Bioimaging of copper alterations in the aging mouse brain by autoradiography, laser ablation inductively coupled plasma mass spectrometry and immunohistochemistry
Li-Ming
Wang
a,
J. Sabine
Becker
*b,
Qi
Wu
a,
Marcus F.
Oliveira
c,
Fernando A.
Bozza
d,
Andrea L.
Schwager
e,
John M.
Hoffman
f and
Kathryn A.
Morton
a
aDepartment of Radiology, University of Utah, Salt Lake City, UT, USA
bCentral Division of Analytical Chemistry, Research Centre Juelich, D-52425 Juelich, Germany. E-mail: s.becker@fz-juelich.de; Web: www.brainmet.com
cLaboratorio de Bioquimica Redox, Instituto de Bioquímica Medica, and Laboratório Associado de Inflamação e Metabolismo, Instituto Nacional de Biologia Estrutural e Bioimagem (INBEB), Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
dInstituto de Pesquisa Clinica Evandro Chagas, Fundação Oswaldo Cruz, and Laboratório Associado de Inflamação e Metabolismo, Instituto Nacional de Biologia Estrutural e Bioimagem (INBEB), Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
eGraduate Program in Neuroscience, University of Utah, Salt Lake City, UT, USA
fDepartments of Radiology and Neurology, University of Utah, Salt Lake City, UT, USA
First published on 1st April 2010
Abstract
Copper may play an important role in the brain in aging and neurodegenerative diseases. We compare the active Cu uptake, Cu-containing enzyme levels, and total Cu distribution in the brains of young and aging mice. 67Cu was administered intravenously to 2, 7-9, and 14 month old mice. Active uptake of 67Cu in the brain was measured at 24 h by digital phosphor autoradiography. Cerebral superoxide dismutase-1 (SOD-1) and cytochrome-C oxidase subunit-1 (CCO-1) levels were analyzed by immunohistochemistry. The total Cu distribution in brain section was determined by imaging laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). In aging mice, active 67Cu uptake and SOD-1 levels were significantly decreased in the brain, whereas blood 67Cu and CCO-1 levels were similar for all mice, irrespective of age. Paradoxically, global Cu cerebral content was increased in aged mice, suggesting that regulation of active Cu uptake by the brain may be linked to total Cu levels in an attempt to maintain Cu homeostasis. However, focal areas of both decreased Cu uptake and Cu content were noted in the striatum and ventral cortex in aging mice. These focal areas of Cu deficit correspond to the regions of greatest reduction in SOD-1 in the aged mice. In aging, dysregulated Cu homeostasis may result in decreased SOD-1 levels, which may contribute to oxidative vulnerability of the aging brain. This study illustrates the importance of a multi-modality approach in studying the biodistribution and homeostasis of Cu in the brain.
1. Introduction
The presence and bioavailability of essential and toxic metals in the brain is important in normal brain function, in the pathogenesis of neurodegenerative diseases and in aging.1 Copper is an essential transition metal that serves as a cofactor of various enzymes. Within the brain, Cu serves a variety of critical functions, both with respect to neurotransmitter modulation, and oxidative protection. Brain levels of Cu-containing enzymes that protect against oxidative damage are high. These include copper–zinc superoxide dismutase (SOD-1), which is located within the cell membrane and cytoplasm, and cytochrome C oxidase subunit-1 (CCO-1), which is located at the mitochondrial inner membrane.A number of enzymes and proteins contribute to the coordinated regulation in uptake, distribution and removal of Cu by the brain. Disregulated Cu homeostasis may result from alterations in any of these molecules. In several neurodegenerative diseases (e.g. Alzheimer's, Parkinson's, Wilson's diseases), abnormal Cu deposition has been observed within specific areas of the brain.2–4 Amyloid precursor protein (APP) and tau protein sequester (and may remove) Cu from normal brain tissue in Alzheimer's disease.4,5 This results in an increase in total brain Cu, but a deficiency in bioavailable Cu. A deficit of bioavailable Cu can also result in other neurodegenerative disorders.6 Menkes disease is a genetic disorder characterized by defects in a P-type ATPase alpha polypeptide that transports Cu across cell membranes, resulting in deficiencies in copper and essential cuproenzymes both systemically and in the brain.7 Conversely, free Cu is damaging to the brain by generation of harmful reactive oxygen species (ROS).
Older individuals may be more susceptible to brain injury than those who are younger. The causes of this vulnerability are incompletely understood and may be multifactorial. However, the generation of reactive oxygen species (ROS) with subsequent oxidative damage to neural tissue is a common injury pathway for many types of cerebral insults.8 The purpose of this study is to determine whether the brains of young and old mice differ in active Cu uptake, Cu content and distribution, and whether these changes are associated with altered levels of Cu-containing enzymes that protect against oxidative damage.
To study Cu distribution in brain tissues (imaging or mapping) different and complementary analytical in vivo and ex vivo techniques are available.9 In vivo positron emission tomography (PET) imaging can be accomplished using 64Cu. Ex vivo techniques include histochemical imaging using cation sensitive fluorescence dyes,10 autoradiography using 67Cu, X-ray fluorescence (XRF), X-ray microanalysis and the more recent technique of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS).11,12 For the current study, phosphor imaging autoradiography was used to study the active uptake of copper after the injection of 67Cu. Imaging LA-ICP-MS was used to measure the total metal distribution in thin tissue sections of brain. Immunohistochemistry was employed to assess SOD-1 and CCO-1 levels in the brains of young and old mice.
2. Experimental
2.1. Animal studies
Six Balb/C mice from each of three age groups (2 months, 7–9 months, and 14 months) were injected by tail vein with a single dose of 2 μCi g−1 body weight (4 × 10−5 mg Cu/g body weight) of 67CuCl2 (Trace Life Sciences, Denton, TX) in 50 μL normal saline. The use of 67Cu (T1/2: 2.58 days) allowed for the assessment of short-term Cu distribution and its comparison to the steady-state Cu distribution, as determined by LA-ICP-MS imaging of brain tissue. Twenty-four hours after injection, mice were euthanized by CO2 inhalation. The brain and a sample of blood were removed. The brain was flash frozen in methylbutane and cut at 20 μm sections by cryomicrotome, mounted on glass slides, and air-dried. Adjacent slices of brain in the same animals were then subjected to either phosphor imaging autoradiography, or were allowed to undergo radioactive decay to background levels (10 half-lives) and were then analyzed by either immunohistochemistry or by imaging LA-ICP-MS.All experiments using live animals were performed in compliance with relevant regulations and institutional guidelines and with approval by the Institutional Animal Care and Use Committee (IACUC) of the University of Utah.
2.2. Copper-67 uptake by the brain
Active uptake of Cu by the brain was determined within the brain slices by digital autoradiography (Fuji BAS-5000, Fujifilm Life Sciences, Stamford, CT). Radioactivity was quantified using Multi Gauge software (Fujifilm Life Sciences), calibrated with 20 μm microtome slices of spiked, flash frozen blood standards, and compared to blood radioactivity from the same animal. Depending on the size of the region, 3–15 individual measurements were made per brain structures per mouse. Selected slices of brain were stained with cresyl violet for anatomic correlation.2.3. SOD-1 and CCO-1 protein levels in the brain
SOD-1 and CCO-1 protein levels were measured in 20 μm cryomicrotomed brains slices by immunohistochemistry. Primary antibodies (Santa Cruz Inc, Santa Cruz, CA) were rabbit polyclonal anti SOD-1 or mouse monoclonal anti CCO-1. Secondary antibodies (goat anti mouse IgG) were conjugated with Alexa Fluor 488®. Imaging of these brain sections was accomplished by digital fluorescence scanning with a 488 nm filter (Fuji LAS-3000, Fijifilm Life Sciences, Stamford, CT). Relative fluorescence (AU mm¬2) was compared in similar brain slices from the different age groups using Multi Gauge software.2.4. Imaging laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS)
Images of the Cu in 20 μm sections of the mouse brains were generated by an established LA-ICP-MS technique, using a quadrupole-based ICP-MS (Agilent 7500ce) coupled to a commercial laser ablation system (New Wave, UP 266).11 The slices of brain were scanned (maximum analyzed area ∼180 mm2) with a focused laser beam (wavelength 266 nm, diameter of laser crater 120 μm and laser power density 1 × 109 W cm−2). The ion intensities of Cu isotopes 63Cu+ and 65Cu+ were measured by LA-ICP-MS (line by line) within the ablated area. Images were generated using new software packages developed at Forschungszentrum Jülich. For quantification purposes, matrix-matched laboratory brain standards with added Cu concentrations (0, 1, 2, 5, 10, 20 μg g−1) were prepared by means of spiking with Cu standard solutions of brain tissue homogenates. Spiked brain homogenates were frozen, cut on a cryostat in 20 μm sections and mounted onto glass slides in the same manner as the brain slices. Details of the calibration procedure are described elsewhere.12 The set of calibration standards were measured together with the brain tissue under the same experimental conditions. Ion images were quantified in LA-ICP-MS by external calibration. The LA-ICP-MS calibration correlation coefficient curves of lab standards for both Cu isotopes were 0.992.2.5. Statistical analysis
Comparisons between groups of animals were made by one-way ANOVA analysis, with application of an a posteriori Bonferroni test.3. Results and discussion
Active uptake of Cu by the brain was measured 24 h after the intravenous injection of 67Cu, and detected by digital high resolution autoradiography. The results are summarized in Fig. 1 and 2. There was no significant difference in blood levels of 67Cu between the groups of mice [2 month (1.81 μCi g−1 + 0.43), 7–9 month (2.21 μCi g−1, +0.53), 14 month (2.30 μCi g−1 + 0.55)]. For all groups, prominent 67Cu uptake was observed in the cerebrospinal fluid (CSF) spaces, with relatively lower uptake in the brain parenchyma (Fig. 1). Compared to 2 month old mice, there was a significant and progressive decline in 67Cu uptake in most parenchymal portions of the brain by 7 months, and in all portions of the brain, including the CSF, by 14 months of age (Table 1, Fig. 2). Uptake of 67Cu by the CSF was 26% of blood levels in 2 month old mice, decreasing to 16% of blood levels (an overall decline of 38%) in 14 month old mice. Uptake of 67Cu by parenchymal regions of the brain was approximately 2.3% of blood levels in 2-month-old mice, decreasing to 0.94% (an overall decline of 58%) in 14-month-old mice.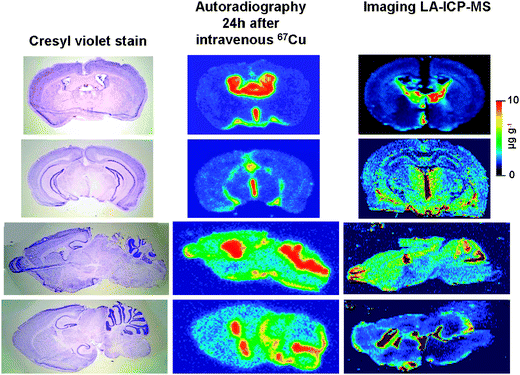 | ||
| Fig. 1 Shown are cresyl violet stained images (left panel) and digital autoradiographic phosphor images (middle panel) of adjacent sections of mouse brain 24 h after intravenous injection of 67Cu. The upper two panels show coronal slices. The lower two panels show sagittal images. The right panel shows similar brain regions in independent specimens analyzed by imaging laser ablation-inductively coupled plasma-mass spectroscopy (LA-ICP-MS). Images demonstrate a similar pattern of Cu uptake and distribution, with significantly greater Cu in the CSF spaces, and relatively lower Cu in the brain parenchyma. | ||
 | ||
| Fig. 2 Coronal (top panel) and sagittal (lower panel) digital autoradiographic phosphor images obtained 24 h after the intravenous injection of 67Cu in 2-month-old, 7-9-month-old, and 14-month-old mice. Images demonstrate a progressive decline in 67Cu uptake in the brain with aging. | ||
| Brain region | 100 × [tissue(μCi g−1)/blood(μCi g−1)] (± SD) | p = ANOVA Bonferroni contrast difference (95% CI) | ||||
|---|---|---|---|---|---|---|
| 2 month | 7–9 month | 14 month | 2 month vs. 7–9 month | 2 month vs. 14 month | 7–9 month vs. 14 month | |
| a statistically significant | ||||||
| CSF | 25.96 | 23.46 | 16.04 | p = 0.20 | p = 0.0001 | p < 0.0001a |
| (1.48) | (1.28) | (1.05) | −0.0250 (−0.0635 to 0.0134) | −0.0992 (−0.1352 to −0.0631)a | −0.0742 (−0.1074 to −0.0409)a | |
| Cortex | 2.48 | 1.85 | 1.19 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| (0.13) | (0.08) | (0.07) | −0.0063 (−0.0093 to −0.0033)a | −0.0129 (−0.0159 to −0.0099)a | −0.0066 (−0.0087 to −0.0045)a | |
| Hippocampus | 2.37 | 1.73 | 1.01 | p = 0.0008 | p < 0.0001 | p < 0.0001 |
| (0.16) | (0.01) | (0.07) | −0.0063 (−0.0099 to −0.0027)a | −0.0135 (−0.0168 to −0.0102)a | −0.0072 (−0.0096 to −0.0048)a | |
| Striatum | 1.85 | 1.41 | 0.8 | p = 0.03 | p < 0.0001 | p < 0.0001 |
| (0.17) | (0.11) | (0.06) | −0.0044 (−0.0084 to −0.0005)a | −0.0095 (−0.0127 to −0.0064)a | −0.0051 (−0.0076 to −0.0027)a | |
| Pons/Medulla | 2.03 | 1.62 | 0.76 | p = 0.11 | p < 0.0001 | p < 0.0001 |
| (0.19) | (0.16) | (0.03) | −0.0040 (−0.0091 to 0.0010) | −0.0126 (−0.0174 to −0.0079)a | −0.0086 (−0.0125 to −0.0047)a | |
| Cerebellum | 2.63 | 2.11 | 0.95 | p = 0.12 | p < 0.0001 | p < 0.0003 |
| (0.22) | (0.24) | (0.04) | −0.0052 (−0.0118 to 0.0014) | −0.0168 (−0.0224 to -0.0113)a | −0.0116 (−0.0175 to −0.0058)a | |
The quantitative Cu distribution was analyzed in selected brain slices of 2 month and 14 month old mice by imaging LA-ICP-MS. Examples of coronal slices of brain in these mice are illustrated in Fig. 3. With aging, Cu distribution becomes more heterogeneous. While global Cu content was increased in older mice, Cu in the striatum and ventral cortex showed an approximately 50% reduction in 14 month, compared to 2 month old mice.
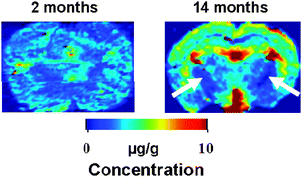 | ||
| Fig. 3 Coronal slices of brain in 2-month-old (left panel) and 14-month-old (right panel) mice imaged by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Images demonstrate relatively homogeneous distribution of Cu in the brains of 2 month old mice. In 14 month old mice, there is a global increase in Cu content in the brain. White-matter regions as the capsula interna (arrows) show low Cu content in aging mice. | ||
As measured by digital fluorescence of immunostained slices of brain, there was a 20% decrease in global brain content of SOD-1 in the brains of the oldest animals examined (14 months), compared to 2 and 7–9 month cohorts (Table 2, Fig. 4), which was predominantly due to focal decreases in SOD in the striatum and ventral cortex. These same regions are those showing more focal decreases in Cu content by LA-ICP-MS. The SOD-1 levels in the brains of 2 and 7–9 month mice were not significantly different. There was no statistical difference in the content of CCO-1 in the brains of any of the cohorts of animals examined. The results of immunostaining approach are illustrated in Fig. 4 and summarized in Table 2.
 | ||
| Fig. 4 Digital fluorescence images of comparable brain sections in 2-month-old and 14-month-old mice show a decrease in immunostaining of superoxide dismutase-1 (SOD-1) in aging mice, most pronounced in the striatum and ventral cortex. There is no change in content or distribution of cytochrome-C oxidase subunit-1 (CCO-1) between young and old mice. | ||
| Enzyme | Fluorescence intensity/AU mm¬2 × 10¬3 | p = ANOVA Bonferroni contrast difference (95% CI) | ||||
|---|---|---|---|---|---|---|
| 2 month | 7–9 month | 14 month | 2 month vs. 7–9 month | 2 month vs. 14 month | 7–9 month vs. 14 month | |
| a statistically significant | ||||||
| SOD-1 | 513.38 | 537.35 | 417.17 | 0.602 | 0.016 | 0.003 |
| (32.19) | (31.86) | (17.83) | 23.97 (−69.95 to 117.90) | −96.21 (−172.52 to −19.89)a | −120.18 (−196.49 to −43.88)a | |
| CCO-1 | 614.72 | 698.06 | 560.43 | 0.76 | 0.57 | 0.18 |
| (75.34) | −80.97 | (58.25) | 83.34 (−146.02 to 312.71) | −54.29 (−251.79 to 143.22) | −137.63 (−344.49 to 69.23) | |
A critical essential metal for many aspects of normal neurological functions, Cu in the brain (at μg g−1 levels) plays an important role in regulating synaptic transmissions and ion channel function in the brain.13,14 As a transition metal with redox behavior, Cu is also a critical cofactor for enzymes, most notably SOD1 and CCO-1, which protect the brain from oxidative injury. Cu abnormalities have been implicated in a number of neurodegenerative disorders, including amytrophic lateral sclerosis (ALS), Menkes disease, and Alzheimer's disease (AD).6 In AD brain, Cu is involved in the formation of beta-amyloid plaques.4
Copper homeostasis in the brain is complex, involving active uptake, excretion, and a complex interplay between bioavailable copper and both free and tissue bound forms. There is a labile pool of Cu in the brain, and Cu is found widely in the brain parenchyma, in multiple cell types.6,10 The mechanisms for Cu uptake in the brain are incompletely understood and may be multifactorial. This is supported by recent evidence that the copper transporter-1 (Ctr-1) may be more highly expressed in the choroid plexus and the ATP7A copper transporter may be more important in the brain parenchyma and its capillaries.15
The CSF functions both in an excretory capacity, for transport of toxins and metabolites from the brain to the blood stream, and in a nutrient capacity, delivering vitamins and other essential molecules to the extracellular fluid (ECF) of the brain parenchyma, where they are then available for uptake by cells. Many substances diffuse freely from the ventricles into the ECF space of the brain parenchyma, and recent studies postulate that blood-CSF (BCSF) barrier constitutes the primary barrier for regulation of Cu uptake in the brain.13 In the present study, there was no change in blood levels of 67Cu with aging. The concentration of 67Cu in the CSF was high (up to 26% of blood levels). There was a decrease in uptake of 67Cu both in the CSF and in the brain parenchyma with aging. However, if the BCSF interface constituted the only barrier to uptake of Cu by the brain, then it would be expected that the decline in brain parenchymal levels of 67Cu would parallel those in the CSF. In fact, brain parenchymal levels show an earlier and more significant decrease in 67Cu uptake than was observed in the CSF. Compared to 2 month old mice, brain parenchyma decreased by 23% in 7–9 month mice, and 58% in 14 month old mice, while that in the CSF decreased by 10% in 7–9 month mice (not statistically different) and by 38% in 14 month old mice. These observations suggest dual mechanisms for modulation of Cu uptake by the brain, one at the blood/CSF level and one at the CSF/brain or blood/brain level, both of which may undergo changes with senescence.
As a function of age, there is an inverse relationship between active Cu uptake (as measured by 67Cu) and total content of Cu in the brain (as measured by LA-ICP-MS). While active Cu uptake is decreased, total Cu content in the brain is globally increased in aging mice. This finding suggests that mechanisms regulating Cu uptake may be linked to overall Cu content in the brain in an attempt to maintain Cu homeostasis at a constant level. With aging, this mechanism may undergo global and regional dysregulation. Heterogeneity is also noted in the distribution of Cu with aging, with more focal decreases both in Cu uptake and content in the striatum and ventral cortex. These specific regions are those most notable for decreased SOD-1 in the aging mice. In the younger animals, Cu is more uniformly distributed throughout the brain. Whether regional decreases in bioavailable Cu are responsible for the decreased SOD-1 levels in these same regions, or whether relative Cu deficiency in these regions is secondary to a deficit in the Cu-containing enzymes in these regions is not yet known.
The lifespan of a mouse is approximately 24 months. Even more dramatic alterations in cerebral Cu uptake and SOD-1 levels may exist in very old animals, although this has yet to be investigated. Previous studies have also supported a decrease in SOD-1 in the brain of aging animals.16,17 However, at least one older study reported that, while SOD decreases in the brains of aging mice, there is actually an increase in total copper content in the brains of older mice.15
4. Conclusions
These studies illustrate the necessity of a multimodality approach to understanding the role of metals, such a Cu, in the brain. Additional research is needed to understand the complex relationship between total Cu content, active uptake and excretion of Cu, and the expression of Cu transporters, chaperones, and Cu-containing molecules in the brain. How these factors contribute to the bioavailability of a labile pool of Cu for synaptic transmission and incorporation into critical cuproenzymes remains to be determined. An example of the complex relationship between bioavailable Cu and total Cu content occurs in Alzheimer’s disease, where total Cu in the brain has been shown to be increased. However, Cu is sequestered by amyloid precursor protein, resulting in a functional deficiency of bioavailable Cu within normal neuronal tissues. This deficiency in bioavailable Cu may contribute to the pathogenesis of AD.18 Further studies will be required to determine whether similar decreases in bioavailable Cu could exist with aging.The addition of newer synergistic imaging approaches, such MALDI-MS (matrix assisted laser desorption/ionization mass spectrometry), may provide additional information regarding the nature of Cu-containing molecules in the brain.19,20 Copper-64, a positron emitter with a convenient half life (12.7 h), may provide an opportunity for non-invasive and longitudinal observations regarding active Cu uptake in the brain in live subjects by positron emission tomography (PET). In summary, this study illustrates the importance of a multi-modality approach to studying the biodistribution and flux of Cu in the brain.
From these experiments it can be concluded that aging may result in both global and regional alterations in Cu uptake, content and distribution, and in SOD-1 levels. This may contribute to the heightened vulnerability of the brain to injury in the aged. A multi-modality approach will be required to understand the significance of these observations.
Acknowledgements
Supported by grants from the NIBIB/NIH (EB 005728) and CNPq (through Institutos Nacionais de Ciência e Tecnologia 2008), FAPERJ (MFO and FAB through Jovens Cientistas do Nosso Estado and Edital de Apoio a Grupos Emergentes de Pesquisa do Rio de Janeiro) and ICGEB. MFO and FAB are research scholars from CNPq.References
- A. Sigel, H. Sigel and R. K. O. Sigel, Neurodegenerative Diseases and Metal Ions, John Wiley & Sons, Ltd Chichester, 2006 Search PubMed.
- G. J. Liu, W. D. Huang, R. D. Moir, C. R. Vanderburg, B. Lai, Z. C. Peng, R. E. Tanzi, J. T. Rogers and X. D. Huang, J. Struct. Biol., 2006, 155, 45–51 CrossRef CAS.
- L. M. Miller, Q. Wang, T. P. Telivala, R. J. Smith, A. Lanzirotti and J. Miklossy, J. Struct. Biol., 2006, 155, 30–37 CrossRef CAS.
- A. I. Bush and R. E. Tanzi, Neurotherapeutics, 2008, 5, 421–432 CrossRef CAS.
- J. Su. Becker, M. Zoriy, M. Przybylski and J. S. Becker, J. Anal. At. Spectrom., 2007, 22, 63–68 RSC.
- P. Zatta and A. Frank, Brain Res. Rev., 2007, 54, 19–33 CrossRef CAS.
- S. G. Kaler, Am. J. Clin. Nutr., 1998, 67, 1002S–1034S.
- C. Kaur and E. A. Ling, Curr. Med. Chem., 2008, 15, 3068–3080 CrossRef CAS.
- J. S. Becker, A. Matusch, C. Palm, D. Salber, K. Morton and J. Su. Becker, Metallomics, 2010, 2, 104–111 RSC.
- M. Kozma, P. Szerdahelyi and P. Kása, Acta Histochem., 1981, 69, 12–17 Search PubMed.
- J. S. Becker, Inorganic Mass Spectrometry: Principles and Applications, John Wiley and Sons, Chichester, 2007 Search PubMed.
- J. S. Becker, M. Zoriy, A. Matusch, D. Salber, C. Palm and J. Su. Becker, Mass Spectrom. Rev., 2010, 29, 156–175 CAS.
- M. S. Horning and P. Q. Trombley, J. Neurophysiol., 2001, 86, 1652–1660 CAS.
- M. L. Schlief and J. D. Gitlin, Mol. Neurobiol., 2006, 33, 81–90 Search PubMed.
- B.-S. Choi and W. Zheng, Brain Res., 2009, 1248, 14–21 CrossRef CAS.
- H. R. Massie, V. R. Aiello and A. A. Iodice, Mech. Ageing Dev., 1979, 10, 93–99 CrossRef CAS.
- A. Gupta, M. Hasan, R. Chander and N. K. Kapoor, Gerontology, 1991, 37, 305–309 CrossRef CAS.
- C. Treiber, A. Simons, M. Strauss, M. Hafner, R. Cappai, T. A. Bayer and G. Multhaup, J. Biol. Chem., 2004, 279, 51958–64 CrossRef CAS.
- J. Su. Becker, S. Mounicou, M. Zoriy, J. S. Becker and R. Lobinski, Talanta, 2008, 76, 1183–8 CrossRef CAS.
- J. S. Becker and N. Jakubowski, Chem. Soc. Rev., 2009, 38, 1969–1983 RSC.
| This journal is © The Royal Society of Chemistry 2010 |
