nanoparticles (0.3 nM) in PBS for 2 h at 37 °C and analyzed as previously described.3. Results and discussion
Synthesis and functionalization of Fe2O3@Au nanoparticles
The synthesis and functionalization of Fe2O3@Au nanoparticles are shown in Fig. 1. The Fe2O3@Au nanoparticles were prepared by the literature procedure.20 With the iterative deposition of Au on Fe2O3 cores, the color of nanoparticles changed from yellow to brownish red gradually, and gave a final surface plasmon resonance band at 558 nm (Fig. 2a). The changing optical properties of the nanoparticles show that uniformly spherical Fe2O3@Au nanoparticles are formed.20 Determined by TEM, the average sizes of the as-prepared Fe2O3 core and Fe2O3@Au nanoparticles are 15.9 ± 5 and 56 ± 14 nm in diameter, respectively (Fig. 2b and Fig. S1 of the ESI† ). In addition, the EDS spectrum shows that the particle contains both Fe and Au, giving a further confirmation of the particle composition (Fig. 2c). | ||
| Fig. 1 Schematic representation of the preparation of R8-Fe2O3@Au and anti-EGFR-Fe2O3@Au nanoparticles. | ||
 | ||
| Fig. 2 (a) UV–visible spectra of the iterative gold deposition on Fe2O3nanoparticles. The inset shows the color of Fe2O3 seeds (left), Fe2O3@Au nanoparticles (middle) and the distribution of Fe2O3@Au nanoparticles under a magnetic field (right). (b) TEM images of the Fe2O3@Au nanoparticles. (c) EDS spectrum of the Fe2O3@Au nanoparticles. (d) UV–visible spectra of Fe2O3@Au nanoparticles modified with biotin-CALNN peptide and subsequently conjugated with biotin-R8peptide or biotin-anti-EGFRvia a streptavidin–biotin bridge. | ||
For bioanalytical and/or biomedical applications, introducing multiple functionalities would be of great value, as it provides more flexibility for multiplexing in applications or detections. Following a previously reported peptide stabilization and functionalization procedure, the peptide, CALNNGK(biotin)G, was chosen to stabilize and functionalize the particles. The peptide-capped Fe2O3@Au nanoparticles were conjugated with biotin-R8 or biotin-anti-EGFR by standard biotin–streptavidin chemistry, respectively (Fig. 1). No significant change of the surface plasmon resonance band was observed after the surface modification of Fe2O3@Au nanoparticles (Fig. 2d). Moreover, this functionalization method endowed the R8-Fe2O3@Au nanoparticles good stability in physiological medium, which was even better than that of CALNNGGRRRRRRRR-modified nanoparticles (Fig. S2† ).28
Tracking the cellular uptake of R8-Fe2O3@Au nanoparticles
The peptidebiotin-R8 was used as a functional group to deliver the Fe2O3@Au nanoparticles into living cells since arginine-rich peptides have an extremely high transmembrane efficiency.29–33 After 24 h incubation with HeLa cells, the R8-Fe2O3@Au nanoparticles exhibited a high level of cellular internalization (Fig. 3). This phenomenon was observable using a normal bright-field microscope for concentrations as low as 0.07 nM of R8-Fe2O3@Au nanoparticles in culture media (Fig. 3d). The uptaken R8-Fe2O3@Au nanoparticles mainly aggregated in the position close to the nucleus, and the number of intracellular particles was increased by increasing the concentration of R8-Fe2O3@Au nanoparticles in the culture medium. | ||
| Fig. 3 Bright-field microscopy images of HeLa cells incubated with R8-Fe2O3@Au nanoparticles for 24 h at concentrations of (a) 0.19 nM, (b) 0.15 nM, (c) 0.11 nM and (d) 0.07 nM. In the control experiment, HeLa cells were incubated with 0.19 nM strepavidin-Fe2O3@Au nanoparticles (e) or biotin-Fe2O3@Au nanoparticles (f) for 24 h. | ||
The internalization process can be easily tracked since the bimetal core–shell structure of the Fe2O3@Au nanoparticles gives rise to a strong plasmon resonance scattering.34Fig. 4 shows time-dependent dark-field microscopy images of HeLa cells after incubated with R8-Fe2O3@Au nanoparticles for a 24 h period. The intracellular light signal is increased with increasing incubation time (Fig. 4d–j). Within 30 min of R8-Fe2O3@Au nanoparticle addition, the cell outer surfaces were visibly stained (Fig. 4d). After 1 h incubation, the R8-Fe2O3@Au nanoparticles had already entered the cell and began to accumulate in the cellular nucleus region (Fig. 4e). After 8 h, most of the nanoparticles had aggregated in perinuclear compartments (Fig. 4h). For comparison, the light scattering intensity of the nanoparticles internalized in HeLa cells vs. incubation time is summarized in Fig. 4j.
 | ||
| Fig. 4 Dark-field microscopy images of HeLa cells incubated with 0.19 nM R8-Fe2O3@Au nanoparticles for (d) 0.5 h, (e) 1 h, (f) 2 h, (g) 4 h, (h) 8 h and (i) 24 h. In the control groups: HeLa cells were cultured in the medium for 8 h without any nanoparticles (a), with 0.19 nM biotin-Fe2O3@Au nanoparticles (b) or strepavidin-Fe2O3@Au nanoparticles (c). (j) Relative light scattering intensity of the HeLa cells treated with 0.19 nM R8-Fe2O3@Au nanoparticles at different incubation times. | ||
Cellular cytotoxicity of R8-Fe2O3@Au nanoparticles
Although gold is an inert metal, its effect on normal cell proliferation cannot be underestimated. On the other hand, the nature of surface-ligands on the nanoparticles could remarkably affect their cytotoxicity. The in vitro cellular cytotoxicity of the R8-Fe2O3@Au nanoparticles at different concentrations was examined (Fig. 5a). The experimental results indicate that the HeLa cells still have more than 90% viability after incubation with as high as 0.22 nM nanoparticles in culture medium for 24 h. During a relatively long period (two weeks) of co-culture with the R8-Fe2O3@Au nanoparticles, no significant change in the viability of the HeLa cells was observed. The results suggest that the R8-Fe2O3@Au system has a low cytotoxicity. However, a significant difference in cellular proliferation was observed after HeLa cells were incubated with standard culture medium or culture medium supplemented with R8-Fe2O3@Au nanoparticles (Fig. 5b). The data showed a dose-dependent effect, a moderate decrease (<10%) in proliferation was observed at a lower concentration of the R8-Fe2O3@Au nanoparticles (0.07 nM) while a significant decrease in proliferation (c.a. 46%) was generated at a higher concentration of the R8-Fe2O3@Au nanoparticles (0.22 nM). In addition, a significant inhibition (> 40%) of cellular proliferation was also observed when HeLa cells were incubated with the culture medium supplemented with CALNNGGRRRRRRRR-capped Fe2O3@Au nanoparticles or the peptidebiotin-R8, respectively (as shown Fig. S3 of the ESI† ). These experimental results indicate that the R8 motif leads to the inhibition of cellular proliferation.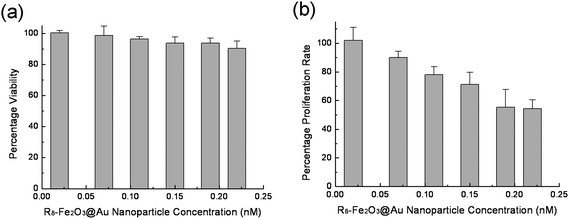 | ||
| Fig. 5 Cytotoxicity induced by R8-Fe2O3@Au nanoparticles in terms of cell viability (a) and proliferation rate (b). The relative cell viability and proliferation rate related to control cells were calculated on the basis of the number of HeLa cells. | ||
Internalization mechanism of R8-Fe2O3@Au nanoparticles
A set of experiments was designed to determine the key factors involved in R8-Fe2O3@Au nanoparticle delivery. When reducing the incubation temperature to 4 °C, it was observed that the cellular uptake of R8-Fe2O3@Au nanoparticles decreased remarkably, suggesting that it is an energy-dependent process (Fig. 6a). | ||
| Fig. 6 (a) Effect of temperature on the internalization of R8-Fe2O3@Au nanoparticles with HeLa cells. HeLa cells were incubated with 0.19 nM R8-Fe2O3@Au nanoparticles for 4 h at 37 °C or 4 °C, respectively. Inset images present the corresponding dark-field micrographs at 37 °C (left) and 4 °C (right). (b) Competition of heparin sulfate. | ||
With increasing heparin concentration, a competitor of HSPGs on cell membranes,35 in culture medium, the cellular uptake of R8-Fe2O3@Au nanoparticles was significantly reduced (Fig. 6b). This phenomenon suggests that the endocytosis process of R8-Fe2O3@Au nanoparticles depends on the cell membrane-associated proteoglycans.
Delivery efficiency of R8-Fe2O3@Au nanoparticles
In order to test the practicability of using the R8-Fe2O3@Au nanoparticles to deliver extracellular materials into cells, biotin-fluorescein was attached to the Fe2O3@Au nanoparticles with or without biotin-R8. For the delivery efficiency comparison between F-R8-Fe2O3@Au nanoparticles and F-Fe2O3@Au nanoparticles, the fluorescence signal of the intracellular Fe2O3@Au nanoparticles was recorded by fluorescence confocal scanning laser microscopy (Fig. 7). The distribution of the intracellularnanoparticles is localized perinuclearly in a punctuate mode, which indicates that the nanoparticles have been trapped inside the endosomal vesicular system.29 Furthermore, the quantitative analysis of the variation of cellular uptake of nanoparticles with incubation time has been studied by ICP-MS (Fig. S4 of the ESI† ). The cellular uptake of R8-conjugated nanoparticles (>80% of total added amount) is much higher than that of F-Fe2O3@Au nanoparticles (<10% of total added amount). The experimental results indicates that the R-conjugated Fe2O3@Au nanoparticles can be used as carriers to deliver materials into cells with a high efficiency. | ||
| Fig. 7 Confocal microscopy images of HeLa cells incubated with 0.19 nM F-Fe2O3@Au nanoparticles (a and c) or F-R8-Fe2O3@Au nanoparticles (b and d) for 8 h, respectively. | ||
Cellular surface antigen targeting of anti-EGFR-Fe2O3@Au nanoparticles
To further extend the application of the Fe2O3@Au nanoparticles, anti-EGFR was used as the model molecule to study antigen–antibody interactions on living cell surfaces. As it is well-known that EGFR is over-expressed in most types of cancer cells, for example, HeLa cells contain approximately 104EGFR molecules on the cell membrane which is more abundant than that of normal cells, e.g., a 293 cell.36–39 The interaction of the HeLa and 293 cells with anti-EGFR-Fe2O3@Au nanoparticles is shown in Fig. 8 and Fig. S5 of the ESI† . After incubation with anti-EGFR-Fe2O3@Au nanoparticles, the relative light intensity of the nanoparticles bound to the HeLa cell membranes is greater than that of the 293 cell. In addition, the light scattering signal was significantly decreased when free anti-EGFR was introduced into the system (Fig. S6 of the ESI† ). This phenomenon confirms that the anti-EGFR is involved in the binding process. These results suggest that the particles could be further conjugated with a variety of antibodies for specific cellular recognition and separation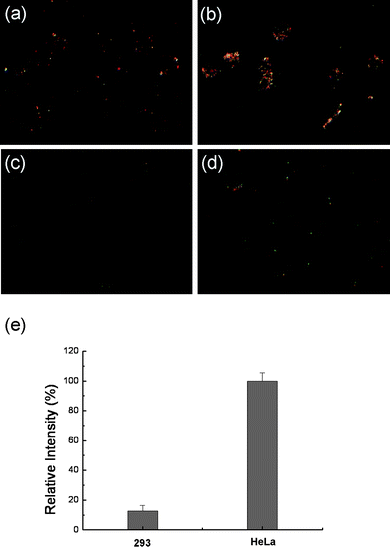 | ||
| Fig. 8 Dark-field microscopy images of 293 cells (a) and HeLa cells (b) incubated with 0.3 nM anti-EGFR-Fe2O3@Au nanoparticles for 2 h. As control samples, cells (293 (c) and HeLa (d)) were incubated with 0.3 nM streptavidin-Fe2O3@Au nanoparticles for 2 h under same experimental conditions. Relative light scattering intensity of anti-EGFR-Fe2O3@Au nanoparticles on the cell membrane (e); the data were obtained by subtraction of the average light scattering intensity from anti-EGFR-Fe2O3@Au nanoparticles on stained cells from that of control sample. | ||
4. Conclusion
In summary, we have developed a simple method for preparing Fe2O3@Au nanoparticles with flexible functionality and excellent biocompatibility. As proof-of-principle experiments, the interactions of living cells with well-known-biomolecule- (peptide R8 or anti-EGFR) functionalized Fe2O3@Au nanoparticles have been carefully studied. The R8-Fe2O3@Au nanoparticles can serve as optical probes for investigating the mechanisms of membrane internalization processes as well as acting as efficient cellular delivery agents. In addition, specific targeting by anti-EGFR-Fe2O3@Au nanoparticles with different cells has been observed. Therefore, this new type of functionalized nanoparticle could open up a new possibility to develop simple sensors for biological or biomedical applications, such as intracellular trafficking processes and specific cell recognition.Acknowledgements
The authors would like to thank the NSFC (Grant No. 20675080), the Chinese Academy of Sciences (Grant No. KJCX2-YW-H11) and the CAS-Bayer Start-up Fund (2008) for financial support.References
- C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature, 1996, 382, 607–609 CrossRef CAS.
- R. C. Mucic, J. J. Storhoff, C. A. Mirkin and R. L. Letsinger, J. Am. Chem. Soc., 1998, 120, 12674–12675 CrossRef CAS.
- M.-C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS.
- N. L. Rosi and C. A. Mirkin, Chem. Rev., 2005, 105, 1547–1562 CrossRef CAS.
- C. J. Murphy, A. M. Gole, J. W. Stone, P. N. Sisco, A. M. Alkiany, E. C. Goldsmith and S. C. Baxter, Acc. Chem. Res., 2008, 41, 1721–1730 CrossRef CAS.
- C. M. Niemeyer, Angew. Chem., Int. Ed., 2001, 40, 4128–4158 CrossRef CAS.
- C.-C. You, M. De and V. M. Rotello, Curr. Opin. Chem. Biol., 2005, 9, 639–646 CrossRef CAS.
- E. Katz and I. Willner, Angew. Chem., Int. Ed., 2004, 43, 6042–6108 CrossRef CAS.
- S. Mornet, S. Vasseur, F. Grasset and E. Duguet, J. Mater. Chem., 2004, 14, 2161–2175 RSC.
- J. A. Ryan, K. W. Overton, M. E. Speight, C. N. Oldenburg, L. Loo, W. Robarge, S. Franzen and D. L. Feldheim, Anal. Chem., 2007, 79, 9150–9159 CrossRef CAS.
- Y. T. Lim, M. Y. Cho, J. M. Lee, S. J. Chung and B. H. Chung, Biomaterials, 2009, 30, 1197–1204 CrossRef CAS.
- M. Lewin, N. Carlesso, C.-H. Tung, X.-W. Tang, D. Cory, D. T. Scadden and R. Weissleder, Nat. Biotechnol., 2000, 18, 410–414 CrossRef CAS.
- C. C. Berry and A. S. G. Curtis, J. Phys. D: Appl. Phys., 2003, 36, R198–R206 CrossRef CAS.
- C. Wilhelm, F. Gazeau, J. Roger, J. N. Pons and J.-C. Bacri, Langmuir, 2002, 18, 8148–8155 CrossRef CAS.
- J. W. M. Bulte, T. Douglas, B. Witwer, S. C. Zhang, E. Strable, B. K. Lewis, H. Zywicke, B. Miller, P. V. Gelderen, B. M. Moskowitz, L. D. Duncan and J. A. Frank, Nat. Biotechnol., 2001, 19, 1141–1147 CrossRef CAS.
- J. M. De la Fuente, C. C. Berry, M. O. Riehle and A. S. G. Curtis, Langmuir, 2006, 22, 3286–3293 CrossRef.
- S. Kumar, N. Harrison, R. Richards-Kortum and K. Sokolov, Nano Lett., 2007, 7, 1338–1343 CrossRef CAS.
- S. Sun, S. Anders, T. Thomson, J. E. E. Baglin, M. F. Toney, H. F. Hamann, C. B. Murray and B. D. Terris, J. Phys. Chem. B, 2003, 107, 5419–5425 CrossRef CAS.
- S. I. Stoeva, F. Huo, J.-S. Lee and C. A. Mirkin, J. Am. Chem. Soc., 2005, 127, 15362–15363 CrossRef CAS.
- J. L. Lyon, D. A. Fleming, M. B. Stone, P. Schiffer and M. E. Williams, Nano Lett., 2004, 4, 719–723 CrossRef CAS.
- J. Lin, W. Zhou, A. Kumbhar, J. Wiemann, J. Fang, E. E. Carpenter and C. J. O'Connor, J. Solid State Chem., 2001, 159, 26–31 CrossRef CAS.
- J. Bai and J.-P. Wang, Appl. Phys. Lett., 2005, 87, 152502 CrossRef.
- L. Wang, J. Luo, Q. Fan, M. Suzuki, I. S. Suzuki, M. H. Engelhard, Y. Lin, N. Kim, J. Q. Wang and C. J. Zhong, J. Phys. Chem. B, 2005, 109, 21593–21601 CrossRef CAS.
- H. L. Liu, C. H. Sonn, J. H. Wu, K.-M. Lee and Y. K. Kim, Biomaterials, 2008, 29, 4003–4011 CrossRef CAS.
- R. Lévy, N. T. K. Thanh, R. C. Doty, I. Hussain, R. J. Nichols, D. J. Schiffrin, M. Brust and D. G. Fernig, J. Am. Chem. Soc., 2004, 126, 10076–10084 CrossRef CAS.
- A. G. Tkachenko, H. Xie, Y. Liu, D. Coleman, J. Ryan, W. R. Glomm, M. K. Shipton, S. Franzen and D. L. Feldheim, Bioconjugate Chem., 2004, 15, 482–490 CrossRef CAS.
- V. Biju, D. Muraleedharan, K. Nakayama, Y. Shinohara, T. Itoh, Y. Baba and M. Ishikawa, Langmuir, 2007, 23, 10254–10261 CrossRef CAS.
- L. Sun, D. Liu and Z. Wang, Langmuir, 2008, 24, 10293–10297 CrossRef CAS.
- M. Zorko and U. Langel, Adv. Drug Delivery Rev., 2005, 57, 529–545 CrossRef CAS.
- S. Futaki, T. Suzuki, W. Ohashi, T. Yagami, S. Tanaka, K. Ueda and Y. Sugiura, J. Biol. Chem., 2001, 276, 5836–5840 CrossRef CAS.
- I. Nakase, A. Tadokoro, N. Kawabata, T. Takeuchi, H. Katoh, K. Hiramoto, M. Negishi, M. Nomizu, Y. Sugiura and S. Futaki, Biochemistry, 2007, 46, 492–501 CrossRef CAS.
- S. M. Fuchs and R. T. Raines, Biochemistry, 2004, 43, 2438–2444 CrossRef CAS.
- A. Ori, M. C. Wilkinson and D. G. Fernig, Front. Biosci., 2008, 4309–4338 CrossRef CAS.
- J. S. Aaron, J. Oh, T. A. Larson, S. Kumar, T. E. Milner and K. V. Sokolov, Opt. Express, 2006, 14, 12930–12943 CrossRef CAS.
- A. Scarpellini, R. Germack, H. Lortat-Jacob, T. Muramatsu, E. Billett, T. Johnson and E. A. M. Verderio, J. Biol. Chem., 2009, 284, 18411–18423 CrossRef CAS.
- T. Maruo, Cancer, 1992, 69, 1182–1187 CAS.
- K. Sokolov, M. Follen, J. Aaron, I. Pavlova, A. Malpica, R. Lotan and R. Richards-Kortum, Cancer Res., 2003, 63, 1999–2004 CAS.
- V. A. Kickhoefer, M. Han, S. Raval-Fernandes, M. J. Poderycki, R. J. Moniz, D. Vaccari, M. Silvestry, P. L. Stewart, K. A. Kelly and L. H. Rome, ACS Nano, 2009, 3, 27–36 CrossRef CAS.
- H. He, C. Xie and J. Ren, Anal. Chem., 2008, 80, 5951–5957 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: TEM images of Fe2O3nanoparticles, stability of peptide conjugated Fe2O3@Au nanoparticles; Inhibition of cellular proliferation by nanoparticles and biotin-R8, ICP-MS analysis of HeLa cells incubated with R8-Fe2O3@Au, F-R8-Fe2O3@Au, or F-Fe2O3@Au nanoparticles as a function of incubation time and anti-EGFR blocking study. See DOI: 10.1039/b9nr00152b |
| This journal is © The Royal Society of Chemistry 2010 |
