R161, K452 and R460 residues are vital for metal–citrate complex transport in CitSc from Streptomyces coelicolor†
Joshua J.
Lensbouer
,
Qi Wen
Li
,
Maggie
Estlinbaum
and
Robert P.
Doyle
*
Department of Chemistry, Syracuse University, Syracuse, New York, 13244-4200, USA. E-mail: rpdoyle@syr.edu
First published on 17th February 2010
Abstract
Recent discoveries have been made that demonstrate Gram-positive bacteria can transport metal–citrate complexes through the CitMHS family of proteins in symport with H+ ions. The CitMHS family of transporters investigated to date have the ability to selectively transport only certain metal–citrate complexes. Despite sharing amino acid sequence similarity as high as 73%; predicting what complexes are transported remains difficult. The iron–citrate transporter from Streptomyces coelicolor has been mutated at three postulated critical sites (R161, K452 and R460) based on activity modeling against the LacY permease. All three mutants eliminate or greatly reduce uptake of metal–citrate complexes tested. The implications of this are discussed.
Introduction
The CitMHS family of proteins belongs within the ion transporter (IT) superfamily.1 Members of this superfamily have been demonstrated only in Gram-positive bacteria to date (see Table 1) and facilitate transport of citrate in complex with metal ions. While there has been extensive research conducted into uncomplexed ‘free’ citrate transport across membranes,2–4 there has been a relative dearth of research into membrane proteins that can transport metal–citrate complexes in an energy independent manner.5–8 This is in part because such metal–citrate uptake systems were not clarified until the ground breaking studies of Lolkema in Bacillus subtilis.5 Lolkema demonstrated that CitM from B. subtilis, designated here as CitBs1, transported citrate in complex with Mg2+, Ni2+, Mn2+, Co2+ and Zn2+ but not in complex with Ca2+, Ba2+ and Sr2+.5 CitH, redesignated here as CitBs2, also from B. subtilis, transported citrate complexed to Ca2+, Ba2+ and Sr2+ but not Mg2+, Ni2+, Mn2+, Co2+ and Zn2+.5 Clearly, the group of metal ions transported by CitBs1 includes the smaller cations, with a Pauling radius of less than ∼0.80 Å.| Bacteria | Protein | Transports |
|---|---|---|
| Bacillus subtilis | CitBs1 | Mg2+, Ni2+, Mn2+, Co2+, Zn2+ |
| Bacillus subtilis | CitBs2 | Ca2+, Ba2+, Sr2+ |
| Streptococcus mutans | CitSm | Fe3+, Mn2+ |
| Enterococcus faecalis | CitEf | Ca2+ |
| Streptomyces coelicolor | CitSc | Fe3+, Ca2+, Ba2+, Pb2+ |
The ions transported by CitBs2 of B. subtilis have radii larger than 0.98 Å. More recently, the work of Cvitkovitch in Streptococcus mutans and Doyle in Streptomyces coelicolor has further expanded this field.6,8 Cvitkovitch et al. functionally characterized a CitBs1 homolog from Streptococcus mutans.6 In this instance citrate complexed to Fe3+ and Mn2+ was transported, while Ca2+, Mg2+ and Ni2+ were not. Cvitkovitch in fact states that iron is the most efficient cofactor for citrate uptake in S. mutans. This suggests the intriguing possibility that, given S. mutans is considered a major etiological agent of dental caries and oral cancer, it may be using the system to access essential iron. Given members of the CitMHS family are postulated in bacteria such as Bacillus anthracis, Neiserria meningitides and Cornybacterium diphtheriae, this has great significance to the medical community.
A fourth system characterized in native membranes is that of the transporter in Enterococcus faecalis.7 High amino acid sequence similarity (73%) to the sequence of CitSm led researchers to believe, as with CitSm, it could be an iron transporter. Instead, the system was shown to be a CitBs2 (B. subtilis) homolog, transporting larger ionic radii metals such as calcium. The transporter of S. mutans itself had been predicted to be a transporter for Mg2+. This unpredictability clearly demonstrates our limited understanding of these systems. Comparative amino acid sequence identity, while a good predictor of the presence of a CitMHS family member, clearly does not yet allow us to predict metal cofactor preference.
To address this problem we must first understand the complex uptake and compare/contrast it to ‘free’ citrate transport systems. To do this, bioinformatics was used to predict a secondary structure and potentially important amino acids for citrate recognition in CitSc. Additionally, we have begun mutagenesis studies using these predictions, and the preliminary results are reported herein.
Experimental
Bacteria strains and growth conditions
E. coli JW4251 ΔfecA758 cells were obtained from the Molecular Cellular and Developmental Biology Department at Yale University (New Haven, CT). JW4251 colonies were selected and grown overnight in 5 mL of Luria-Bertani (LB) broth containing 35 μg/mL of kanamycin at 37 °C and 250 rpm. Cells harbouring pET-25b(+) cells containing the CitSc, K452C, R460C, or R161A mutant were grown in LB broth containing 35 μg/mL kanamycin and 50 μg/mL ampicillin.Media systems
Buffer and broth ingredients were purchased from Sigma-Aldrich, Becton Dickerson and Company, or Merck and were of biological grade. [14C]-Citrate was purchased from MP Biomedical at 50–100 mCi/mmol. Metal salts were purchased from Sigma-Aldrich and were of 99% purity or higher. DNA was isolated using the Promega Wizard Plus SV miniprep kit. Buffers and broths were made using water that was distilled and deionized to 18.6 MΩ using a Barnstead Diamond ultrapurification system. Chelex was added to all buffers at 15 g/L, and the resulting suspension was stirred overnight. The Chelex was then removed by vacuum filtration. This was to ensure the removal of metal ion impurities in the buffers. All cells were incubated in a Barnstead MaxQ 5000 shaker with a digital temperature display. Antibiotics came from EMD or Sigma-Aldrich. The pUC18 and pET-25b(+) vectors were purchased from Novagen. All primers were ordered from Integrated DNA Technologies (Coralville, IA). Cells were centrifuged with a Sorvall Legend RT tabletop centrifuge at 4000 rpm for 10 min at 25 °C. Cloning was performed with a Techne TC-312 thermal cycler. All restrictive enzymes were purchased from or provided pro gratis by Promega (Madison, WI). Scintillation fluid used was PerkinElmer Ultima Gold. Scintillation vials were purchased from Laboratory Product Sales and VWR. All radiation samples were analyzed using a PerkinElmer tri-carbon 2900 TR liquid scintillation analyzer. Culture density was determined by optical density (600 nm) measurements using a Cary 50 Bio UV-visible-light spectrophotometer in a 1 ml quartz cuvette (Sigma-Aldrich).Cloning of a putative ORF for CitSc
The S. coelicolor A3(2) cosmid St10A9 containing the open reading frame (ORF) encoding CitSc was obtained from the John Innes Centre, Norwich, United Kingdom. The ORF was cloned by PCR using Deep Vent polymerase (New England Biolabs) and the following primers:Forward
5′-GCACGGATCCATGCTGACCATCCTCGG-3′
Reverse
5′-CTGCAAGCTTTCAGATGATGCCGAACAGG-3.
PCR conditions used were as follows: 34 cycles of denaturing at 95 °C for 1 min, primer annealing at 61 °C for 1 min, and PCR at 74 °C for 1.5 min. The forward primer introduced a BamHI restrictive site upstream of the ATG start codon of CitSc. The reverse primer introduced a HindIII site immediately downstream of the stop codon. The PCR product was purified using electrophoresis on a 1% agarose gel and excised using the Omega-biotech DNA gel extraction kit. Following purification the DNA was digested with BamHI and HindIII, purified as mentioned above, and ligated (T4 ligase, 16 °C, overnight) into the pUC18 plasmid, which had previously been digested with BamHI, HindIII, and calf intestinal phosphatase. The resulting plasmid was designated pUC18-CitSc. The correct insertion was confirmed by sequencing performed at the SUNY Upstate Medical University’s DNA Core Facility, Syracuse, NY. The pUC18-CitSc plasmid was chemically transformed (30 min at 4 °C, 45 s at 42 °C, 2 min on ice and recovery for 1 h in SOC broth at 37 °C) into DH5α E. coli cells.
Mutant construction
With the pUC18_CitSc construct PCR was used to introduce point mutations at the K452 and R460 codons. Both amino acids were changed to cysteine resulting in the K452C and R460C mutants. The mutations were introduced using the following primers:K452C forward
5′-CATGGCCTGCGTCGAGTTC-3′
K452C reverse
5′-AACTCGACGCAGGCCATGCCG-3′
R460C forward
5′-CGACCACACGTGCTTCGTG-3′
R460C reverse
5′-CACCACGAAGCACGTGTG-3′.
Taq polymerase (New England Biolabs) was used. PCR conditions were as follows: 14 cycles of denaturing at 95 °C for 1 min, primer annealing at 61 °C for 1 min, and PCR at 74 °C for 4.5 min. The PCR product was digested with DpnI to remove methylated DNA template and transformed into DH5α E. coli cells. Colonies were selected and the plasmid DNA isolated and sequenced. Mutant constructs and CitSc were digested out of the pUC18 vector with BamHI and HindIII and ligated into pET-25b(+) (T4 ligase, 16 °C overnight), which had previously been digested with BamHI, HindIII, and calf intestinal phosphatase. pET-25b(+) inserts a pelB sequence at the N-terminus of CitSc and mutants for greater membrane insertion. Ligations were chemically transformed into DH5α E. coli cells using the protocol already described. pET-25b(+)_CitSc, pET-25b(+)_K452C, and pET-25b(+)_R460C vectors were isolated and sequenced. pET-25b(+)_CitSc, pET-25b(+)_K452C, and pET-25b(+)_R460C vectors were transformed into JW4251 E. coli cells for [14C]-citrate transport studies. Mutant R161A was constructed by Retrogen, Inc. (San Diego, CA, USA) from the pET-25b(+)_CitSc vector. Sequence was verified using an ABI 3730 automated sequencer at Retrogen.
Metal speciation
Speciation for all transport assays was calculated using the Visual MINTEQ 2.51 program designed by the Environmental Protection Agency. MINTEQ values were calculated at a pH of 6.5 and a temperature of 37 °C. Studying uptake of iron–citrate complexes in particular is challenging when the confounding effects of mono-, di- and poly-nuclear complex formation in media occurs. The complexes produced however can be predicted by calculations (MINTEQ) and validated by experiment (speciation, corroborated by mass spectrometry and electronic absorption spectroscopy).9 It is important to note here then that these issues can be overcome by controlling buffer composition, pH and iron to citrate ratios.Transport in E. coli
E. coli JW4251-2 cultures were grown in LB broth as described previously. Protein expression for transport assays was induced at 30 °C with 0.5 mM isopropyl-D-thiogalactopyranoside (IPTG) at an OD600 of 1.0 for 1 h. Subsequent flux assays followed the protocol previously noted in the literature with the following adaptations: cells were resuspended with 10 ml of 50 mM PIPES buffer at pH 6.5 for all metals, and the transport assay was conducted at 37 °C. Non-induced controls were included as well as JW4251 no plasmid controls.8 Concentrations of metal ion and citrate were chosen to maximize the formation of the desired metal–citrate complex. The final calcium concentration was 10 mM with 4.4 μM [14C]-citrate, for magnesium studies the final magnesium concentration was 1 mM with 4.4 μM [14C]-citrate, and for iron studies the final ferric concentration was 2 μM with 4.4 μM citrate (Iron and citrate were precomplexed). Experiments were performed using at least three independent cultures, and samples at each time point were collected in triplicate per run. Incubation of the metal ion and pre-complexation of the metal with [14C]-citrate prior to cell addition yielded similar results.Secondary protein structure
Known permeases LacY, NdhA, and GlpT from E. coli were compared to CitSc.10–12 Kyte–Doolittle plots of CitSc, LacY, NdhA, and GlpT were conducted using the San Diego Super Computer Biology Workbench (SDSC-BW) website, which uses the hydropathic algorithms of Kyte and Doolittle, as well as the rapid FastP and FastA modelling of Pearson.13–15 TMHMM modelling was conducted using the SDSC-BW website.16 Other membrane prediction programs (e.g. PredictProtein program, TMpred, SOSUI and HMMTop) were used to compare and predict the best secondary structure that fit known LacY, NdhA, and GlpT permease structures. Conserved amino acids and domains of the five CitMHS members were identified using the SDSC-BW CLUSTALW multiple sequence alignment program.17Structural conformation
Cultures were prepared as in transport studies. After spinning down the 50 mL culture, cells were resuspended in buffer (50 mM Tris, 150 mM NaCl, pH 8.5). A Fisher sonic dismembrator model 100 was used to rupture the cells. The cells were pulsed at a setting of 5 for ten repetitions of 2 s on ice. Pulsing was repeated two more times. Cultures were spun down (10 min; 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g) and the supernatant was loaded onto 10-15% polyacrylamide gels (Fig. S-1, ESI).†
000 g) and the supernatant was loaded onto 10-15% polyacrylamide gels (Fig. S-1, ESI).†
Results and discussion
Protein prediction and modelling
No CitMHS crystal structures are reported to date. Therefore, known permease structures (LacY, NdhA, and GlpT) were used to provide a model for the secondary CitMHS structure.10–12 Each permease contains twelve transmembrane α-helices with the N- and C-termini exposed to the cytoplasm. Kyte–Doolittle plots of the three permeases and CitSc show there is a significant resemblance of CitSc to LacY and GlpT.13–15 Further similarity is seen using TMHMM predictions. LacY is predicted to have eleven transmembrane helices, but the LacY crystal structure has twelve helices (Fig. 1).16 The TMHMM prediction program incorrectly fuses the ninth and tenth transmembrane helices, which is seen as a coalescent tall and short peak. The same event occurs in the CitSc TMHMM prediction. The tenth and eleventh transmembrane helices appear as a coalescent tall and short peak. Other programs (e.g. Predict Protein, TMpred, SOSUI and HMMTop) correctly predict twelve membrane helices for LacY and/or CitSc.13–16 | ||
| Fig. 1 TMHMM prediction of LacY permease from E. coli and secondary metal–citrate transporter CitSc from S. coelicolor. | ||
The Predict Protein program predicts thirteen transmembrane helices for CitSc.18 However, HMMTop, SOSUI, and TMpred predict twelve transmembrane helices for LacY and CitSc.19–21 The thirteenth helix predicted by the PredictProtein program is a small hydrophobic region likely leftover from a gene duplication event. The hydrophobic region may be a reentrant loop that has similarly been predicted in the 2-hydroxycarboxylate transporter between the fifth and sixth transmembrane segments.22
A CLUSTALW multiple sequence alignment of the five known CitMHS members reveals a very highly conserved amino acid profile (Fig. S-2, ESI†).17 The highest conservation exists in the third, fourth and fifth α-helices as well as the ninth, tenth, and eleventh/twelfth helices. These regions are 39–43% similar. The greatest amino acid disparity exists in the gene duplication site with the putative reentrant loop. It is possible that not every CitMHS member contains this reentrant loop. With the putative α-helices known, critical amino acids for substrate recognition and transport can be proposed.
Metal–citrate complexes are negatively charged and need positively charged domain to attract them, as seen with the FecA ferric-citrate protein from E. coli and the Tcp chemoreceptor from Salmonella enterica.24,25 Two conserved regions in the third and eleventh/twelfth α-helices contain positively charged residues in CitSc. The positively charged region in the third α-helix is believed to be part of a salt bridge between two aspartic acids, D75 and D80, and an arginine, R161. The eleventh/twelfth α-helical domain is believed to be involved with citrate binding.
When comparing the eleventh/twelfth domains of the five CitMHS members HTR460 is seen in CitSc, but HQK is seen for CitBs1 and CitBs2. CitEf and CitSm show a sequence of WQK. Arginine residues are critical for binding to metal complexes that contain carboxylic acids, carbonates, or siderophores as ligands. A common profile found in these systems is TR.24,25 Enterobactin, an iron siderophore, contains a HTR, and the Tcp chemoreceptor, a metal–citrate sensor, contains an ETR sequence.23 Iwama et al. demonstrated that the E was not vital for citrate sensing, T was vital for citrate sensing, and R was vital for citrate binding.2
A TR sequence is also found in human lactoferrin and the E. coli 2-methyl-citrate lyase.26,27 Human lactoferrin binds ferric ions with a carbonate ligand and the TR coordinates to the carbonate. This strong relationship plus the additional positive charges in the eleventh/twelfth domain suggests this area is vital for metal–citrate binding and recognition, and the TR may be critical for Fe3+-citrate binding.
Using the TMHMM diagram, Predict Protein Program, TMpred, SOSUI, HMMTop, CLUSTAW, known permease structures, and additional information provided previously, a putative secondary structure of CitSc with the highlighted amino acids mentioned earlier was constructed (see Fig. 2). Based on this information, the R161A, K452C, and R460C mutants have been constructed and their activity tested.
 | ||
| Fig. 2 Cartoon diagram of putative secondary structure of CitSc. N- and C-termini are exposed to the cytoplasm. Twelve transmembrane helices are predicted. A re-entrant loop is seen between the sixth and seventh transmembrane domains. D75 and D80 in transmembrane helix three are predicted to be involved with salt bridge formation. R161 in transmembrane helix five is predicted to be involved in citrate binding or salt bridge formation. K452, R460, and K465 are predicted in the eleventh/twelfth domain are thought to be critical amino acids for citrate attraction and binding. | ||
Transport properties of CitSc mutants
To investigate the influence of the eleventh/twelfth positively charged domain on metal–citrate transportation, K452C and R460C mutants were built. Mutant R161A was also constructed to test a positively charged residue away from the eleventh and twelfth domain that may play a role in salt-bridge formation. The three mutants and the wild type gene were inserted into pET-25b(+) and overexpressed in E. coli JW4215-2 ΔfecA758 cells.Using a Gram-negative host to overexpress the Gram-positive protein may introduce limitations or difficulties. However, E. coli is the most widely studied and mutated Gram-negative bacterium. It can be noted that proteins from Gram-positive bacteria, as well as from mammalian sources, have been functionally characterized in E. coli including other CitMHS members.28–30 Flux assays were conducted with ‘free’ citrate, Ca2+-, Fe3+-, and Mg2+-citrate to test for metal-[1,5-14C]citrate and or “free-citrate” accumulation (see Fig. 3–5). Additional controls included flux assays in E. coli cells without plasmid and non-induced cultures.
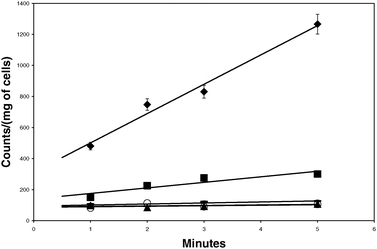 | ||
| Fig. 3 Ca2+-citrate uptake by the wild type CitSc overexpressed in JW4251 E. coli cells. Solid diamonds represent wild type, solid squares represent uptake with non-induced wild-type, closed circles represent untransformed JW4251 cells as baseline control, open circles represent free citrate uptake control (in wild type), and closed triangles represent Mg2+-citrate transport. Standard deviations are included with error bars. | ||
 | ||
| Fig. 4 Ca2+-citrate uptake in wild type, K452C, R460C, and R161A. Closed diamonds represent wildtype, open diamonds represent R161A, closed triangles represent R460C, close squares represent K452C, open squares represent free citrate control, and asterisks (hidden by the other baselines) represent JW4251 cells without plasmid. R161A, R460C, free citrate control, and no plasmid control overlap. Error bars are not seen except for the wild type CitSc due to shrinking the graph. | ||
 | ||
| Fig. 5 Fe3+-citrate uptake in wild type, K452C, R460C and R161A. Closed diamonds represent wild type, open diamonds represent R161A, closed triangles represent R460C, close squares represent K452C, open squares represent free citrate control, and asterisks represent JW4251 cells without plasmid. Experiments were performed at least in triplicate. | ||
Fig. 3 shows that as previously reported for the CitSc system, Ca2+-citrate was transported by the wild type but no ‘free-citrate’ or Mg2+-citrate was transported. Untransformed JW4251 cells did not show any uptake of ‘free’ citrate or Mg2+-citrate.
Fig. S-3 (ESI)† shows the uptake of Ca2+-citrate for the K452C mutant. Clearly, there is statistical uptake compared to the noninduced wildtype. No magnesium citrate was observed to be transported. Fig. 5 is a comparison of the wild type CitSc to the mutants K452C, R460C, and R161A. Mutants K452C and R460C show a complete disruption of Ca2+-citrate transportation up to three minutes. At five minutes, R460C showed statistical accumulation but well below that of the wild type. Mutant R161A showed moderate disruption of Ca2+-citrate transportation when compared to the wild type.
Fig. 5 compares the transport of Fe3+-citrate by the wild type CitSc to that of the mutants K452C, R460C, and R161A. Unlike previously published results, a lower citrate activity was used to visualize possible kinetics of Fe3+-citrate uptake. Previously, citrate activity was twenty fold higher making underlying processes invisible.8 By using the same citrate concentration but a lower activity (1 (nCi/mmol)/mL stock solution) a biphasic uptake pattern was observed. This same pattern was observed by Colton and Braun with the ferrichrome uptake in E. coli.27 They suggested limited binding sites as a potential biphasic uptake patter, but new research into iron speciation may indicate a much more complex explanation involving pH, concentration, and temperature.9 Mutants K452C and R460C show a significant reduction in Fe3+-citrate, but a much smaller biphasic pattern is still distinguishable. The R161A mutant shows a different pattern, which is indicative that we have not affected the metal–citrate binding but more likely the salt bridge. These results suggest that the positive charge on the eleventh/twelfth domain from K452 and R460C are critical for citrate recognition and uptake in CitSc. Loss of that positive charge prevents recognition or binding and prevents transport. Additionally, the R161A mutant may play a less critical role in metal–citrate acquisition such as supporting a salt bridge as seen with LacY or NhaA.
Conclusion
The positively charged amino acids R161, K452, and R460 play a critical role in metal–citrate recognition and transportation for CitMHS proteins. This study demonstrates that loss of these critical charges by point mutation decreased the amount of metal–citrate accumulated by the JW4152 Δfec E. coli cells. These are the first mutant constructs of CitMHS proteins.Many factors contribute to the recognition between CitMHS members and the metal–citrate complexes that they transport. Using secondary structure and multiple sequence alignment programs we were able to construct a putative secondary structure for CitSc. This information indicated that there are many amino acids that are important for allowing this protein to function but that the positively charged loop in the eleventh/twelfth domain is critical for citrate binding. Therefore, K452 and R460 were the most likely candidates involved in citrate recognition and binding.
K452C showed a drastic decrease in Ca2+-citrate and Fe3+-citrate transportation compared to wild type but still above the free citrate control (see Fig. 2). Mg2+-citrate was not observed to be transported by the K452C mutant. R460C showed a very similar pattern to K452C but had a statistically higher uptake at five minutes with the Ca2+-citrate. R161A showed a much lower accumulation of Ca2+-citrate compared to the wild type, but R161A showed more accumulation than K452C and R460C. Additionally, R161A showed an alternative uptake pattern for Fe3+-citrate when compared to the wild type, K452C and R460C mutants.
The decreased uptake of metal–citrate was not observed to be the same in all mutants suggesting that the K452 and R460 amino acids play a more critical role in substrate recognition and binding, while the less affected R161 amino acid is more likely involved with the translocation of protons. This is not surprising given that the R161 amino acid is buried within the fifth α-helix with flanking hydrophobic amino acids, while the K452 and R460 amino acids are predicted to be solvent exposed and share adjacent amino acid profiles seen in other metal–carbonyl binding systems (e.g. Tcp). Additionally, no different metal–citrate complexes were observed to be transported, indicating that these positive charges do not dictate complex recognition only citrate attraction and binding.
The five CitMHS members characterized to date recognize different metal–citrate complexes except for CitBs2 and CitEf, which indicates that even though these five proteins share high similarity their activity is unpredictable. Therefore, the differences in the amino acid MSA represent key sites for future mutagenesis. One particular difference of note is that CitBs1 is the only member that recognizes and transports Mg2+-citrate. Looking at the CLUSTAW MSA two key differences are noticeable: the aspartic acid in the eleventh/twelfth domain that replaces the glycine at the 456th position of CitSc, and the replacement of the tyrosine at the 445th position in CitSc to a histidine in CitBs1. These two differences may significantly impact what complexes are recognized. Looking at the MgtE magnesium transporter there is a series of aspartic acids that are involved in Mg2+ sensing.31 The extra aspartic acid seen in CitBs1 could be for Mg2+ sensing and binding. Additionally, the histidine that replaces the 445th tyrosine seen in CitSc, which is conserved in the other members, is an important amino acid difference due to the fact that tyrosine is known to complex to Ca2+ and Fe3+ as seen in the calcium binding protein LipL32 of Leptospira interrogans and the iron binding protein human lactoferrin.26,32 More experiments are in progress to attempt to further deconvolute this little explored family of proteins.
Acknowledgements
The author acknowledges support for this work from the Office of the Vice-president for Research at Syracuse University and the iLEARN Program.Notes and references
- S. Prakash, G. Cooper, S. Singhi and M. H. Saier Jr., Biochim. Biophys. Acta, Biomembr., 2003, 1618, 79–92 CrossRef CAS.
- N. Ishiguro, H. Izawa, M. Shinagawa, T. Shimamoto and T. Tsuchiya, J. Biol. Chem., 1992, 267, 9559–9564 CAS.
- J. S. Lolkema, H. Enquist and M. E. van der Rest, Eur. J. Biochem., 1994, 220, 469–475 CrossRef CAS.
- C. Marty-Teysset, J. S. Lolkema, P. Schmitt, C. Vivies and W. N. Konings, J. Biol. Chem., 1996, 270, 25370–25376.
- B. P. Krom, J. B. Warner, W. N. Konings and J. S. Lolkema, J. Bacteriol., 2000, 182, 6374–6381 CrossRef CAS.
- B. Korithoski, K. Krastel and D. G. Cvitkovitch, J. Bacteriol., 2005, 187, 4451–4456 CrossRef CAS.
- V. S. Blancato, C. Magni and J. S. Lolkema, FEBS J., 2006, 273, 5121–5130 CrossRef CAS.
- J. J. Lensbouer, A. Patel, J. P. Sirianni and R. P. Doyle, J. Bacteriol., 2008, 190, 5616–5623 CrossRef CAS.
- A. M. N. Silva, X. Kong, M. C. Parkin, R. Cammack and R. C. Hider, Dalton Trans., 2009, 40, 8616–8625 Search PubMed.
- J. Abramson, I. Smirnova, V. Kasho, G. Verner, H. R. Kaback and S. Iwata, Science, 2003, 301, 610–615 CrossRef CAS.
- E. Padan, M. Venturi, Y. Gerchman and N. Dover, Biochim. Biophys. Acta, Bioenerg., 2001, 1505, 144–157 CrossRef CAS.
- Y. Huang, M. J. Lemieux, J. Song, M. Auer and D. Wang, Science, 2003, 301, 616–620 CrossRef CAS.
- J. Kyte and R. F. Doolittle, J. Mol. Biol., 1982, 157, 105–132 CAS.
- W. R. Pearson and D. J. Lipman, Proc. Natl. Acad. Sci. U. S. A., 1988, 85, 2444–2448 CAS.
- W. R. Pearson, Methods Enzymol., 1990, 183, 63–98 CAS.
- TMHMM Protein Prediction, San Diego Super Computer Work Bench, Department of Bioengineering, at University of California, San Diego, 2009, http://workbench.sdsc.edu/.
- J. D. Thompson, D. G. Higgins and T. J. Gibson, CLUSTALW, Protein Prediction, San Diego Super Computer Work Bench, Department of Bioengineering, at University of California, San Diego, 2009, http://workbench.sdsc.edu/ Search PubMed.
- B. Rost, G. Yachdav and J. Liu, The PredictProtein Server. Nucleic Acids Research 32 (Web Server issue): W321-W326, 2004, http://www.predictprotein.org/.
- G. E. Tusnády, HMMTOP, Institute of Enzymology, Hungarian Academy of Sciences, Budapest, Hungary, 2009, http://www.enzim.hu/hmmtop/html/document.html Search PubMed.
- T. Hirokawa, S. Boon-Chieng and S. Mitaku, Bioinformatics, 1998, 14, 378–379 CrossRef CAS . http://bp.nuap.nagoya-u.ac.jp/sosui/sosui_submit.html.
- K. Hoffman and W. Stoffel, Biol. Chem., 1993, 374, 166.
- A. Dobrowolski and J. S. Lolkema, Biochemistry, 2009, 48, 7448–7453 CrossRef CAS.
- W. W. Yue, S. Grizot and S. K. Buchanan, J. Mol. Biol., 2003, 332, 353–368 CrossRef CAS.
- T. Iwama, Y. Ito, H. Aoki, H. Sakamoto, S. Yamagata, K. Kawai and I. Kawagishi, J. Biol. Chem., 2006, 281, 17727–17735 CrossRef CAS.
- H. R. Faber, T. Bland, C. L. Day, G. E. Norris, J. W. Tweedy and E. N. Baker, J. Mol. Biol., 1996, 256, 352–363 CrossRef CAS.
- S. Liu, Z. Lu, Y. Han, E. Melamud, D. Dunaway-Mariano and O. Herzberg, Biochemistry, 2005, 44, 2949–2962 CrossRef CAS.
- J. W. Coulton and V. Braun, J. Gen. Microbiol., 1979, 110, 211–220 CAS.
- W. Haihong and J. E. Crohan, J. Biol. Chem., 2004, 279, 34489–34495 CrossRef.
- A. Dumoulin, U. Grauschopf, M. Bischoff, L. Thony-Meyer and B. Berger-Bachi, Arch. Microbiol., 2005, 184, 117–128 CrossRef CAS.
- M. H. Lee, A. Nittayajarn, R. P. Ross, C. B. Rothschild, D. Parsonage, A. Claireborn and C. E. Rubens, J. Bacteriol., 1999, 181, 5790–5799 CAS.
- R. Ishitani, Y. Sugita, N. Dohmae, N. Furuya, M. Hattori and O. Nureki, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 15393–15398 CrossRef CAS.
- P. Hauk, C. R. Guzzo, H. R. Ramos, P. L. Ho and C. S. Farah, J. Mol. Biol., 2009, 390, 722–736 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SDS-PAGE gels of E. coli expressing each mutant and a no vector control. Protein sequence homologies of CitMHS family members explored to date and Ca2+-citrate uptake by K452C. See DOI: 10.1039/b920689b |
| This journal is © The Royal Society of Chemistry 2010 |
