Parallel on-line detection of a methylbismuth species by hyphenated GC/EI-MS/ICP-MS technique as evidence for bismuth methylation by human hepatic cells†
Markus
Hollmann
*a,
Jens
Boertz‡
a,
Elke
Dopp
b,
Joerg
Hippler
a and
Alfred Vitalis
Hirner
a
aInstitute of Environmental Analytical Chemistry, University of Duisburg-Essen, Universitaetsstrasse 3-5, 45141 Essen, Germany. E-mail: markus_hollmann@uni-due.de; Fax: +49 (0)201 1833951; Tel: +49 (0)201 1833238
bInstitute of Hygiene and Occupational Medicine, University of Duisburg-Essen, Hufelandstrasse 55, 45122 Essen, Germany. E-mail: elke.dopp@uni-due.de; Fax: +49 (0)201 7234546; Tel: + 49( 0)201 7234578
First published on 6th October 2009
Abstract
Methylation of metal(loid)s by bacteria or even mammals is a well known process that can lead to increased toxicity for humans. Nevertheless, reliable analytical techniques and tools are indispensable in speciation analysis of trace elements, especially since environmental or biological samples are usually characterised by complex matrices. Here the methylating capability of hepatic cells was observed in vitro. HepG2 cells were incubated with colloidal bismuth subcitrate, bismuth cysteine and bismuth glutathione, respectively for a period of 24 h. For identification the cell lysate was ethylated by sodium tetraethyl borate under neutral conditions. After cryo focussing by purge and trap, the bismuth speciation was carried out viaGC/EI-MS/ICP-MS. Colloidal bismuth subcitrate and bismuth cysteine were methylated by HepG2 cells, while no methylated bismuth species was detected after incubation with bismuth glutathione.
1. Introduction
Inorganic bismuth compounds are known to show low toxicity,1 so bismuth is commonly used as a lead substitute, e.g. in paintings or alloys.2 Furthermore, it has been used in medicine for many decades as an anti-gastritic and anti-ulcer agent.3 In contrast, organic bismuth compounds like the permethylated trimethylbismuth (TMBi) may cause encephalopathic symptoms4 and are proven to be highly toxic as shown in experiments with cats and dogs.5 After application of inorganic bismuth compounds many cases of bismuth poisoning are well documented, e.g. epidemic-like encephalopathic diseases in France and Australia.6,7 It is commonly assumed that the observed symptoms were caused by conversion of inorganic bismuth to TMBi.8 This can be caused by intestinal microbes as shown by Michalke et al.8,9 Boertz et al. determinated TMBi in the human body after ingestion of colloidal bismuth subcitrate.10 Von Recklinghausen et al. observed the uptake of bismuth glutathione and bismuth citrate by human erythrocytes, lymphocytes and hepatocytes.11 The authors assumed that biomethylation of inorganic bismuth compounds occurs in the human intestinum, however, the methylation of bismuth in the liver is also imaginable. As Styblo et al. have shown for arsenic, the lighter homologue of bismuth, hepatomic cells (HepG2) can methylate inorganic arsenic.12It is already known that heavy metals in living organisms form complexes with sulfur containing molecules like cysteine and glutathione.13,14 Especially the formation of methylmercury cysteine in fish15 as a biologically active substance indicates the importance of small “biomolecules” for transport processes in animals. The formation of bismuth cysteine and bismuth glutathione complexes is also possible as shown by Burford and co-workers.16,17
Since alkylated bismuth compounds tend to decompose in water, the importance of bismuth cysteine complexes in the field of bismuth methylation is emphasized by a study proving the existence of methylbismuth cysteine in aqueous solution.18
Speciation analysis of bismuth compounds is usually carried out by liquid chromatography coupled to mass spectrometry17 as well as LT-GC/ICP-MS.10,19–22 For quantification of bismuth in alloys, stable volatile ethyl derivatives are used for quantitative determination of bismuth by ICP-AES.23 The very same technique has been used for volatilization and determination of methylated bismuth species in human blood samples after ingestion of bismuth(III) citrate–hydroxide complex.24
In the present qualitative study, the transformation of inorganic bismuth to methylated bismuth species by human hepatic cells (HepG2) is investigated by derivatization of these species with sodium tetraethyl borate. Then the volatile bismuth species are detected simultaneously by EI-MS and ICP-MS after gas chromatographic separation, revealing all bismuth containing compounds directly through correlation of both detector signals. This unique analytical system has recently proven its usefulness in the determination of volatile arsenic species generated by the (intestinal) microflora in human feaces.25
2. Experimental
2.1 Reagents and standards
All reagents used were of analytical grade or better and are either purchased from Sigma Aldrich (Buchs, Switzerland) or from Alfa Aesar (Karlsruhe, Germany). Milli-Q water (Millipore, Milford, MA, USA) was used for the preparation of bismuth glutathione and bismuth cysteine. Colloidal bismuth subcitrate (CBS) was prepared according to Asato et al.26 The bismuth complexes of glutathione and cysteine were prepared according to Burford et al.17 In brief: bismuth cysteine and bismuth glutathione were synthesized by adding solid bismuth(III) chloride (BiCl3) to a saturated solution of L-cysteine or glutathione, in ultra-pure water in a molar bismuth to ligand ratio of 2 : 1. The mixture was stirred at room temperature (20 °C) under an ultra pure argon atmosphere.In a further step we isolated both complexes by adding small amounts of methanol until precipitation of a (slightly) yellow solid occurred. For isolation the crystalline product was subsequently filtered through a fibreglass filter. Finally the solid was dried in a vacuum desiccator containing silica gel for several days.
Colloidal bismuth subcitrate was prepared as follows: to a solution of bismuth citrate in aqueous ammonia (25%) and Dulbecco’s phosphate buffered saline (D-PBS, Gibco®, Invitrogen™, Karlsruhe, Germany) hydrochloric acid (37%, p.a., Riedel-de-Haën, Seelze, Germany) was added dropwise until a pH value of 8.5 was reached. The resulting CBS-suspension was used without further treatment and characterization.
The solution of each bismuth compound was prepared separately by dissolving CBS, bismuth cysteine or bismuth glutathione in D-PBS yielding a solution of 2000 mg bismuth compound per kg.
2.2 Instrumentation
The 6890 N gas chromatographic system (Agilent Technologies, Waldbronn, Germany) was equipped with a UNIS 2000 inlet system (Joint Analytical Systems, Moers, Germany) for programmed temperature vaporisation (PTV) after purge and trap sampling.Two detection systems were used simultaneously: a 5973 N EI-MS (Agilent Technologies, Waldbronn, Germany), which served as a molecule selective detector for species conformation, and a 7500a ICP-MS (Agilent Technologies) for sensitive and element selective detection of the analytes. Working parameters for all instruments are listed in Table 1.
| GC conditions | |
| Column | DB-5 MS, 30 m 250 μm 25 μm |
| Initial head pressure | 254.8 kPa |
| Inlet Conditions PTV | |
| Split | 1 : 50 |
| Initial temperature | −100 °C for 10 min |
| Heating rate | 800 °C/min |
| Final temperature | 250 °C for 11 min |
| Oven programme | |
| Initial temperature | 60 °C |
| Cooling rate 1 | −100 °C/min |
| Final temperature 1 | 35 °C for 10 min |
| Heating rate 2 | 30 °C/min |
| Final temperature 2 | 230 °C for 10 min |
| EI-MS parameters | |
| Mass window | 200–400 amu |
| Transfer line temperature | 280 °C |
| MS Quad temperature | 150 °C |
| Ionisation energy | 70 eV |
| ICP-MS parameters | |
| Argon flow | 15 l/min |
| Carrier gas | 0.79 l/min |
| Makeup gas | 0.23 l/min |
| RF-Power | 1540 W |
| Sampling depth | 5.0 mm |
| Isotopes monitored (dwell time) | 203Tl, 205Tl, 209Bi (0.1 s) |
More details of this unique system are described elsewhere.27
2.3 Chromatographic and analytical conditions
To analyze alkylated bismuth species, constant flow separation was employed on a DB-5 MScapillary column (30 m 250 μm 25 μm, J&W Scientific, Agilent Technologies, Waldbronn, Germany). The mobile phase was helium (5.0, Air Liquide, Duesseldorf, Germany). Internal standardization of ICP-MS was done by continuously adding a solution of 10 ng/ml thallium.2.4 Cell culture
Tumorigenic human hepatocellular carcinoma cells (HepG2, HB 8065, ATCC, USA) were cultured in minimal essential medium (MEM, CC-Pro, Neustadt, Germany) with Earle’ BSS and sodium bicarbonate (CC-Pro) supplemented with 10% heat-inactivated foetal calf serum (FCS, Gibco®, Invitrogen™, Karlsruhe, Germany), nonessential amino acids (MEM-NEAM, 0.1 mM), sodium pyruvate (1 mM), and gentamycin (10 mg/mL, all reagents from CC-Pro) at 37 °C under 5% CO2 (99.7%, Air Liquide, Duesseldorf, Germany) and 95% air in a water-jacket incubator (Thermo Forma, Ohio, USA). HepG2 is an adherent cell line that grows as a monolayer.2.5 Incubation of cells
In a 25 cm2 cell culture flask 106 HepG2 cells were cultivated in 5 ml Minimum Essential Medium (MEM) for 24 h. Following substitution of the medium with 10 ml HEPES and evaluation of the cell viability vialight microscopy the cells were exposed to 1 ml of each solution containing either CBS, bismuth cysteine or bismuth gluthathione for 24 h at 37 °C. The final Bi concentrations for incubation were 105 mg/kg (CBS), 93 mg/kg (BiCys2) and 122 mg/kg (BiGSH2), respectively. Cell culture flasks were closed with gas tight caps and Teflon tape. A butyl-rubber septum was glued on the gas tight cap to avoid the loss of volatile compounds during sample transfer.2.6 Sample transfer & derivatization
Transfer and derivatization procedures were slightly modified as described by Boertz et al.10 After incubation the cell media flasks were stored at −80 °C for three hours and subsequently warmed to room temperature for lysis of the cells. Each liquid phase was transferred by a 10 ml syringe, penetrating both septum and cap of the cell media flask, into an empty 25 ml glass vial closed with a butyl-rubber septum. Subsequently the vial was placed in a helium purge gas flow and 1 ml of sodium tetraethylborate solution (1% w/w) (Galab, Geesthacht, Germany, stabilized by 2% (w/w) KOH-solution) and 100 μl Antifoam 289® were added for ethylation. Finally the headspace was purged for 10 minutes to a packed liner that was cooled to −100 °C.3. Results and discussion
3.1 Standard identification
For identification of bismuth cysteine and bismuth glutathione ESI-HR-TOF-MS (positive mode) was performed. The spectrum of bismuth cysteine shows dominant ions at m/z = 449.0033 [M + H]+, which are consistent with the expected molecular ion (calculated 449.0037 amu) indicating the formula BiC6H12N2O4S2 (BiCys2). BiGSH2 was identified at m/z = 822.1396 [M + H]+ in good agreement with the calculated value of the chemical formula BiC20H33N6O12S2 (calculated 822.1402 amu). No significant impurities could be detected in ESI-MS.3.2 Bismuth detection after incubation
Correlation of the ICP-MS signal and TIC of EI-MS revealed all bismuth containing compounds. As shown in Fig. 1a, two bismuth containing compounds (peaks b and d) were detected by ICP-MS as well as EI-MS after incubation of HepG2 with both bismuth cysteine and CBS. | ||
| Fig. 1 (a) Ethylation of hepatic cells incubated with BiCys2 revealed two bismuth containing compounds b and d. (b) Using BiGSH2 for incubation instead, peak b does not occur in the chromatogram. | ||
A magnified view of the TIC (EI-MS) gave six representative peaks which were named as shown in Fig. 1. Taking the ICP-MS signal into account only peaks b and d represented bismuth compounds. All other peaks had their origin in siloxanes which could be derived from either the GC column or the Antifoam agent, which was added during sample preparation.
Peaks b and d were identified by interpreting the fragmentation of these compounds as shown in Fig. 2a and b.
 | ||
| Fig. 2 (a) Fragmentation of peak b indicating diethyl methyl bismuth. (b) Fragmentation of peak d indicating triethyl bismuth. | ||
Consequently, these compounds could be identified as methylbismuth and an inorganic bismuth species forming diethyl methylbismuth and triethyl bismuth after derivatization by sodium tetraethyl borate.
Both chromatograms and mass spectra of bismuth cysteine and CBS, were identical. Peaks a, c, e and f could be identified as siloxanes D3, D4, D5 and D6 by matching the spectra with the NIST database. Considering the blank values (ESI,† Fig. S2 and Fig. S4) their origin is probably the added Antifoam agent.
In contrast, incubation with bismuth glutathione did not lead to the methylated bismuth compound as shown in Fig. 1b.
The methylbismuth species giving peak b did not occur in the chromatogram. For ethylation it is known that artifact formation can happen.28 So we observed carefully the formation of monomethyl species during ethylation of inorganic bismuth compounds.
As Fig. 3 demonstrates, no methylated species occurred during ethylation of the cell culture medium without hepatic cells containing bismuth compounds, nor did methylated bismuth species occur after adding sodium tetraethyl borate to a non-incubated sample of HepG2 in cell culture medium. For ethylation of bismuth it turned out to be important to buffer the solution to a pH value of about 7–8.
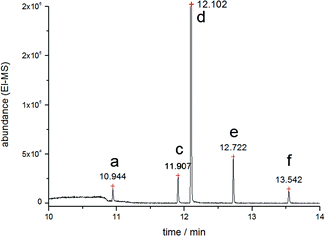 | ||
| Fig. 3 Ethylation of inorganic bismuth compounds in HEPES showing no methylated species. Peak b at tr = 11.564 min is not observed. | ||
Many independent studies have shown that TMBi is a volatile bismuth species detected in biological and environmental samples10,19 although the heat of formation ΔHf = 194 kJ/mol29 is rather high. This means permethylation of bismuth is an energetically inappropriate process. Nevertheless TMBi was detected, which can be explained by catalytically mediated biomethylation. Likewise, an exchange of methyl groups by monomethylated bismuth species as well as a stepwise methylation in the human liver is possible. An earlier work of Hirner and co-workers underlines the time shifted transfer of methyl groups to bismuth by monitoring the increase and decrease of methylated bismuth species in the blood of bismuth exposed probands.24
Nevertheless, further investigations have to be carried out to close the gap between mono- and trimethylated bismuth species both in vitro and in vivo.
One possible explanation of why bismuth cysteine enters the cells is that it uses amino acid carriers. Since bismuth glutathione is a possible excretion of HepG2, an intake into these cells is very unlikely. This mechanism is well studied for mercury cysteine by Clarkson et al.30 The intake of CBS can be explained by the possible formation of an amino acid complex of bismuth by amino acids that are part of the MEM solution.
Due to the lack of suitable bismuth standards and reference materials a quantitative reflection of the results is hardly reliable. Nevertheless, assuming that both methylated and non-methylated bismuth species have similar derivatization efficiencies, comparison of their resulting peak areas in ICP-MSchromatograms shows that approximately 2–3% of the inorganic bismuth species were methylated.
The values listed in Table 2 are given to make a rough estimate of bismuth conversion rates by hepatic cells. Since there are no reliable standards available for analytical balancing, we had to assume that there are no different effects on methylated and non-methylated bismuth compounds during derivatization and ionization, respectively.
Furthermore, to prevent the potential loss of volatile bismuth species, no gas was exchanged during incubation, although HepG2 cells require proper gas exchange for viability. So the methylation yields could also be limited by cell lifetime without oxygen.
Since this study was planned as a qualitative experiment only, we did not determine LODs. Typical LODs for volatile bismuth species on our analytical system are 0.1 to 0.3 ng per m3 of gaseous sample.10
Conclusions
In summary, it was shown that CBS as well as bismuth cysteine is methylated by HepG2 cells, in contrast to bismuth glutathione which is not methylated. This indicates a species dependent intake of bismuth into human hepatic cells and supports the findings of von Recklinghausen et al.11 that no significant uptake of bismuth gluthathione was observed.Further investigations for clarification of which is the dominant species in the cells will be carried out. Likewise it has to be specified whether the monomethyl bismuth species were excreted by the cells or if they were still inside the cells and could only be detected because of the lysis. With respect to the analytics used, the sensitivity and ability of elemental detection of ICP-MS hyphenated to GC/EI-MS providing structural information is a powerful tool in bismuth speciation analysis.
The ICP-MS signal directly indicates where to look for bismuth-containing compounds. Otherwise an identification of bismuth compounds by EI-MS spectra alone would be more complicated, since bismuth is a monoisotopic element which subsequently has no characteristic isotope pattern. Moreover selected ion monitoring at m/z = 209 is not significant since this fragment is dominated by siloxane fragmentation originating from the Antifoam agent or from the GC column.
Besides this, we could show that hepatoma cells are able to methylate CBS and bismuth cysteine but not bismuth glutathione. These results show that human hepatoma cells have the potential to methylate bismuth and that the permethylated species is not generated within the observed period of time. If methylation was not observed as in the case of bismuth glutathione, this might result from the low uptake of this compound into the hepatoma cells. In conclusion, this study shows that bismuth is methylated by human hepatic cells in vitro. It appears from the result that both the intestinal microflora and the liver could be involved in bismuth biotransformation in the human body. In future studies, after suitable standards for method validation are available, further investigations concerning the transformation process and the cellular distribution of bismuth compounds should be done.
Acknowledgements
We would like to thank Prof. A. W. Rettenmeier (Institute of Hygiene and Occupational Medicine, University of Duisburg-Essen, Germany) for providing the facilities to perform the cell culture experiments. We would also like to thank the team of technical assistants, especially Mrs Zimmer for helping us in different ways while performing the cell culture experiments.This work was financed by the German Research Foundation (Deutsche Forschungsgemeinschaft; DFG) “Synthesis and analysis of bismuth species with biological relevance” (“Synthese und Analyse biologisch relevanter Bismutspezies”).
References
- Y. Palmieri, Bismuth: The amazingly “green” environmentally-minded element, Bulletin Bismuth Institute, Special Issue, Brussels, 1993 Search PubMed.
- E. Wiberg, N. Wiberg and A. F. Holleman, Inorganic Chemistry, Academic Press, De Gruyter, San Diego, Berlin, New York, 2001 Search PubMed.
- H. Z. Sun, L. Zhang and K. Y. Szeto, Met. Ions Biol. Syst., 2004, 41, 333–378 CAS.
- E. M. Grandjean, E. Ducommun, G. Gauthier and B. Courvoisier, Schweizerische Medizinische Wochenschrift, 1976, 106, 1006–1011 Search PubMed.
- T. Sollmann and J. Seifter, J. Pharmacol. Exp. Ther., 1939, 67, 17–49 Search PubMed.
- R. J. Burns, D. W. Thomas and V. J. Barron, Aust. N. Z. J. Med., 1974, 4, 109–110 Search PubMed.
- A. Buge and C. Rancurel, Sem. Hop.: Ther., 1976, 52, 514–514 Search PubMed.
- K. Michalke, J. Meyer, A. V. Hirner and R. Hensel, Appl. Organomet. Chem., 2002, 16, 221–227 CrossRef CAS.
- K. Michalke, A. Schmidt, B. Huber, J. Meyer, M. Sulkowski, A. V. Hirner, J. Boertz, F. Mosel, P. Dammann, G. Hilken, H. J. Hedrich, M. Dorsch, A. W. Rettenmeier and R. Hensel, Appl. Environ. Microbiol., 2008, 74, 3069–3075 CrossRef CAS.
- J. Boertz, L. M. Hartmann, M. Sulkowski, J. Hippler, F. Mosel, R. A. Diaz-Bone, K. Michalke, A. W. Rettenmeier and A. V. Hirner, Drug Metab. Dispos., 2009, 37, 352–358 CrossRef CAS.
- U. von Recklinghausen, L. M. Hartmann, S. Rabieh, J. Hippler, A. V. Hirner, A. W. Rettenmeier and E. Dopp, Chem. Res. Toxicol., 2008, 21, 1219–1228 CrossRef CAS.
- M. Styblo, L. M. Del Razo, E. L. LeCluyse, G. A. Hamilton, C. Q. Wang, W. R. Cullen and D. J. Thomas, Chem. Res. Toxicol., 1999, 12, 560–565 CrossRef CAS.
- C. C. Bridges and R. K. Zalups, Chem. Res. Toxicol., 2006, 19, 1117–1118 CrossRef CAS.
- E. M. Krupp, B. F. Milne, A. Mestrot, A. A. Meharg and J. Feldmann, Anal. Bioanal. Chem., 2008, 390, 1753–1764 CrossRef CAS.
- H. H. Harris, I. J. Pickering and G. N. George, Science, 2003, 301, 1203 CrossRef CAS.
- G. G. Briand, N. Burford, M. D. Eelman, N. Aumeerally, L. Chen, T. S. Cameron and K. N. Robertson, Inorg. Chem., 2004, 43, 6495–6500 CrossRef CAS.
- N. Burford, M. D. Eelman, D. E. Mahony and M. Morash, Chem. Commun., 2003, 146–147 RSC.
- J. Hippler, M. Hollmann, H. Juerling and A. V. Hirner, Chem. Pap., 2009, 63, 742–744 Search PubMed.
- J. Feldmann, E. M. Krupp, D. Glindemann, A. V. Hirner and W. R. Cullen, Appl. Organomet. Chem., 1999, 13, 739–748 CrossRef CAS.
- U. M. Gruter, J. Kresimon and A. V. Hirner, Fresenius J. Anal. Chem., 2000, 368, 67–72 CrossRef CAS.
- J. Kresimon, U. M. Gruter and A. V. Hirner, Fresenius J. Anal. Chem., 2001, 371, 586–590 CrossRef CAS.
- E. M. Krupp, R. Grumping, U. R. R. Furchtbar and A. V. Hirner, Fresenius J. Anal. Chem., 1996, 354, 546–549 CAS.
- M. V. Tagle, M. R. F. delaCampa and A. SanzMedel, Anales De Quimica, 1996, 92, 213–218.
- A. V. Hirner et al., in Organic Metal and Metalloid Species in the Environment, ed. A. V. Hirner and H. Emons, Springer, Berlin, 1st edn, 2004, ch. 4, pp. 181–204 Search PubMed.
- R. A. Diaz-Bone, M. Hollmann, O. Würfel and D. Pieper, J. Anal. At. Spectrom., 2009 Search PubMed.
- E. Asato, K. Katsura, M. Mikuriya, U. Turpeinen, I. Mutikainen and J. Reedijk, Inorg. Chem., 1995, 34, 2447–2454 CrossRef CAS.
- J. Kosters, J. Hippler, R. A. Diaz-Bone and A. V. Hirner, J. Anal. At. Spectrom., 2005, 20, 996–999 RSC.
- A. V. Hirner, Anal. Bioanal. Chem., 2006, 385, 555–567 CrossRef CAS.
- C. Elschenbroich, Organometallics, Wiley-VCH, Weinheim, 2006 Search PubMed.
- T. W. Clarkson, J. B. Vyas and N. Ballatorl, Am. J. Ind. Med., 2007, 50, 757–764 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Fig. S1–S5, EI-MS spectra. See DOI: 10.1039/b911945k |
| ‡ New correspondence address: European Commission, Joint Research Centre, Institute for Reference Materials and Measurements (IRMM), Reference Materials Unit, Retieseweg 111, B-2440 Geel, Belgium. Fax: +32 (0)14571548; Tel: +49 (0)14573005; E-mail: jens.boertz@uni-due.de |
| This journal is © The Royal Society of Chemistry 2010 |
