Zinc release of Zn7-metallothionein-3 induces fibrillar type amyloid-β aggregates†
Jade
Durand‡
ab,
Gabriele
Meloni§
c,
Christine
Talmard
ab,
Milan
Vašák
c and
Peter
Faller
*ab
aCNRS, LCC (Laboratoire de Chimie de Coordination), 205, route de Narbonne, F-31077 Toulouse, France. E-mail: peter.faller@lcc-toulouse.fr; Fax: 0033/561553003
bUniversité de Toulouse, UPS, INPT, LCC, F-31077 Toulouse, France
cDepartment of Biochemistry, University of Zürich, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland
First published on 21st October 2010
Abstract
The reactive oxygen species H2O2 promotes the Zn7-metallothionein-3 induced Aβ40 aggregation of fibrillar type structures via slow cysteine oxidation and Zn2+ release, whereas amorphous aggregates are formed by addition of Zn2+ to Aβ40.
In the central nervous system, specifically the brain, relatively high concentrations of zinc are found with regional selectivity. While approximately 80% of zinc in the brain is protein-bound zinc, the remainder is concentrated in presynaptic vesicles of zinc-enriched glutamergic neurons, containing millimolar Zn2+ concentrations. This vesicular zinc is released into the synaptic cleft upon neuronal activity, where it can through an interaction with many different postsynaptic targets modulate their activity.1
In Alzheimer's disease (AD) altered Zn2+ homeostasis is well documented. This observation along with the presence of Zn2+ in senile plaques, where its concentration can be as high as ∼1 mM, indicated that this metal could play an important role in this neurodegenerative disorder.2–4 Aggregation of the peptide amyloid-β (Aβ) has been proposed to be a key event in AD. Aggregates are believed to promote a neuronal dysfunction, and later on dementia via the production of reactive oxygen species (ROS).5 Studies on AD animal models have also shown that genetic ablation of synaptic Zn2+ greatly reduces the amount of amyloid plaques.6In vitro studies showed that Zn2+ ions bind stoichiometrically to Aβ with an apparent dissociation constant of 1–10 μM at pH 7.4. (for review see ref. 7). Zn2+ can induce Aβ aggregation within time scale of milliseconds8 and can promote synaptic targeting of oligomeric Aβ.4 Generally, Zn2+ induced Aβ aggregates are rather amorphous with a lower content of fibrils and are less reactive with the amyloid marker thioflavin T (ThT) than aggregates of Aβ alone (reviewed in ref. 7 and 9). Although Zn2+ induces Aβ aggregation and destabilizes oligomers, it is not clear if Zn2+ has a protective or an exacerbating effect on the neurotoxicity of Aβ.9,10
In the brain, a molecule involved in zinc metabolism and linked to AD is a small metalloprotein metallothionein-3 (Zn7MT-3).11–13 Zn7MT-3 is mainly expressed in hippocampal glutamergic neurons that release Zn2+ from synaptic vesicles.14 Evidence has been provided that MT-3 occurs intra- and extracellularly.15 Structural studies revealed that the protein can bind 7 Zn2+ ions via its 20 cysteines, forming two metal-thiolate clusters a Zn3–Cys9 cluster in the N-terminal β-domain and a Zn4–Cys11 cluster in the C-terminal α-domain.13,16
Oxidative stress is implicated in the pathogenesis and/or progression of neurodegenerative diseases. In general, ZnMTs, including the mammalian Zn7MT-3, Zn7MT-2 and Zn7MT-1 isoforms, through quenching of reactive oxygen and nitrogen species (ROS and RNS) protect cells from oxidative stress. This reaction leads to cysteine oxidation/modification and Zn2+ release (for recent review see ref. 12). However, structural differences among Zn7MT-3 and Zn7MT-1/-2 are responsible for their differing reactivity and biological activity.17 Zn7MT-3, but not Zn7MT-1 and Zn7MT-2 protects cultured neurons from the toxicity of Aβ.18 This protective effect has been accounted for by the metal swap between Zn7MT-3 and Cu2+–Aβ, leading to the suppression of ROS production caused by the redox cycling of Cu2+–Aβ.19 These literature reports strongly indicate that ZnMT-3 and Aβ are co-localized near the synapses of zinc-enriched glutamergic neurons and are linked to AD and oxidative stress.
Here, we investigated if Zn2+ transfer from Zn7MT-3 to Aβ40 is possible in the absence and presence of H2O2. The term transfer was used in the sense to describe the Zn2+ movement from Zn7MT-3 to Aβ40 without an interaction between the two peptides. We found that Zn7MT-3 donates Zn2+ to Aβ40 not only in the presence of H2O2, but also in its absence. Moreover, we could show that Zn2+ transfer from Zn7MT-3 modulates Aβ40 aggregation differently than the addition of free Zn2+.
First, we checked by absorption spectroscopy whether Zn2+ may be released from Zn7MT-3 upon addition of H2O2. We have followed the CysS–Zn2+ charge-transfer band at 230 nm over time as a function of different H2O2 concentrations. The observation that the charge-transfer band decreased with time in a concentration dependent manner indicated that Zn2+ is slowly released from the Zn2+-thiolate clusters in Zn7MT-3 (Fig. 1A).
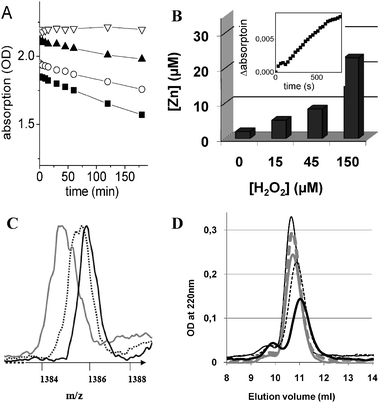 | ||
| Fig. 1 (A) Time dependence of the Zn7MT-3 absorption at 230 nm as a function of different H2O2 concentrations. The Cys–Zn2+ charge-transfer band at 230 nm was followed over 3 h. Conditions: Zn7MT-3 20 μM, Hepes 20 mM, NaCl 20 mM, at pH 7.4. H2O2 concentrations used 0 μM (▽), 50 μM (▲), 100 μM (○), and 200 μM (■). (B) H2O2 induced Zn2+ release from Zn7MT-3 monitored by complexing dye zincon. The Zn2+ release from Zn7MT-3 as a function of different concentrations of H2O2 after a 1 day incubation. Conditions: 20 μM Zn7MT-3, H2O2: 0, 15, 45 or 150 μM, Hepes 20 mM, NaCl 20 mM, pH 7.4. Prior to measurement the sample was 20 times diluted with the same buffer containing 20 μM zincon. The presented Zn2+ release relates to the original Zn7MT-3 concentration of 20 μM; Inset: Time dependence Zn2+ release from 10 μM Zn7MT-3 in the absence of H2O2 monitored trough the absorption of 20 μM zincon at 620 nm. (C) Mass spectra of apoMT-3 after incubation with different H2O2 concentrations. Conditions: 40 μM Zn7MT-3 in 20 mM Hepes, 20 mM NaCl, at pH 7,4 was incubated for 1 day with 0 (black), 40 (dotted), and 200 μM (gray)) H2O2. Prior to analyses the samples were diluted with two volumes of H2O–acetonitrile (50% v/v) containing 0.1% formic acid. The measurements were performed at pH 3, which induced the release of Zn2+. Only the 5 times charged peaks are shown. (D) Size exclusion chromatography (SEC) of Zn7MT-3 after reaction with H2O2. The Zn7MT-3 sample was incubated for 1 day with 0 (thick dotted gray), 20 (thick solid gray), 100 (thin dotted black), or 200 μM (thick solid black) H2O2. A freshly prepared solution of Zn7MT-3 without H2O2 was used as a control (thin solid). Conditions: 20 μM Zn7MT-3, 20 mM Hepes, 20 mM NaCl, at pH 7.4. | ||
To obtain a more direct measure of Zn2+ release from Zn7MT-3 in the presence of H2O2, the protein sample was incubated with increasing concentrations of H2O2 and the Zn2+ release monitored by the complexing dye zincon. Fig. 1B shows the H2O2 concentration dependent Zn2+ release from 20 μM Zn7MT-3, obtained by following the formation of Zn2+–zincon complex at 620 nm over 1 day. We also observed that a small amount of Zn2+ (about 0.1 mole equivalent) was apparently released even in the absence of H2O2. To confirm this result the kinetics of Zn2+ release was measured directly using a higher Zn7MT-3 concentration (10 μM) (Fig. 1B, inset). Indeed, the absorption band of the Zn2+–zincon complex at 620 nm increased linearly with time. A similar Zn2+ release from Zn7MT-3 in the presence of increasing zincon concentrations has also been reported by Chen et al.17
To examine whether the Zn2+ release from Zn7MT-3 by H2O2 is due to a cysteine oxidation and not its removal by zincon, a mass spectrometry (ESI-MS) was applied. We analyzed the apoMT-3 form after the Zn2+ depletion of the holoprotein at acidic pH. Although no deconvoluted peaks could be obtained, the results suggest that cysteine oxidation occurred by adding H2O2 (Fig. 1C). The apoMT-3 form has a theoretical mass of 6927.1 Da. In the absence of H2O2, an average mass of 6928.0 Da was obtained. However, upon addition of H2O2 a shift to smaller average masses was seen, in agreement with the formation of disulfide bonds (loss of two protons leads to a decrease of mass by 2 Da). In addition, a broadening of the mass peak was also observed indicating a mass heterogeneity due to the formation of a varying number of disulfide bonds in different protein molecules. Consequently, the Zn7MT-3 structure is susceptible to oxidation by H2O2. Taken together, the results of zincon, ESI-MS, and the CysS–Zn2+ charge-transfer band absorption measurements all indicate that H2O2, through cysteine oxidation to disulfides, releases Zn2+ from Zn7MT-3 in a concentration dependent manner.
Next we used size-exclusion chromatography (SEC) to examine whether the disulfide bridges formed upon oxidation of Zn7MT-3 by H2O2 were intra- or intermolecular. In general, while in the case of intermolecular disulfide bridges the formation of dimers or higher polymers of Zn7MT-3 should be observed, in the case of intramolecular disulfide bridges a decrease of the hydrodynamic radius of ZnMT-3, due to a more compact disulfide bridged structure would be expected.20Fig. 1D shows the results of the SEC of Zn7MT-3 after a 1 day incubation with different concentrations of H2O2. The freshly prepared Zn7MT-3 eluted at about a 10.5 ml volume, a typical value for monomeric metal loaded Zn7MT-3 with an apparent molecular mass of about 22 kDa due to its ellipsoid shape.21 A small amount of ZnMT-3 dimers, eluting with a volume of 9.7 ml, was also observed in the control experiments as reported.20 Incubation of Zn7MT-3 with increasing H2O2 concentrations resulted in two effects. First, the monomeric peak shifted to a higher elution volume. The latter effect reflects a decrease in the apparent molecular mass of the protein consistent with the increase in structure compactness brought about by a disulfide formation and Zn2+ release.20 Second, an increase in size of the dimeric peak, in line with the formation of some intermolecular disulfide bridges, was also observed. However, since the monomeric peak even at 200 μM H2O2 was still much higher, this indicates that the disulfide bridges are predominantly formed intramolecularly. This conclusion is in agreement with the ESI-MS data (see above), where molecular masses consistent with predominately intramolecular disulfide bonds were obtained. Note that the apparent decrease in peak intensity seen in the elution profile of Zn7MT-3 at a higher H2O2 concentration does not reflect a protein loss, but is due mainly to the decrease in the molar extinction coefficient of the CysS–Zn2+ charge-transfer band upon protein oxidation (see above).
The above results indicate that cysteine oxidation in Zn7MT-3 by H2O2 results in a release of Zn2+ from the protein. Next we investigated whether the released Zn2+ can stimulate aggregation of the Aβ40 peptide. The aggregation process was followed by turbidimetry, ThT assay, and transmission electron microscopy. The turbidimetry is a simple, but crude assay to follow the formation of aggregates. The turbidity experiments were performed at 300 nm over 100 min and are summarized in Fig. 2A. Aβ40 alone showed a very small turbidity increase under the conditions used (Fig. 2A, thin solid line). In contrast, the Zn2+–Aβ40 complex, formed upon addition of 1 mole equivalent of Zn2+ solution, exhibited a fast and dramatic increase in turbidity (Fig. 2A, thick solid line), in agreement with the aggregation accelerating effect of Zn2+ described in the literature.9,22 Aβ40 in the presence of Zn7MT-3 and without H2O2 showed a small but significant turbidity increase compared to that of Aβ40 alone (Fig. 2A, dotted line). However, upon addition of H2O2 to the latter sample the turbidity increase was much higher (Fig. 2A, dashed line), but did not reach the intensity and rapidity of aggregation of the Zn2+–Aβ40 complex (Fig. 2A, thick solid line). The latter result indicates that the Zn2+ release from Zn7MT-3 with H2O2 results in an intermediate turbidity between Aβ40 alone and Zn2+–Aβ40. Furthermore, in accordance with the Zn2+ release from Zn7MT-3 in the absence of H2O2 (see above), the aggregation promoting effect has also been seen.
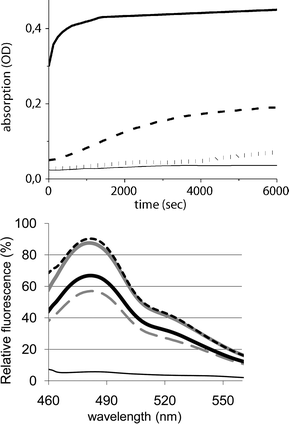 | ||
| Fig. 2 (Upper panel) Turbidity assay: Turbidity of Aβ40 was measured at 300 nm over 100 min (thin solid line), Zn-Aβ (thick solid), Aβ40 and Zn7MT-3 (dotted grey), and Aβ40, Zn7MT-3 and H2O2 (dashed black) Conditions: Aβ 30 μM, Zn7MT-3 20 μM, 100 μM H2O2, Hepes 20 mM, NaCl 20 mM, pH 7.4. (Lower panel) Thioflavin T fluorescence: Emission spectra of ThT (10 μM), excitation at 435 nm. ThT was added after sample incubation for 1 day to Aβ40 (gray dashed), Aβ and 1 equiv Zn2+ (gray solid), Aβ40 and Zn7MT-3 (black thick solid), Aβ40, Zn7MT-3 and H2O2 (black dotted), ThT alone (thin solid); Conditions: 30 μM Aβ40, 20 μM Zn7MT-3, 100 μM H2O2, 20 mM Hepes, 20 mM NaCl, at pH 7.4. | ||
The measurement of turbidity only provides information about the global aggregation state. In contrast, the fluorescence dye ThT is relatively specific for amyloid structures such as that of the fibrils of Aβ. The corresponding ThT fluorescence spectra were measured after 1 day of sample incubation (Fig. 2B). In this case, Aβ40 alone (Fig. 2B, gray dashed line) showed ThT fluorescence that increased upon binding of 1 mole equivalent of Zn2+ solution (Fig. 2B, gray solid line) in line with the Zn2+ amyloid promoting effect. Interestingly, although Zn2+ release from Zn7MT-3 with H2O2 resulted in an intermediate turbidity between Aβ40 alone and Zn2+–Aβ40 (see above), the corresponding ThT fluorescence (Fig. 2B, black dotted line) revealed a value closely similar to that of the Zn2+–Aβ40 complex prepared upon addition of Zn2+ solution, suggesting that the amounts of fibrilar structure are similar.¶ Moreover, since a considerable ThT fluorescence of aggregated Aβ40 was also seen in the presence of Zn7MT-3 without H2O2 (Fig. 2B, black solid line), this indicates that released Zn2+ under these conditions is also able to promote significantly the formation of Aβ40 fibrils (see below).
Transmission electron microscopy was used to investigate the Aβ40 aggregates formed under the different conditions (Fig. 3). The estimated amount of aggregates follows the order Zn2+–Aβ40 > Aβ40 plus Zn7MT-3 plus H2O2 > Aβ40 plus Zn7MT-3 > Aβ40 alone. These results parallel the turbidity measurements as expected, since turbidity measures the Aβ40 aggregation. However, the content of fibrillar structures reflects more the ThT fluorescence. Thus, the solution of Aβ40 and Zn7MT-3 in the presence and absence of H2O2 showed the highest content of fibrillar structures, explaining why the ThT fluorescence was as high as for the Zn2+–Aβ40 complex, although the latter form is more aggregated. This further suggests that while in the former case Aβ40 aggregates slowly form substantial amounts of fibrillar structures, in the latter case fast Zn2+ binding to Aβ40 results in more amorphous type aggregates.
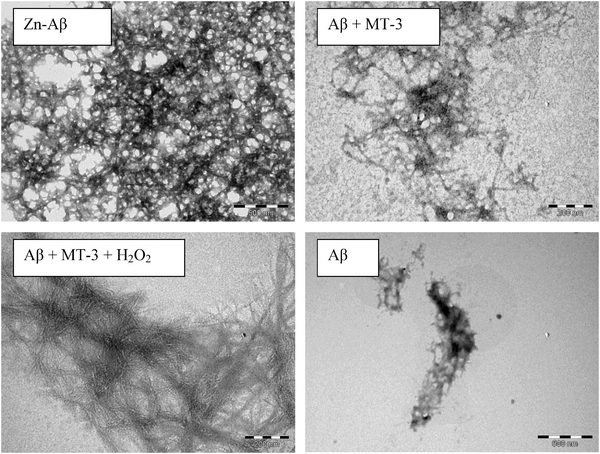 | ||
| Fig. 3 Transmission electron microscopy (negatively stained) of Aβ40, Zn2+–Aβ40, Aβ40 plus Zn7MT-3, and Aβ40, Zn7MT-3 plus H2O2 after 1 day incubation. Concentrations: 30 μM Aβ40, 30 μM ZnSO4, 20 μM Zn7MT-3, and 100 μM H2O2. | ||
In summary, the results presented above suggest that cysteine oxidation in Zn7MT-3 in the presence and absence of H2O2 leads to Zn2+ release, which in turn can induce aggregation of Aβ40. Moreover, the slow release of Zn2+ promotes the formation of amyloid-type fibrils in contrast to amorphous Aβ40 aggregates formed by the addition of Zn2+. As a consequence it is the rate of cysteine oxidation in MT-3 that regulates the assembly of Aβ40 aggregates and their morphology. The results are in line with the observation that zinc concentrations modulate the rate of assembly and the type of morphology of Aβ40 aggregates. (See e.g.ref. 8 and 23). This effect may have biological relevance since both Aβ and Zn7MT-3 have been linked to AD, oxidative stress and are likely to be co-localized in the brain. Zn7MT-3 might be involved in the protection of cells not only by its capacity for ROS scavenging,24 but also via Zn2+ release and subsequent binding to Aβ. However, at present it is not clear whether Zn2+ binding has a beneficial or rather detrimental effect on Aβ toxicity. Thus, the biological significance of the in vitro findings remains to be established.
Acknowledgements
This work was supported by the programme blanc NT09-488591 (Neurometals) of Agence national de la recherche (ANR) (P.F.) and Swiss National Science Foundation Grant 3100A0-111884 (M.V.). We would like to thank Cathy Claperols and Vincent Colliere (LCC Toulouse) for measuring mass spectrometry and transition electron microscopy, respectively. Dr Fatima Bousejra-El Garah (LCC) is acknowledged for measuring effect of H2O2 on ThT fluorescence.References
- C. J. Frederickson, Int. Rev. Neurobiol., 1989, 31, 145–238 CAS.
- P. A. Adlard and A. I. Bush, J. Alzheimers Dis., 2006, 10, 145–163.
- P. Zatta, D. Drago, S. Bolognin and S. L. Sensi, Trends Pharmacol. Sci., 2009, 30, 346–355 CrossRef CAS.
- A. Deshpande, H. Kawai, R. Metherate, C. G. Glabe and J. Busciglio, J. Neurosci., 2009, 29, 4004–4015 CrossRef CAS.
- D. G. Smith, R. Cappai and K. J. Barnham, Biochim. Biophys. Acta, Biomembr., 2007, 1768, 1976–1990 CrossRef CAS.
- J.-Y. Lee, T. B. Cole, R. D. Palmiter, S. W. Suh and J.-Y. Koh, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 7705–7710 CrossRef CAS.
- P. Faller and C. Hureau, Dalton Trans., 2009, 1080–1094 RSC.
- D. Noy, I. Solomonov, O. Sinkevich, T. Arad, K. Kjaer and I. Sagi, J. Am. Chem. Soc., 2008, 130, 1376–1383 CrossRef CAS.
- M. P. Cuajungco and K. Y. Faget, Brain Res. Rev., 2003, 41, 44–56 CrossRef CAS.
- E. Mocchegiani, C. Bertoni-Freddari, F. Marcellini and M. Malavolta, Prog. Neurobiol., 2005, 75, 367–390 CrossRef CAS.
- J. Hidalgo, M. Aschner, P. Zatta and M. Vašák, Brain Res. Bull., 2001, 55, 133–145 CrossRef CAS.
- S. G. Bell and B. L. Vallee, ChemBioChem, 2009, 10, 55–62 CrossRef CAS.
- Z. C. Ding, Q. Zheng, B. Cai, F. Y. Ni, W. H. Yu, X. C. Teng, Y. Gao, F. Liu, D. Chen, Y. Wang, H. M. Wu, H. Z. Sun, M. J. Zhang, X. S. Tan and Z. X. Huang, J. Inorg. Biochem., 2008, 102, 1965–1972 CrossRef CAS.
- B. A. Masters, C. J. Quaife, J. C. Erickson, E. J. Kelly, G. J. Froelick, B. P. Zambrowicz, R. L. Brinster and R. D. Palmiter, J. Neurosci., 1994, 14, 5844–5857 CAS.
- M. A. Lynes, K. Zaffuto, D. W. Unfricht, G. Marusov, J. S. Samson and X. Yin, Exp. Biol. Med. (Maywood), 2006, 231, 1548–1554 Search PubMed.
- M. Vašák, J. Trace Elem. Med. Biol., 2005, 19, 13–17 CrossRef CAS.
- Y. Chen, Y. Irie, W. M. Keung and W. Maret, Biochemistry, 2002, 41, 8360–8367 CrossRef CAS.
- Y. Irie and W. M. Keung, Biochem. Biophys. Res. Commun., 2001, 282, 416–420 CrossRef CAS.
- G. Meloni, V. Sonois, T. Delaine, L. Guilloreau, A. Gillet, J. Teissie, P. Faller and M. Vašák, Nat. Chem. Biol., 2008, 4, 366–372 CrossRef CAS.
- G. Meloni, P. Faller and M. Vašák, J. Biol. Chem., 2007, 282, 16068–16078 CrossRef CAS.
- P. Faller, D. W. Hasler, O. Zerbe, S. Klauser, D. R. Winge and M. Vašák, Biochemistry, 1999, 38, 10158–10167 CrossRef CAS.
- A. I. Bush, W. H. Pettingell, G. Multhaup, M. Paradis, J. P. Vonsattel, J. F. Gusella, K. Beyreuther, C. L. Masters and R. E. Tanzi, Science, 1994, 265, 1464–1467 CrossRef CAS.
- M. Innocenti, E. Salvietti, M. Guidotti, A. Casini, S. Bellandi, M. L. Foresti, C. Gabbiani, A. Pozzi, P. Zatta and L. Messori, J. Alzheimers Dis., 19, 1323–1329 Search PubMed.
- P. J. Thornalley and M. Vašák, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 1985, 827, 36–44 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials and Methods. See DOI: 10.1039/c0mt00027b |
| ‡ Present address: IPBS, 205, route de Narbonne, F-31077 Toulouse. |
| § Present address: Division of Chemistry and Chemical Engineering, and Howard Hughes Medical Institute, California Institute of Technology, 114-96, Pasadena, CA 91125, USA. |
| ¶ We verified that H2O2 does not affect the ThT fluorescence under the conditions used here. Thus the increase of ThT fluorescence in samples containing Aβ40, Zn7MT-3 and H2O2 can not be ascribed to a direct interaction of H2O2 with ThT. This supports the view that the increase in ThT fluorescence is due to increased Zn-release from Zn7MT-3 upon reaction with H2O2. |
| This journal is © The Royal Society of Chemistry 2010 |
