Protein phosphorylation studies of cerebral spinal fluid for potential biomarker development
Karolin K.
Kroening
a,
Julia
Kuhlmann
a,
Renee
Easter
a,
Joseph F.
Clark
b,
Gail
Pyne-Geithman
b and
Joseph A.
Caruso
*a
aUniversity of Cincinnati/Agilent Technologies Metallomics Center of the Americas, Department of Chemistry, University of Cincinnati, Cincinnati, OH 45221-0172, USA
bDepartment of Neurology, University of Cincinnati, Cincinnati, Ohio 45267-0536, USA
First published on 15th April 2010
Abstract
Subarachnoid hemorrhage (SAH) followed by cerebral vasospasm (CV) leads to severe debilitation or death of an estimated one million people worldwide every year. A biomarker that would predict the onset of CV after a SAH would be useful in informing treatment protocols, but has yet to be found. The focus of this study is to explore differences in protein phosphorylation in cerebral spinal fluid (CSF) among healthy patients, SAH patients and SAH-CV patients. A significant difference in phosphorylation among the three sample types could be an important step towards the discovery of a diagnostic marker. The identification and validation of phosphorylated protein differences for study is manifested in the nature of signaling involved in the pathological events seen post SAH. Capillary liquid chromatography (cap-LC) coupled to inductively coupled plasma mass spectrometry (ICPMS) and nano-liquid chromatography-CHIP/ion trap mass spectrometry (nanoLC-CHIP/ITMS) are used to identify and measure protein phosphorylation changes in the CSF of the aforementioned groups. ICPMS represents a suitable method for screening ultra-trace phosphorus levels at the natural isotope, 31P, while nano-LC-CHIP/ITMS is used to identify phosphoproteins by searching appropriate protein databases.
Introduction
Mass spectrometry is a powerful tool for the identification of protein phosphorylation and phosphorylation sites that might be an indication of cell signaling associated with certain pathologies. Our point of departure is to investigate phosphorylation in the cerebral spinal fluid (CSF) of subarachnoid hemorrhagic stroke (SAH) patients for signaling changes prior to the deadly complications that may arise from the cerebral vasospasm condition (CV), with the ultimate goal of developing appropriate biomarkers.The American Heart Association reported in 2009 that hemorrhagic stroke accounts for about 15% of all stroke cases. Hemorrhagic stroke results from a weakened artery that ruptures and bleeds into the surrounding brain tissue. The blood then accumulates and can lead to increases in intracranial pressure. There are two types of hemorrhagic stroke: intracerebral hemorrhage and subarachnoid hemorrhage.1
Our attention focuses on subarachnoid hemorrhage where the ruptured vessel is located in the subarachnoid space: the interval between the arachnoid membrane and pia mater in the central nervous system, which is filled with cerebral spinal fluid. In 85% of the cases of spontaneous SAH, the cause is rupture of a cerebral aneurysm. An aneurysm is a weakness and/or ballooning in the wall of an artery in the brain. In 15 to 20% of cases of spontaneous SAH, no aneurysm is detected on the first angiogram.2,3
After SAH, cerebral vasospasm post subarachnoid hemorrhage is the cause of death in 40 to 50% of patients.4 “Vaso” refers to blood vessel and “spasm” refers to the vessel's “spastic”, or “constricted” physical state.5 The term “cerebral vasospasm” is commonly used to refer to both the clinical picture of delayed onset of ischemic neurological deficits associated with aneurysmal SAH (“symptomatic vasospasm”) and the narrowing of cerebral vessels documented by angiography or other studies (“angiographic or arterial vasospasm”). Arterial vasospasm typically appears three to four days after rupture and reaches a peak in incidence and severity at seven to ten days. The incidence and time course of symptomatic vasospasm (CV in this study) parallels that of arterial vasospasm. The most important factors in determining the clinical effect of vasospasm are the severity and extent of vessel narrowing. Symptomatic vasospasm typically begins four to five days after the hemorrhage and is characterized by the insidious onset of confusion and a decreasing level of consciousness. When the arterial narrowing is marked, with concomitant ischemia, these symptoms may progress to focal neurological deficits, infarction, coma and death. In less severe cases, neurological recovery can be expected as the arterial narrowing resolves. The exact mechanism(s) by which SAH induces arterial vasospasm continues to be a subject of considerable research and debate.6–8 Therefore, the development of biomarkers that may predict CV after SAH is an interesting, but insufficiently studied research area.
Our focus in this study is to explore protein phosphorylation differences in cerebral spinal fluid between patients that have suffered from post-SAH CV (CSF V) and the ones that did not have CV post-SAH (CSF C); thereby expanding our preliminary study.4 CSF samples from non-hemorrhage patients were also available and were used as a control (CSF Control). The phosphorylation of proteins on the amino acid residues of serine, threonine and tyrosine is estimated to affect 30% of the proteome and is a major regulatory mechanism that controls many basic cellular processes.9 Phosphoprotein analysis in complex protein mixtures, such as CSF, involves identification and sequencing of phosphoproteins and phosphopeptides. The application of capillary liquid chromatography coupled to inductively coupled plasma mass spectrometry (capLC-ICPMS) combined with nano-liquid chromatography-electrospray ionization, ion trap mass spectrometry (nanoLC-CHIP/ITMS) represents a suitable technique for quantification of phosphoproteins and their phosphorylation degree by detecting the natural isotope 31P. It also is a preferred method, since it avoids the use of the radioactive 32P and 33P labeling, often used in identifying protein phosphorylation.
Because of the complexity of CSF, the subdivision between two different regions (<5 kDa and 5–50 kDa) has been chosen to facilitate protein identification. The CSF samples were studied in the 5–50 kDa molecular weight range, which represents the common weight range for many proteins, but also the region <5 kDa has been taken in consideration. Important differences in protein identifications are shown in this study and are promising for the development of biomarkers in order to predict the onset of CSF V.
Materials and methods
The CSF samples were obtained through the Department of Neurology at the University of Cincinnati with appropriate institutional review board approvals. Double deionized (DDI) water was prepared by passing distilled water through a NanoPure (18 MΩ) treatment system (Barnstead, Boston, MA, USA). Acetonitrile (Tedia Company, Fairfield, OH, USA) has been used for mobile phase B in the capLC-ICPMS analysis. Formic Acid and Trifluoroacetic acid for the mobile phases A and B in the capLC-ICPMS separation have been purchased from Fisher Scientific (Fairlawn, NJ, USA). Solvents being used for the nanoLC-CHIP/ITMS, water and acetonitrile, were of high purity and have been purchased from Burdick and Jackson (Muskegon, MI, USA). Formic acid reagent grade (LOT: LB68266) for the ion trap and Spin Concentrators for Proteins (5 kDa MWCO and 50 kDa MWCO) are from Agilent (Agilent Technologies, Santa Clara, CA, USA). Top-Tip porous titanium dioxides tips were purchased from Glygen Corp. (Columbia, MD, USA).Bovine β-Casein, Iodoacetamide and dithiothreitol were acquired from Sigma-Aldrich (St. Louis, MO, USA). Ammonium bicarbonate was purchased from Fisher Scientific. Urea was from Mallinckrodt Baker (Canada). Sequence grade modified Trypsin and the suspension buffer acetic acid was obtained from Promega (Madison, WI, USA). Spin-X centrifuge tube filters (22 μm) were acquired Corning Inc. (Corning, NY, USA). Protein amounts in the cerebral spinal fluid samples determinations have been performed with the Pierce BCA assay (Pierce Chemical Company, Rockford, IL).
All information related to protein identifications are originated from Spectrum Mill rev. A. 03.03, Agilent Technologies and UniProtKB, the protein knowledge base, which consists of two sections: SwissProt and TrEMBL.
Sample preparation
The CSF samples were received at −80 °C and slowly thawed on ice before sample preparation in order to avoid protein degradation. Subsequently, the samples were loaded and spun on a 5 kDa followed by a 50 kDa spin concentrator at 5000 g and 4 °C for 20 min in order to obtain the desired MW regions for sample analysis. The samples were spun at 5000 g for 20 min at 4 °C. Based on the protein amount present, previously determined by the Pierce BCA assay in the different samples received, a tryptic digestion was performed. The calculated amounts (1 mg for Bovine β-Casein, 12 μL for CSF C, 20 μL for CSF V, 15 μL for CSF Control) were dissolved in water in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. 20 μL of a 0.4 M ammonium bicarbonate and 8 M urea (pH 7.5) and 5 μL of 45 mM dithiothreitol were added and incubated at 50 °C for 15 min in order to reduce the protein. After cooling the sample to room temperature, 5 μL of 100 mM iodoacetamide were added and left in the dark for 15 min, 5 μL of trypsin were added and the protein solution was incubated at 37 °C for 22 h. To halt the trypsin activity, formic acid was added. Samples were filtered through 0.22 μm, filters prior to the capLC separation.
1 ratio. 20 μL of a 0.4 M ammonium bicarbonate and 8 M urea (pH 7.5) and 5 μL of 45 mM dithiothreitol were added and incubated at 50 °C for 15 min in order to reduce the protein. After cooling the sample to room temperature, 5 μL of 100 mM iodoacetamide were added and left in the dark for 15 min, 5 μL of trypsin were added and the protein solution was incubated at 37 °C for 22 h. To halt the trypsin activity, formic acid was added. Samples were filtered through 0.22 μm, filters prior to the capLC separation.
After the fractions from the capLC-ICPMS were collected, they were passed through the TopTip titanium dioxide tips in order to enrich the phosphoproteins. Particular attention was focused on this part of the sample preparation. The sample and the elution buffer had to be pushed through the tip very slowly in order to give the phosphopeptides time to bind to the stationary phase.
Instrumentation
Capillary liquid chromatography (capLC)
An Agilent (Agilent Technologies, Santa Clara, CA, USA) 1200 series capillary liquid chromatography system equipped with a high pressure binary gradient pump, chilled autosampler, vacuum degasser, column compartment with Peltier heating/cooling, and UV detector was used to identify the phosphoprotein species being investigated in this study. An Agilent Zorbax SB-C18 column (0.5 × 150 mm, 5 μm) was used for the separation under gradient conditions with mobile phase A (1% FA, 0.1% TFA (v/v) in water) and B (80% ACN, 1% FA, 0.1% TFA (v/v) in water). The gradient system was the following: 0–5 min, 3% B; 5–10 min, 5% B; 10–15 min, 10% B; 15–20 min, 45% B; 20–50 min, 75% B; 50–60 min, 10% B; 60–90 min, 0% B. The column was re-equilibrated for 30 min after each run. 2 μL of digested CSF sample were injected into the capLC-ICPMS at a flow rate of 5.0 μL min−1. The autosampler temperature was held at 4 °C to prevent degradation of the protein samples.Inductively coupled plasma mass spectrometer (ICPMS)
An Agilent 7500cx ICPMS (Agilent Technologies, Santa Clara, CA) equipped with a shield torch system and a collision/reaction cell was used for element specific detection of phosphorus. Coupling of the ICPMS with the LC was accomplished through the use of a PEEK coated silica tubing and a DS-5 capillary nebulizer (CETAC Technologies, Omaha, NE). ICPMS detection of the only phosphorus isotope (m/z = 31) was carried out using helium as a reaction gas at a flow rate of 3.7 mL min−1 and a quadrupole and octopole bias at −16 V and −18 V, respectively, for a net energy discrimination voltage of +2 V.NanoLC-CHIP/ITMS
An Agilent 6300 Series HPLC-CHIP/Ion Trap XCTsystem (Agilent Technologies, Santa Clara, CA) coupled to a 1200 LC, equipped with both a capillary and nano pump was used for mass identification. The sample was loaded via the capillary pump on the on-chip enrichment column. The chip used, contained a Zorbax 300SB C18 enrichment column (4 mm × 75 μm, 5 μm) and a Zorbax 300SB C18 analytical column (43 mm × 75 μm, 5 μm). Samples were loaded on the enrichment column at a flow rate of 4 μL min−1 with a 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of solvent A (0.1% FA (v/v) in water) and B (90% ACN, 0.1% FA (v/v) in water). After the enrichment column was loaded, the on-chip microfluidics switched to the analytical column at a flow rate of 0.4 μL min−1. The following gradient conditions were used in the analysis: 0–5 min, 3% B; 5–50 min, 40% B; 50–60 min, 70% B; 60–70 min, 50% B; 70–90 min, 0% B.
3 ratio of solvent A (0.1% FA (v/v) in water) and B (90% ACN, 0.1% FA (v/v) in water). After the enrichment column was loaded, the on-chip microfluidics switched to the analytical column at a flow rate of 0.4 μL min−1. The following gradient conditions were used in the analysis: 0–5 min, 3% B; 5–50 min, 40% B; 50–60 min, 70% B; 60–70 min, 50% B; 70–90 min, 0% B.
The MS conditions have been adopted from Ellis et al.4 The analysis was achieved using nitrogen as a drying gas at a flow rate of 4 L min−1 and a temperature of 350 °C. The capillary voltage was set to 1900 V, with a skimmer voltage of 30.0 V (capillary exit: 70.0 V; trap drive: 85.0 V). Two averages were taken for each precursor ion. The target number of ions was 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 with a maximum accumulation time of 150 ms. The MS scan range was 50–2200 m/z in standard-enhanced scan mode. MS2 experiments were performed with five precursor ions per cycle and fragmentation amplitudes of 1.30 V. ESI-MS, MS/ESI-MS2, and MS/ESI-MS3 experiments were completed as well.
000 with a maximum accumulation time of 150 ms. The MS scan range was 50–2200 m/z in standard-enhanced scan mode. MS2 experiments were performed with five precursor ions per cycle and fragmentation amplitudes of 1.30 V. ESI-MS, MS/ESI-MS2, and MS/ESI-MS3 experiments were completed as well.
Results and discussion
Analysis of standards for method confirmation
A study of detection limit (LOD) for 31P has been performed to assess the efficiency of the chromatographic method coupled to ICPMS. A tryptic digest of bovine β-casein was the standard of choice for the LOD determination. The concentration range of this protein was 0, 5, 10, 25, 50 ng mL−1 and R2 for the calibration curve was 0.989. The linearity of the calibration curve has been verified with Mandel's fitting test. Detection limits (3σ) based on three times the standard deviation of seven replicates of the blank areas (IUPAC definition) for the analysis of 31P of bovine β-casein, based on 2 μL injection volume, was 10 ng mL−1. The 31P fraction in the chromatogram was collected offline and taken to the nanoLC-CHIP/ITMS for further identification. The base peak chromatogram (BPC) shows a peak at 30.4 min. Fragmentation analysis confirms the presence of bovine β-casein showing MS fragments at m/z 278.4, m/z 147.2, m/z 284.3 and m/z 749. These are some examples of the characteristic fragments at the sequence positions 2, 44, 122 and 128 from bovine β-casein after tryptic digestion.The method of using capLC-ICPMS and nanoLC-CHIP/ITMS has been confirmed to be an excellent tool for phosphorylation site identification in previous studies, where phosphopeptide standards such as Pp60-src and P60 c-src showed validity for the techniques applied in this study.10
Analysis of CSF samples (5-50 kDa) with capLC-ICPMS and nanoLC-CHIP/ITMS
The chromatograms of CSF Control, CSF V and CSF C samples show distinct differences. Although the separations are not exemplary, the aim of this study was the investigation of differences in phosphorylation and to determine which proteins those differences are related to. However, not only the differences are interesting to see, but also the similarities on certain samples, since they can indicate a disease state as well.The CSF V sample presented a peak at 67.2 min as shown in Fig. 1. This peak was not present in the CSF Control or in the CSF C sample and therefore, indicates a difference in phosphorus containing species. Furthermore, a peak at 74.6 min was present in the CSF Control, but not in the CSF samples from patients that had suffered from subarachnoid hemorrhage, CSF C, with subsequent vasospam, CSF V. All chromatograms were based on n = 3, where n represents the number of independent analytical replicates.
 | ||
| Fig. 1 CSF Control, CSF C, CSF V (5–50 kDa molecular weight region) capLC-ICPMS chromatograms. A peak at 67.2 min in the CSF V is different from CSF Control and CSF C. Also, a peak at 74.6 min in the CSF Control sample is not present in the samples from patients that have suffered a SAH. | ||
Both peaks were collected offline, Fraction #1 for the peak present in the CSF V sample and Fraction #2 for the peak present in the CSF Control, and analyzed with nanoLC-CHIP/ITMS for further mass identification. A relatively long time span of 30 min with a gradient from 45% to 75% ACN was chosen in order maintain spray stability. The base peak chromatogram (BPC) and average mass spectrum for the collected fraction (Fraction #1) of the peak at 67.2 min are shown in Fig. 2. The BPC of this peak shows high intensity peaks (×109) between 12.1 and 18.6 min.
 | ||
| Fig. 2 Base peak chromatogram and mass spectrum of Fraction #1 from CSF V sample of chromatogram of Fig. 1. | ||
The results of the protein database search of the region of high intensity with Spectrum Mill, with the SwissProt database, are presented in Table 1, based on statistical matches as score (which reflects information content, such as the amount of useful fragmentation), spectrum intensity, and Fwd-Rev score (which is the difference between scores for top hits from a database search of the matched peptide sequence, both for the forward sequence reading direction and the reverse direction). The phosphorylation sites are listed in column 4. Not all the proteins shown are necessarily related to stroke complications; on the other hand they may be an indication that a protein, whose function is not currently known to be related to SAH, may actually play a role.
| Score | Fwd-Rev Score | Spectrum Intensity | Variable Sites | Sequence | Ret. Time/min | Accession # | Name |
|---|---|---|---|---|---|---|---|
| 7.86 | 1.76 | 4.88E+05 | S879s | (R)sLNEELGDEDSEKKR(K) | 43.66 | O14924 | Regulator of G-protein signaling 12 (RGS 12) Homo Sapiens (Human) |
| 6.57 | −0.83 | 2.56E+05 | T1123t | (K)VKTDEVVTLtPRIGPK(V) | 41.53 | P13611 | Versican core protein precursor (Large fibroblast proteoglycan) (Chondroitin sulfate proteoglycan core protein 2) (PG-M) (Glial hyaluronate-binding protein) (GHAP)—Homo sapiens (Human) |
| 5.95 | 0 | 4.14E+05 | S2032s | (K)KTGSKNLCAVELPSsVK(L) | 37.9 | Q9NZJ4 | Sacsin—Homo sapiens (Human) |
| 5.1 | 0.39 | 6.90E+05 | S4077s | (R)sSRRTGPQSPCER(T) | 40.88 | Q12955 | Ankyrin-3 (ANK-3) (Ankyrin-G)—Homo sapiens (Human) |
| 5.01 | 0.12 | 1.23E+06 | T129t | (K)AERIGEtPELCALTGPFER(G) | 54.63 | O60294 | Leucine carboxyl methyltransferase 2 (EC 2.1.1.-) (p21WAF1/CIP1 promoter-interacting protein) (tRNA wybutosine-synthesizing protein 4 homolog)—Homo sapiens (Human) |
| 5 | 0.47 | 8.46E+05 | S129s | (K)GAAVNGPVSHSSLTKTsNMNK(G) | 44.14 | Q9H582 | Zinc finger protein 644 (Zinc finger motif enhancer-binding protein 2) (Zep-2)—Homo sapiens (Human) |
| 4.8 | −0.09 | 3.38E+05 | S358s S360s | (K)QRNAEALAELsEsLRNR(A) | 42.69 | Q9H1B7 | RING finger protein C14orf4—Homo sapiens (Human) |
| 4.44 | 4.44 | 5.88E+05 | S59s | (K)GPNANs(−) | 50.96 | P62861 | 40S ribosomal protein S30—Homo sapiens (Human) |
The results with highest statistical validity for the CSF V sample (Table 1) show the regulator of G-protein signaling 12, homo sapiens. It inhibits signal transduction by increasing the GTPase activity of G-protein α-subunits, thereby, driving them into their inactive GDP-bound form. Isoform-3 of this protein is brain specific. It is phosphorylated upon DNA damage, probably by ATM, ataxia telangiectasia mutated, or ATR, ATM and Rad3-related, and the database SwissProt reports phosphorylation on serine 879 in the sequence. G-protein-coupled receptors (GPCRs) are major targets for drug discovery. The regulator of G-protein signaling (the RGS)-protein family has important roles in GPCR signal transduction. RGS proteins represent a large set of tantalizing new central nervous system (CNS) drug targets with potential therapeutic actions in a wide range of clinical situations, such as Alzheimer's and Parkinson's Diseases, Spasticity and Epilepsy.11
The following significant protein is Sacsin, which is highly expressed in the central nervous system. It may function in chaperone-mediated protein folding and it is known that chaperones, especially the stress inducible Hsp70, have been studied for their potential to protect the brain from ischemic injury. Giffard et al. showed that over expression of Hsp70 in vivo by, either viral or transgenic induction, before ischemia protects neurons from injury. The protection may be mediated by one or more of the many activities ascribed to Hsp70, including refolding denatured proteins and preventing unfolded and damaged proteins from aggregating, or by a direct anti-apoptotic mechanism.12
Of interest is also the 40S ribosomal protein S30 protein (Fau). Fau is a ribosomal protein synthesized as a C-terminal extension protein (CEP) of an ubiquitin-like protein. This may be of importance, since transient cerebral ischemia activates various post-translational protein modifications, such as phosphorylation and ubiquitin conjugation. These are believed to play a major role in the pathological process triggered by an interruption of blood supply and culminating in cell death.13
These aforementioned are a few examples of the proteins that have been identified in the BPC of the peak at 67.2 min of the CSF V sample. None of the above mentioned proteins were found in the CSF Control or CSF C; thus indicating a different phosphorylation profile in the CSF V patients' CSF compared to CSF C or CSF Control which may be diagnostic, prognostic or mechanistically relevant to the clinical condition. The authors are aware that for these examples and those below, only proof of concept is suggested. A statistically valid study to trigger clinical interventions or enhance treatments will require 30 patients in the SAH CSF C group and 30 additional in the SAH CSF V group. A research proposal has been submitted requesting funding for such a study.
The CSF Control collected peak, Fraction #2, gave the following results: The protein FAM38A (score: 8.25, peak intensity: 4.55 × 106 cps indicating relatively high statistical validity), which is a membrane protein induced by β-amyloid treatment, has been identified. It is expressed in numerous tissues. In normal brain tissue, it is expressed exclusively in neurons, but not in astrocytes. It was found to be expressed in about half of the activated astrocytes located around classical senile plaques in Alzheimer's disease.14 This protein gave an eminent value of 3.32 for the Fwd-Rev score, which indicates the difference between scores for top hits from a database search of the matched peptide sequence both in forward sequence reading direction and the reverse direction. The larger the score the greater the confidence in the sequence reported, accordingly to the score and spectrum intensity.
The Cystatin-SA precursor (Cystatin-S5) has also been identified. Cystatin-SA (CST2) is a human gene and a member of the family of cysteine protease inhibitors. Although cystatin-SA has not been associated with stroke, elevated cystatin-C levels were independently associated with both ischemic and hemorrhagic stroke, while cystatin-C is used as predictor for the risk of cardiovascular events and death.15
Although it has a slightly lower score and peak intensity and a negative value of the Fwd-Rev score (−0.02), an uncharacterized protein KIAA1383 has been identified. KIAA genes are protein-coding sequences of uncharacterized human genes. Since the protein is uncharacterized and its function and location is not known, it may trigger high interest.
Analysis of CSF samples (<5 kDA) with capLC-ICPMS and nanoLC-CHIP/ITMS
A significant difference between the CSF SAH non vasospasm sample, CSF C, and the SAH vasospasm, CSF V, and normal sample, CSF Control, in the molecular weight region below 5 kDa can be seen as shown in Fig. 3. The peak from 12.1–31.0 min is only present in the CSF C sample, which is the CSF SAH non vasospasm sample, and might be indication of important phosphoproteins. This peak was collected for further capLC-CHIP-ITMS analysis (Fraction # 3).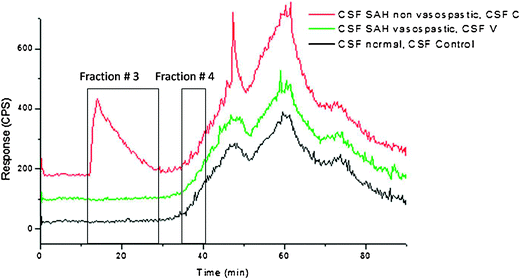 | ||
| Fig. 3 CSF Control, CSF C, CSF V capLC-ICPMS chromatograms. A peak at 12.1 min in the CSF C is being distinguished from CSF Control and CSF V. Also, a peak at around 38 min in the CSF Control and CSF C samples is not present in the samples from patients who have suffered SAH with vasospasm. | ||
The database search resulted in a large number of brain protein phosphorylation hits for Fraction # 3: A very good hit (Score: 12, Spectrum Intensity: 1786325) at retention time 35.54 min was uncharacterized protein C11orf63. The cytolocation of this protein is unknown. C11orf63 is homo sapiens chromosome 11 open reading frame 63, type: mRNA, gene: C11orf63. As of this writing, the protein is uncharacterized and its function and location is not known.
Another protein match with the highest phosphorylation hits is protein73 which is a p53-like transcription factor (p53-related protein) and signifies enhanced signaling or protein activation. It participates in the apoptotic response to DNA damage. Isoforms containing the transactivation domain are pro-apoptotic, isoforms lacking the domain are anti-apoptotic and block the function of p53 and transactivating p73 isoforms. It is present in brain tissue as well as in other organs like kidney, placenta, colon, heart, liver; mainly in almost every organ and, therefore, very important in regulating apoptosis.
Further interesting proteins found include the Microtubule-associated serine/threonine-protein kinase 1. These suggest at this point that CSF C may exhibit similar phosphorylation responses to other neurodegenerative diseases. Accumulating evidence indicates that serine/threonine protein phosphatases (PPs), such as PP1, PP2A and PP2B, participate in the neurodegenerative progress in Alzheimer's disease. The general characteristics and pathologic changes of PP1, PP2A and PP2B in Alzheimer's, and their relations with microtubule-associated proteins, focusing mainly on tau protein (τ-protein), neurofilament (NF), amyloid precursor protein (APP) processing and synaptic plasticity is a topic of active investigation and effective therapeutic intervention in the future.16
The Ubiquitin protein has been found in this fraction and was also reported in a previous study (J. Ellis et al.).10 It is an important protein since it has been linked to neurodegenerative diseases like Parkinson's and Alzheimer's. Alzheimer's disease is the most common form of dementia and is characterized by degeneration of neurons and their synapses. The dementia can be sometimes attributed to a vascular dementia associated with perfusion deficits. Thus, there is a pre-existing cerebral vascular pathology in a subset of Alzheimer's patients. A higher number of senile plaques (SP) and neurofibrillary tangles (NFT) compared to non-demented individuals of the same age are often found post mortem. NFT are composed of a hyperphosphorylated and ubiquitinated forms of τ-protein. Previous studies have shown that both tau and ubiquitin concentrations are increased in the cerebrospinal fluid (CSF) in Alzheimer's disease.17
An additional difference can be seen at 34.0–41.0 min as highlighted in the capLC-ICPMS chromatogram in Fig. 3. CSF C and CSF Control seem to present low counts peaks that are not shown in the CSF V chromatogram. Fractions of these low intensity peaks were collected offline (Fraction # 4) for further analysis with capLC-CHIP-ITMS, since low count signals might be of high interest, such as for signaling proteins with low copy numbers.
Results for Fraction # 4 are the following: This fraction from CSF C sample (SAH non vasospasm) has reported only 4 proteins with phosphorylation sites. The first one is the Gamma-aminobutyric acid (GABA) receptor subunit beta-2. GABA is a major inhibitory neurotransmitter in the vertebrate brain and mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor which leads to the opening of an integral chloride channel. Generally serine/threonine phosphorylation of GABA receptors has been found to reduce GABA receptor activity, and conversely, dephosphorylation of the receptor is often associated with the enhanced receptor function.18,19 The concentration of GABA in CSF has been studied extensively and has been shown to be related to Parkinson's disease as well as epileptic seizures.20,21 The GABA level in CSF was found to be reduced in patients with idiopathic Parkinson's disease when compared with age matched controls, but the difference was not significant. However, GABA levels in CSF were lower in patients treated with levodopa than in untreated patients and controls. There was no difference in plasma GABA levels between Parkinsonian patients and controls.19
Another protein that was found is ubiquilin-4, which has been also identified in Fraction # 3, and was also reported in a previous study Ellis et al.10 As mentioned earlier, this protein is important in the stabilization of other brain proteins. It has also emerged as a therapeutic target for Huntington's disease, as it binds to protein aggregates found in Huntington's, Alzheimer's, and Parkinson's Diseases.22
Protein and protein phosphorylation identification with analytical tools
In confirming one example from the above described proteins, Regulator of G-protein signaling 12 (RGS12) was selected. The mass spectrum shows evidence of the characteristic peaks that may follow tryptic digestion. For example in Fig. 4 the masses m/z 391.2 corresponding to the peptides fragments ATK, m/z 575.3: AQSNR, m/z 728.4: m/z GWLKPK, m/z 1193.6: LAFSAVCPDDR, m/z 1796.9: INLDEAEEFFELISK etc. | ||
| Fig. 4 Regulator of G-protein signaling 12 (RGS12) average mass spectrum showing characteristic masses as ATK, m/z 575.3: AQSNR, m/z 728.4: m/z GWLKPK, m/z 1193.6: LAFSAVCPDDR, m/z 1796.9: INLDEAEEFFELISK etc., being indication of peptide fragmentation. | ||
Serine at the sequence position 879 was identified as a phosphorylation site. The loss of –PO3 is indicated in the mass spectrum at m/z 1544.8 with a low intensity of 6130 counts (Fig. 4). The peak m/z 1464.6 at higher intensity indicating the loss of −78 Da. Therefore, even if Spectrum Mill gives a statistical indication at what position the loss of the phosphate group can occur, the ESI mass spectrum analysis permits a precise and simplified identification of the suggested results from Spectrum Mill, thereby providing further verification. It is clear that mass spectrometry for peptide identification and the identification of phosphorylation sites can be very powerful when combining direct mass spectral information with an appropriate database search. In almost all the other identified proteins the phosphorylation sites from the mass spectra matched with the ones suggested from the database. In most of the mass spectra the neutral loss of a phosphate group or a phosphate group plus water (H3PO4) occurred at phosphoserine and phosphotyrosine containing phosphopeptides.
Conclusion
Phosphoproteins in CSF and particularly the lower copy numbers of many signaling proteins are difficult to detect, but mass spectrometry is an excellent tool for the identification of phosphoproteins and the indication of phosphorylation sites. The capLC-ICPMS method permits a simplified screening of differences in 31P in CSF Control or diseased SAH/CV post SAH patients at low level for phosphorus containing species. NanoLC-CHIP/ITMS provides a relatively modestly expensive option to generate MSn spectra for the database search engines and, thereby, a route to confirm statistically based results via the losses or gains to the mass spectra. In this study differences have been shown in the variety of the two molecular weight regions, <5 KDa or 5–50 kDa, of three different sample types, CSF V, CSF C and CSF Control, and the approach is promising for future studies to inform the identification of biomarkers with putative implications for therapeutic targets.References
- Association, A.H. American Heart Association, Inc. 2009, available from: \http://www.americanheart.org/.
- G. J. Rinkel and J.v.G.a.E.W., Subarachnoid hemorrhage without detectable aneurysm. A review of the causes, Stroke, 1993, 24, 1403–1409 CAS.
- J. van Gijn, R. S. Kerr and G. J. E. Rinkel, Subarachnoid haemorrhage, Lancet, 2007, 369(9558), 306–318 CrossRef.
- J. Ellis et al., A Preliminary Study of Metalloproteins in CSF by CapLC-ICPMS and NanoLC-CHIP/ITMS, J. Proteome Res., 2008, 7(9), 3747–3754 CrossRef CAS.
- G. Khurana, Brain aneurysm, 2006, available from: http://www.brain-aneurysm.com/ Search PubMed.
- D, H.E.D.M.J.M.M.E., Therapeutic potential of the lazaroids (21-aminosteroids) in acute central nervous system trauma, ischemia and subarachnoid hemorrhage. Advances in pharmacology, 1994, 28, 221–68.
- E. D. Hall, Efficacy and mechanisms of action of the cytoprotective lipid peroxidation inhibitor tirilazad mesylate in subarachnoid haemorrhage, European Journal of Anaesthesiology, 1996, 13(3), 279–289 Search PubMed.
- L. Sarah, M. A. Smith, M. Heidi, B. S. Scherch and Edward D. Hall, PhD, Protective effects of tirilazad mesylate and metabolite U-89678 against blood-brain barrier damage after subarachnoid hemorrhage and lipid peroxidative neuronal injury, Journal of Neurosurgery, 1996, 84(229–33) Search PubMed.
- J. Ptacek, et al., Global analysis of protein phosphorylation in yeast, Nature, 2005, 438(7068), 679–684 CrossRef CAS.
- J. Ellis et al., Studying Protein Phosphorylation in Low MW CSF Fractions with capLCâ^'ICPMS and nanoLCâ^'CHIP-ITMS for Identification of Phosphoproteins, J. Proteome Res., 2008, 7(11), 4736–4742 CrossRef CAS.
- R. R. Neubig and D. P. Siderovski, Regulators of G-protein signalling as new central nervous system drug targets, Nat. Rev. Drug Discovery, 2002, 1(3), 187–197 CrossRef CAS.
- R. G. Giffard et al., Chaperones, protein aggregation, and brain protection from hypoxic/ischemic injury, J. Exp. Biol., 2004, 207(18), 3213–3220 CrossRef.
- W. Yang et al., Cerebral ischemia/stroke and small ubiquitin-like modifier (SUMO) conjugation—a new target for therapeutic intervention?, J. Neurochem., 2008, 106(3), 989–999 CrossRef.
- K. Satoh et al., A novel membrane protein, encoded by the gene covering KIAA0233, is transcriptionally induced in senile plaque-associated astrocytes, Brain Res., 2006, 1108(1), 19–27 CrossRef.
- L. Ni et al. and C. Cystatin, Associated With Hemorrhagic and Ischemic Stroke, Is a Strong Predictor of the Risk of Cardiovascular Events and Death in Chinese, Stroke, 2007, 38(12), 3287–3288 CrossRef.
- Q. Tian and J. Wang, Role of Serine/Threonine Protein Phosphatase in Alzheimer's Disease, Neurosignals, 2002, 11(5), 262–269 CrossRef CAS.
- I. Skoog et al., A Population-based Study of tau Protein and Ubiquitin in Cerebrospinal Fluid in 85-year-olds: Relation to Severity of Dementia and Cerebral Atrophy but not to the Apolipoprotein E4 Allele, Neurodegeneration, 1995, 4(4), 433–442 CrossRef.
- I. B. Levitan, Modulation of ion channels by protein phosphorylation and dephosphorylation, Annu. Rev. Physiol., 1994, 56, 193–212 CrossRef CAS.
- L. A. Raymond, C. D. Blackstone and R. L. Huganir, Phosphorylation of amino acid neurotransmitter receptors in synaptic plasticity, Trends Neurosci., 1993, 16(4), 147–153 CrossRef CAS.
- R. J. Abbott, I. F. Pye and S. R. Nahorski, CSF and plasma GABA levels in Parkinson's disease, J. Neurol., Neurosurg. Psychiatry, 1982, 45(3), 253–256 CrossRef CAS.
- L. Wolfgang, Relationship Between GABA Concentrations in Cerebrospinal Fluid and Seizure Excitability, J. Neurochem., 1982, 38(1), 293–295 CrossRef.
- A. L. Mah et al., Identification of Ubiquilin, a Novel Presenilin Interactor That Increases Presenilin Protein Accumulation, J. Cell Biol., 2000, 151(4), 847–862 CrossRef.
| This journal is © The Royal Society of Chemistry 2010 |
