DOI:
10.1039/C003414B
(Paper)
Metallomics, 2010,
2, 407-411
Iron-binding and mobilization from ferritin by polypyridyl ligands
Received
19th February 2010
, Accepted 9th April 2010
First published on
6th May 2010
Abstract
Polypyridyl ligands were investigated for their ability to bind and mobilize iron(II) from ferritin in aqueous solution. Association constants with iron(II) (pFeII) were determined for the pentadentate ligands N4Py (pFeII = 14.4) and Bn-TPEN (pFeII = 13.7) using a competition method with the hexadentate ligand TPEN (pFeII = 14.6, 0.1 M KNO3). Ferrous complexes were formed using the polypyridyl ligands and ferritin as the sole iron source in the presence of reductant. The observed rates of iron mobilization from ferritin were dependent on reductant and were higher in the presence of ascorbate than dithiothreitol. TPEN, N4Py and Bn-TPEN demonstrated comparable and in some cases faster rates and higher levels of iron mobilization when compared to the iron(II) chelator 1,10-phenanthroline, particularly at low concentrations of chelator.
Introduction
Ferritin is a crucial protein in biology that stores and releases cellular iron in a controlled fashion.1 This 480 kDa complex consists of a spherical shell that aggregates iron in its core as ferrihydrite. In animals, two peptides are found within the 24 subunit ferritin complex, the L (light/liver) and H (heavy/heart) chains. While both subunits have been implicated in iron storage, only the heavy chain has been shown, in animals, to have ferroxidase activity.2 This subunit catalyzes the oxidation of ferrous ion to a diferric oxo bridged intermediate, the initial step in iron oxide core formation. At full capacity one ferritin complex can hold up to 4500 Fe atoms. Because ferritin constitutes a large percentage of the overall cellular iron it is generally considered to be a potential loci for obtaining iron from a cell. The generation of iron complexes using ligands and ferritin as a source of iron is well documented.1–25 However, polypyridyl ligands that bind tightly to Fe(II) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 fashion have not been investigated. Recent reports have implicated the gated pores of ferritin as the limiting factor in iron release.26 Later work found the rate of iron release to be affected by small peptides.16
1 fashion have not been investigated. Recent reports have implicated the gated pores of ferritin as the limiting factor in iron release.26 Later work found the rate of iron release to be affected by small peptides.16
Polypyridyl ligands shown in Fig. 1 have been used extensively in biological and biomimetic research. The hexadentate ligand TPEN is a membrane permeable chelator that is often described as “ZnII-specific” (pZnII = 18.0) even though it binds tightly to iron (pFeII = 14.6) and copper (pCuII = 20.6).27, 28 Recent studies where gene expression was monitored in E. coli after treatment with TPEN confirmed that the chelator caused the up-regulation of Fur-regulated transcripts and down-regulation of the cytoplasmic ferritin transcript, suggesting that TPEN lowered the intracellular concentration of iron in addition to zinc.29 As yet underrepresented in biological research, other polypyridyl ligands, including the pentadentate ligands Bn-TPEN and N4Py, have been used to produce iron(IV)-oxo species, also known as ferryls, that mimic the structure and reactivity of non-heme iron enzymes found in nature.30, 31 In addition to their ability to oxidize organic substrates,32–40 recent progress from our laboratory has shown that such biomimetic ferryls can be used to oxidize the amino acid backbone and side chains with substantial selectivity.41, 42 Despite all that is known about the polypyridyl ligands in Fig. 1, their ability to mobilize iron from ferritin has not been described. The present study is aimed at better understanding these ligands by (1) quantifying their association constants with iron and (2) gaining a better understanding of how these ligands interact with ferritin. The second study was undertaken because mobilizing iron from ferritin is one way to deplete intracellular iron.
 |
| | Fig. 1 Penta- and hexadentate polypyridyl ligands. | |
Materials and methods
Materials
TPEN, Bn-TPEN and N4Py were synthesized according to literature methods.43, 44,45 1,10-Phenanthroline (Phen), ferrous ammonium sulfate, and horse spleen ferritin were purchased from Sigma-Aldrich, 99+%. 2-(N-morpholino)ethanesulfonic acid (MES) and potassium nitrate purchased from Fisher 98+%. Potassium phthalate was purchased from Mallinckrodt Chemicals 99+%. Dithiothreitol was purchased from Acros chemicals 99+%. All chemicals were used as received.
Association constant determination
Association constants were elucidated spectrophotometrically using competition experiments with equal concentrations of TPEN, Fe(II) and the ligand of interest. All experiments were performed under anaerobic conditions in 0.1 M KNO3, in order to compare measurements directly with previous studies where the association constant of [FeII(TPEN)]2+ was determined (pFeII = 14.6).27 Argon was bubbled through each solution for 15 min prior to use and solutions were kept anaerobic for the duration of the experiment. Iron(II) association constants with N4Py and Bn-TPEN (denoted as L) were elucidated by the ligand competition method, using TPEN as the competing ligand in both cases. Three solutions were prepared: (1) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of Fe(II) and TPEN; (2) 1
1 molar ratio of Fe(II) and TPEN; (2) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of L and Fe(II); (3) 1
1 ratio of L and Fe(II); (3) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of L, TPEN and Fe(II). These solutions were used to obtain the molar absorptivity for the respective ferrous complexes at a series of three wavelengths (Fig. 2). A least-squares analysis of eqn (1) was performed using measurements from solution 3 and calculated values from the standards. The minimum value was solved for using Microsoft Excel® Solver function. This analysis produced [FeII(TPEN)]2+and [FeII(L)]2+which was used to solve [TPEN] and [L] (eqn (2) and eqn (3)). The equilibrium constant (Keq) and the [FeII(L)]2+ association constant (KFe(L)) were solved using eqn (4) and (5), respectively.
1 mixture of L, TPEN and Fe(II). These solutions were used to obtain the molar absorptivity for the respective ferrous complexes at a series of three wavelengths (Fig. 2). A least-squares analysis of eqn (1) was performed using measurements from solution 3 and calculated values from the standards. The minimum value was solved for using Microsoft Excel® Solver function. This analysis produced [FeII(TPEN)]2+and [FeII(L)]2+which was used to solve [TPEN] and [L] (eqn (2) and eqn (3)). The equilibrium constant (Keq) and the [FeII(L)]2+ association constant (KFe(L)) were solved using eqn (4) and (5), respectively.| | | Atotal = ε[Fe(TPEN)] + ε[Fe(L)] | (1) |
| | | CTPEN = [Fe(TPEN)] + [TPEN] | (2) |
| | | Keq = ([Fe(TPEN)][L])/([Fe(L)][TPEN]) | (4) |
| | | Keq = KFe(TPEN)/KFe(L) | (5) |
Iron mobilization from ferritin
The concentration of iron in the ferritin stock solution was determined by a literature method using Phen, potassium phthalate and ferrous ammonium sulfate.46 Absorbance was measured at 398 and 512 nm for three trials as well as a set of six known solutions to generate a standard curve. Total iron content in all trials presented here was determined to be 2.2 mM. Ferritin mobilization trials were prepared by adding 100 μL of ligand solution with 100 μL of a solution with ferritin and reductant. UV-Vis spectra of these solutions were measured on a Tecan Infinite M200 Microplate reader using a Corning 96 well plate. Spectra were obtained every 5–15 min over a four-hour period. Ligand stock solutions (2 or 8 mM) were prepared by dissolving the solid ligand in the desired volume using 100 mM MES buffer (pH 6.5). Ascorbate or dithiothreitol (DTT) stock solutions were prepared immediately prior to each use by dissolving in MES buffer to the desired concentration (1–25 mM). The ferritin stock solution (19 μL) was added to a 1 mL aliquot of these solutions. The resulting solutions were then added to 100 μL of the ligand solution. Rates of mobilization were determined using the initial slope method where data over the first 5% of the reaction were considered. Percentages for iron mobilzation were calculated by determining the concentration of FeIIL and comparing that with the total concentration of Fe at 2.2 mM.
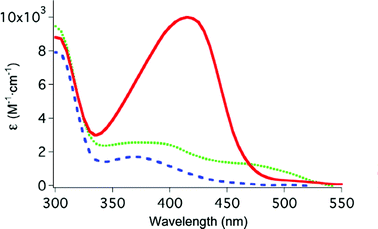 |
| | Fig. 2 UV-vis spectra of ferrous complexes of TPEN ( ), N4Py ( ), N4Py ( ) and Bn-TPEN ( ) and Bn-TPEN ( ). Concentration = 1 mM, 0.1 M KNO3. ). Concentration = 1 mM, 0.1 M KNO3. | |
Results and discussion
Ferrous ion association constants
Before the iron mobilization studies with ferritin were conducted, association constants were determined spectrophotometrically for ferrous complexes of the pentadentate ligands Bn-TPEN and N4Py in order to correlate the strength of iron-binding with the mobilization data. Spectral data for the iron complexes are shown in Fig. 2. Ferrous complexes of the ligands TPEN and Bn-TPEN showed single absorptions at 416 nm (ε = 10600 M−1 cm−1) and 385 nm (ε = 1670 M−1 cm−1) respectively. The ferrous complex of N4Py showed two distinct absorptions at 390 nm (ε = 2750 M−1 cm−1) and 450 nm (ε = 1500 M−1 cm−1), with the second band being less intense than the first. The competition method was used with the hexadentate ligand TPEN whose association constant was predetermined (pFeII = 14.6, 0.1 M KNO3). Association constants for both N4Py and Bn-TPEN were found to fall within one order of magnitude of TPEN (Table 1), indicating that loss of one coordinating group in the ligand scaffold in moving from hexadentate to pentadentate did not lower the association constant dramatically. These results are in direct contrast with the association constant observed with Fe(II) and the tetradentate ligand TPA (tris-(2-pyridylmethyl)amine) under the same conditions, which is significantly lower (pFeII = 8.7) than Bn-TPEN, N4Py and TPEN (pFeII = 13.7–14.6).28
Table 1 Association constants for iron(II)-chelate complexes
| Ligand |
pFeII |
|
Average of three runs, errors as standard deviations are given in parentheses.
Data from literature.27
Data from literature.47
|
| TPEN |
14.6b |
| Bn-TPEN |
13.7a (1) |
| N4Py |
14.4a (1) |
| Phen |
21.0c |
Rate of iron mobilization
The release of iron from the ferritin core is known to be dependant on reductant concentration.1,17,23 Of the various reductants, ascorbate has been used frequently for iron release from ferritin.18,20 Ascorbate and dithiothreitol (DTT) were chosen over more naturally relevant flavanoids due to relative rate of reduction.18,19 Our data indicate that rates of iron mobilization from the ferritin core using ascorbate as the reducant for the various chelators are similar to those reported in the past for ligands 2,2-bipyridine and Phen (Fig. 3).18,20 The rates of mobilization approach saturation at approximately 15 mM ascorbate. Of the four ligands, TPEN shows the highest rates of iron mobilization between 5–25 mM ascorbate at a ligand concentration of 1 mM. Rates observed in the presence of DTT are slower than in the presence of ascorbate and show a similar pattern of saturation at higher reductant concentrations. At full saturation, rates of iron mobilization are approximately 50% lower for TPEN in the presence of DTT when compared with ascorbate, whereas for Bn-TPEN, N4Py and Phen the rates are roughly 30% lower. In both cases TPEN showed superior rates over Phen.
Percentage iron mobilized
The percentage of the total iron mobilized by the polypyridyl ligands as ferrous complexes was comparable with data reported in the literature (Table 2).18 The amount of total iron mobilized after 5 h was slightly higher for TPEN than Phen using ascorbate as the reductant, suggesting that TPEN was a superior chelator when compared to Phen at the same ligand concentration (1 mM). The pentadentate ligands Bn-TPEN and N4Py showed lower percentages of iron mobilized in the presence of ascorbate, whereas with N4Py the percentage was higher than that observed with TPEN and Phen using DTT as the reductant. The total percentages of iron mobilized was higher for TPEN and Phen in the presence of ascorbate than in the presence of DTT, consistent with the rate data shown in Fig. 3. The ligand N4Py showed total percentages equal within error in the presence of ascorbate and DTT. The percentage of iron mobilized by Bn-TPEN was significantly lower than the other chelators in the presence of DTT. We have observed that the ferrous complex of Bn-TPEN is less stable under aerobic conditions than the ferrous complex of N4Py, which may account for the lower percentage observed under the reaction conditions.
Table 2 Percentage of total iron liberated from ferritin in the presence of 15 mM reductant and 1 mM ligand
| Ligand |
% Fe as FeIIL after 5 ha |
|
Data represent the average of three runs, errors as standard deviations are given.
|
|
|
Asc |
DTT |
| TPEN |
6.6 ± 0.5 |
3.4 ± 0.3 |
| Phen |
5.8 ± 0.6 |
3.4 ± 0.4 |
| N4Py |
4.3 ± 0.4 |
4.2 ± 0.4 |
| Bn-TPEN |
4.2 ± 0.4 |
2.5 ± 0.3 |
Ligand dependence
Rates of iron mobilization were compared under varying ligand concentrations with the chelators TPEN and Phen in the presence of 10 mM ascorbate (Fig. 4). Both ligands demonstrate a full saturation of the rate, approximately 0.7 μmole/min, at a ligand concentration of 500 μM or higher under these conditions. However the rate for TPEN reaches full saturation at a significantly lower concentration, approximately 10 μM, when compared with Phen, which becomes saturated above 200 μM. Although data for the ligands N4Py and Bn-TPEN display a similar saturation behavior they are not presented because they are more scattered, due to large experimental errors associated with observing formation of the complexes with low extinction coefficients at low concentrations. The discrepancy between TPEN and Phen at low concentrations of the chelators confirms that TPEN can mobilize iron at a faster rate than Phen under the right conditions, despite the fact that Phen has an overall association constant that is over six orders of magnitude higher then TPEN (Table 1). Considering that TPEN and Phen reached the same maximum velocity at high ligand concentrations (1 mM), it is unlikely that TPEN is interacting with ferritin and opening its gated pores as compared with Phen. Therefore, the faster rates for iron mobilization by TPEN over Phen may be due to a faster or less reversible binding to ferrous ion by the hexadentate ligand TPEN as compared with the bidentate ligand Phen, which needs to react with two additional equivalents of Phen before the stable, low-spin ferrous complex [FeII(Phen)3]2+ is obtained. Interestingly, the concentration of TPEN used in cell studies for the selective chelation of Zn(II) (ca. 5 μM) is in the same range as where TPEN reaches full saturation for iron mobilization from ferritin. Therefore, the possibility that TPEN is mobilizing iron from ferritin in addition to chelating zinc in biological studies should also be considered.
 |
| | Fig. 3 Calculated rates of iron mobilization from ferritin (μmole/min) using the polypyridyl ligands TPEN ( ), Bn-TPEN ( ), Bn-TPEN ( ), N4Py ( ), N4Py ( ) and 1,10-phenanthroline ( ) and 1,10-phenanthroline ( ) in the presence of the reductants ascorbate (graph a) or dithiothreitol (graph b). Conditions: 100 mM MES buffer, pH = 6.5, NaOH, 1 mM ligand, 1 μM Horse spleen ferritin, 2.2 mM total Fe. Each data point represents the average of three runs, with error bars indicating the standard deviation of the data set. ) in the presence of the reductants ascorbate (graph a) or dithiothreitol (graph b). Conditions: 100 mM MES buffer, pH = 6.5, NaOH, 1 mM ligand, 1 μM Horse spleen ferritin, 2.2 mM total Fe. Each data point represents the average of three runs, with error bars indicating the standard deviation of the data set. | |
![Calculated μmole/min of mobilization of ferritin using UV-Vis spectrum of [FeII(L)]2+ where L is polypyridyl ligands TPEN () and 1,10-phenanthroline (). Trials conditions were 100 mM MES pH6·NaOH, 10 mM ascorbate, 1 μM Horse spleen ferritin, 2.2 mM total iron. Each data point represents the average of three runs, with error bars indicating the standard deviation of the data set.](/image/article/2010/MT/c003414b/c003414b-f4.gif) |
| | Fig. 4 Calculated μmole/min of mobilization of ferritin using UV-Vis spectrum of [FeII(L)]2+ where L is polypyridyl ligands TPEN ( ) and 1,10-phenanthroline ( ) and 1,10-phenanthroline ( ). Trials conditions were 100 mM MES pH6·NaOH, 10 mM ascorbate, 1 μM Horse spleen ferritin, 2.2 mM total iron. Each data point represents the average of three runs, with error bars indicating the standard deviation of the data set. ). Trials conditions were 100 mM MES pH6·NaOH, 10 mM ascorbate, 1 μM Horse spleen ferritin, 2.2 mM total iron. Each data point represents the average of three runs, with error bars indicating the standard deviation of the data set. | |
Conclusion
Data presented herein indicate that polypyridyl ligands bind iron tightly and mobilize iron from ferritin under reducing conditions. The pentadentate ligands N4Py and Bn-TPEN have large Fe2+ association constants, which correspond to dissociation constants in the pM-fM range. All of the ligands examined in this study mobilize iron from ferritin under reducing conditions with rates that are competitive with the standard ferrous chelator Phen. In the case of TPEN, the rate of iron mobilization reaches saturation at a lower concentration than Phen and in the same range that it is typically used at in cell studies. Therefore these findings corroborate other data that TPEN is capable of acting as potent iron sequestering agent. While TPEN forms more stable complexes with ZnII, CuII and NiII, TPEN also forms strong complexes with FeII which should not be overlooked.
Acknowledgements
We would like to thank David Rorabacher and Timothy Stemmler for helpful discussions and Wayne State University for its generous support of this research.
References
- E. C. Theil, X. S. Liu and T. Tosha, Inorg. Chim. Acta, 2008, 361, 868–874 CrossRef CAS.
- A. S. Pereira, P. Tavares, S. G. Lloyd, D. Danger, D. E. Edmondson, E. C. Theil and B. H. Huynh, Biochemistry, 1997, 36, 7917–7927 CrossRef CAS.
- R. F. Boyer, T. W. Grabill and R. M. Petrovich, Anal. Biochem., 1988, 174, 17–22 CAS.
- D. L. Jacobs, G. D. Watt, R. B. Frankel and G. C. Papaefthymiou, Biochemistry, 1989, 28, 1650–1655 CrossRef CAS.
- R. Topham, M. Goger, K. Pearce and P. Schultz, Biochemical J., 1989, 261, 137–143 CAS.
- O. Bando and K. Aki, J. Biochem., 1991, 109, 450–454.
- H. F. Bienfait and M. L. Van den Briel, Biochim. Biophys. Acta, Gen. Subj., 1980, 631, 507–510 Search PubMed.
- R. J. Ulvik and I. Romslo, Biochim. Biophys. Acta, Bioenerg., 1981, 635, 457–469 CrossRef CAS.
- H. P. Monteiro and C. C. Winterbourn, Arch. Biochem. Biophys., 1989, 271, 536–545 CrossRef CAS.
- H. P. Montiero, G. F. Vile and C. C. Winterbourn, Free Radical Biol. Med., 1989, 6, 587–591 CrossRef.
- S. Ahmad, V. Singh and G. S. Rao, Chem.-Biol. Interact., 1995, 96, 103–111 CrossRef CAS.
- S. D. Aust, Toxicol. Lett., 1995, 82–83, 941–944 CrossRef CAS.
- S. L. Baader, E. Bill, A. X. Trautwein, G. Bruchelt and B. F. Matzanke, FEBS Lett., 1996, 381, 131–134 CrossRef CAS.
- M. E. M. Rocha, A. M. D. C. Ferreira and E. J. H. Bechara, Free Radical Biol. Med., 2000, 29, 1272–1279 CrossRef CAS.
- R. Agrawal, P. K. Sharma and G. S. Rao, Toxicology, 2001, 168, 223–230 CrossRef CAS.
- X. S. Liu, L. D. Patterson, M. J. Miller and E. C. Theil, J. Biol. Chem., 2007, 282, 31821–31825 CrossRef CAS.
- S. Sirivech, E. Frieden and S. Osaki, Biochem. J., 1974, 143, 311–315 CAS.
- J. Dognin and R. R. Crichton, FEBS Lett., 1975, 54, 234–236 CrossRef CAS.
- F. Felix, L. Jean-Pierre, R. C. Robert and S. Walter, Eur. J. Biochem., 1985, 152, 167–172 CAS.
- R. F. Boyer and C. J. McCleary, Free Radical Biol. Med., 1987, 3, 389–395 CrossRef CAS.
- M. C. Brady, K. S. Lilley, A. Treffry, P. M. Harrison, R. C. Hider, P. D. Taylor and R. C. Hider, J. Inorg. Biochem., 1989, 35, 9–22 CrossRef CAS.
- J. P. Laulhere and J. F. Briat, Biochem. J., 1993, 290, 693–699 CAS.
- A. Treffry, C. Hawkins, J. M. Williams, J. R. Guest and P. M. Harrison, JBIC, J. Biol. Inorg. Chem., 1996, 1, 49–60 CrossRef CAS.
- K. Sakurai, A. Nabeyama and Y. Fujimoto, BioMetals, 2006, 19, 323–333 Search PubMed.
- P. M. Harrison and P. Arosio, Biochim. Biophys. Acta, Bioenerg., 1996, 1275, 161–203 CrossRef.
- H. Takagi, D. Shi, Y. Ha, N. M. Allewell and E. C. Theil, J. Biol. Chem., 1998, 273, 18685–18688 CrossRef CAS.
- G. Anderegg, E. Hubmann, N. G. Podder and F. Wenk, Helv. Chim. Acta, 1977, 60, 123–140 CrossRef CAS.
- K. L. Haas and K. J. Franz, Chem. Rev., 2009, 109, 4921–4960 CrossRef CAS.
- T. K. Sigdel, J. A. Easton and M. W. Crowder, J. Bacteriol., 2006, 188, 6709–6713 CrossRef CAS.
- L. Que, Acc. Chem. Res., 2007, 40, 493–500 CrossRef CAS.
- W. Nam, Acc. Chem. Res., 2007, 40, 522–531 CrossRef CAS.
- M. Klopstra, R. Hage, R. M. Kellogg and B. L. Feringa, Tetrahedron Lett., 2003, 44, 4581–4584 CrossRef CAS.
- K. Nehru, Y. Jang, S. Oh, F. Dallemer, W. Nam and J. Kim, Inorg. Chim. Acta, 2008, 361, 2557–2561 CrossRef CAS.
- Y.-M. Lee, H. Kotani, T. Suenobu, W. Nam and S. Fukuzumi, J. Am. Chem. Soc., 2008, 130, 434–435 CrossRef CAS.
- Y. J. Jeong, Y. Kang, A.-R. Han, Y.-M. Lee, H. Kotani, S. Fukuzumi and W. Nam, Angew. Chem., Int. Ed., 2008, 47, 7321–7324 CrossRef CAS.
- K. Nehru, M. S. Seo, J. Kim and W. Nam, Inorg. Chem., 2007, 46, 293–298 CrossRef CAS.
- K. Nehru, Y. K. Jang, M. S. Seo, W. Nam and J. Kim, Bull. Korean Chem. Soc., 2007, 28, 843–846 CAS.
- S. P. de Visser, K. Oh, A.-R. Han and W. Nam, Inorg. Chem., 2007, 46, 4632–4641 CrossRef CAS.
- N. Y. Oh, Y. Suh, M. J. Park, M. S. Seo, J. Kim and W. Nam, Angew. Chem., Int. Ed., 2005, 44, 4235–4239 CrossRef CAS.
- J. Kaizer, E. J. Klinker, N. Y. Oh, J.-U. Rohde, W. J. Song, A. Stubna, J. Kim, E. Münck, W. Nam and L. Que, Jr., J. Am. Chem. Soc., 2004, 126, 472–473 CrossRef CAS.
- A. R. Ekkati and J. J. Kodanko, J. Am. Chem. Soc., 2007, 129, 12390–12391 CrossRef CAS.
- A. I. Abouelatta, A. A. Campanali, A. R. Ekkati, M. Shamoun, S. Kalapugama and J. J. Kodanko, Inorg. Chem., 2009, 48, 7729–7739 CrossRef CAS.
- H. Tang, N. Arulsamy, M. Radosz, Y. Shen, N. V. Tsarevsky, W. A. Braunecker, W. Tang and K. Matyjaszewski, J. Am. Chem. Soc., 2006, 128, 16277–16285 CrossRef CAS.
- L. Dueland, R. Hazell, C. J. McKenzie, L. P. Nielson and H. Toftlund, J. Chem. Soc., Dalton Trans., 2001, 152–156 RSC.
- G. Roelfes, M. Lubben, S. W. Leppard, E. P. Schudde, R. M. Hermant, R. Hage, E. C. Wilkinson, L. Que and B. L. Feringa, J. Mol. Catal. A: Chem., 1997, 117, 223–227 CrossRef CAS.
- W. Lovenberg, B. B. Buchanan and J. C. Rabinowitz, J. Biol. Chem., 1963, 238, 3899–3913.
-
A. E. Martell and R. M. Smith, Critical stability constants, Plenum Press, New York, 1974 Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 fashion have not been investigated. Recent reports have implicated the gated pores of ferritin as the limiting factor in iron release.26 Later work found the rate of iron release to be affected by small peptides.16
1 fashion have not been investigated. Recent reports have implicated the gated pores of ferritin as the limiting factor in iron release.26 Later work found the rate of iron release to be affected by small peptides.16

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of Fe(II) and TPEN; (2) 1
1 molar ratio of Fe(II) and TPEN; (2) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of L and Fe(II); (3) 1
1 ratio of L and Fe(II); (3) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of L, TPEN and Fe(II). These solutions were used to obtain the molar absorptivity for the respective ferrous complexes at a series of three wavelengths (Fig. 2). A least-squares analysis of eqn (1) was performed using measurements from solution 3 and calculated values from the standards. The minimum value was solved for using Microsoft Excel® Solver function. This analysis produced [FeII(TPEN)]2+and [FeII(L)]2+which was used to solve [TPEN] and [L] (eqn (2) and eqn (3)). The equilibrium constant (Keq) and the [FeII(L)]2+ association constant (KFe(L)) were solved using eqn (4) and (5), respectively.
1 mixture of L, TPEN and Fe(II). These solutions were used to obtain the molar absorptivity for the respective ferrous complexes at a series of three wavelengths (Fig. 2). A least-squares analysis of eqn (1) was performed using measurements from solution 3 and calculated values from the standards. The minimum value was solved for using Microsoft Excel® Solver function. This analysis produced [FeII(TPEN)]2+and [FeII(L)]2+which was used to solve [TPEN] and [L] (eqn (2) and eqn (3)). The equilibrium constant (Keq) and the [FeII(L)]2+ association constant (KFe(L)) were solved using eqn (4) and (5), respectively.
 ), N4Py (
), N4Py ( ) and Bn-TPEN (
) and Bn-TPEN ( ). Concentration = 1 mM, 0.1 M KNO3.
). Concentration = 1 mM, 0.1 M KNO3.
 ), Bn-TPEN (
), Bn-TPEN ( ), N4Py (
), N4Py ( ) and 1,10-phenanthroline (
) and 1,10-phenanthroline ( ) in the presence of the reductants ascorbate (graph a) or dithiothreitol (graph b). Conditions: 100 mM MES buffer, pH = 6.5, NaOH, 1 mM ligand, 1 μM Horse spleen ferritin, 2.2 mM total Fe. Each data point represents the average of three runs, with error bars indicating the standard deviation of the data set.
) in the presence of the reductants ascorbate (graph a) or dithiothreitol (graph b). Conditions: 100 mM MES buffer, pH = 6.5, NaOH, 1 mM ligand, 1 μM Horse spleen ferritin, 2.2 mM total Fe. Each data point represents the average of three runs, with error bars indicating the standard deviation of the data set.![Calculated μmole/min of mobilization of ferritin using UV-Vis spectrum of [FeII(L)]2+ where L is polypyridyl ligands TPEN () and 1,10-phenanthroline (). Trials conditions were 100 mM MES pH6·NaOH, 10 mM ascorbate, 1 μM Horse spleen ferritin, 2.2 mM total iron. Each data point represents the average of three runs, with error bars indicating the standard deviation of the data set.](/image/article/2010/MT/c003414b/c003414b-f4.gif)
 ) and 1,10-phenanthroline (
) and 1,10-phenanthroline ( ). Trials conditions were 100 mM MES pH6·NaOH, 10 mM ascorbate, 1 μM Horse spleen ferritin, 2.2 mM total iron. Each data point represents the average of three runs, with error bars indicating the standard deviation of the data set.
). Trials conditions were 100 mM MES pH6·NaOH, 10 mM ascorbate, 1 μM Horse spleen ferritin, 2.2 mM total iron. Each data point represents the average of three runs, with error bars indicating the standard deviation of the data set.