PRIMACENES: novel non-cytotoxic primaquine-ferrocene conjugates with anti-Pneumocystis carinii activity†
Joana
Matos
ab,
Nuno
Vale
ab,
Margaret S.
Collins
cd,
Jiri
Gut
e,
Philip J.
Rosenthal
e,
Melanie T.
Cushion
cd,
Rui
Moreira
f and
Paula
Gomes
*ab
aCentro de Investigação em Química, Universidade do Porto, R. do Campo Alegre, 687, P-4169-007, Porto, Portugal. E-mail: pgomes@fc.up.pt; Fax: +351 220402659; Tel: +351 220402563
bDepartamento de Química e Bioquímica, Faculdade de Ciências, Universidade do Porto, R. do Campo Alegre, 687, P-4169-007, Porto, Portugal. E-mail: pgomes@fc.up.pt; Fax: +351 220402659; Tel: +351 220402563
cResearch Services, Veterans Affairs Medical Center, Cincinnati, OH 45220, USA
dDivision of Infectious Diseases, Department of Internal Medicine, University of Cincinnati, OH 45267-0560, USA
eDepartment of Medicine, San Francisco General Hospital, University of California, CA 94143-0811, USA
fiMed.UL, Faculdade de Farmácia, Universidade de Lisboa, Av. Professor Gama Pinto, P-1600-083, Lisboa, Portugal
First published on 8th July 2010
Abstract
Primacenes, novel ferrocene–primaquine conjugates, were synthesized and screened for their antimalarial and anti-pneumocystis activity. Primacenes obtained by coupling primaquine amino acid derivatives to ferrocenoic acid were significantly active against Pneumocystis carinii and devoid of cytotoxicity, thus being more selective than the parent drug.
Primaquine (PQ, 1 in Scheme 1) is an antimalarial drug with potent gametocytocidal and modest blood-schizontocidal activity, against which no clinically relevant resistance has been associated to date, as the few PQ-tolerant strains of Plasmodium vivax that were so far identified were considered as most likely to present a priori differences regarding their sensitivity to PQ than true development of resistance to the drug.1 PQ is also useful for the treatment of Pneumocystis infections1–4 caused by Pneumocystis jirovecii (formerly P. carinii f. sp. hominis2,3), which is a common cause of pneumonia in immunocompromised individuals and frequently the first serious illness encountered by HIV-infected patients.4–8P. jirovecii also infects other immunocompromised individuals such as those undergoing cancer therapy and organ and bone marrow transplants.9 The mechanisms by which PQ exerts its activity against either of these two pathogens are yet unveiled,1,4,10 but interference with electron transport in the respiratory chain, oxidative stress and impairment of mithocondrial function have been considered.1,10
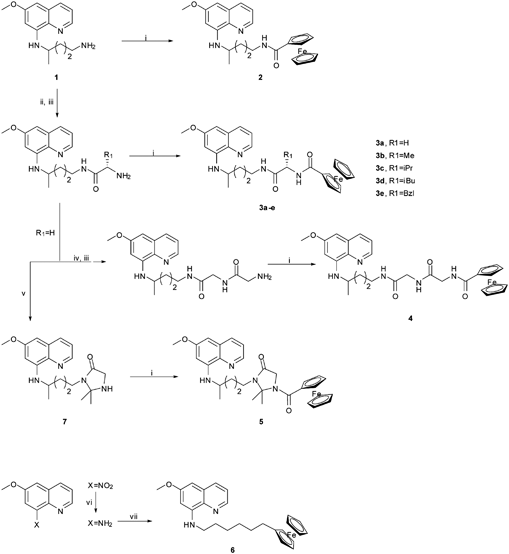 | ||
| Scheme 1 Synthetic routes to Primacenes 2–6: (i) 1 eq. ferrocenecarboxylic acid, 1.1 eq. 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl), 1.1 eq. triethylamine (Et3N), dry CH2Cl2, 90 min in ultra-sound bath (USB) at r.t.; (ii) 1.1 eq. Nα-tert-butoxycarbonyl-protected amino acid N-hydroxy-succinimide ester (BocAA1OSu), 1.1 eq. Et3N, dry CH2Cl2, 24 h at r.t.; (iii) neat CF3COOH, 30 min at r.t., then drop-wise aq. Na2CO3 to pH 11 followed by extraction with CHCl3; (iv) 1.1 eq. BocGlyOSu, dry CH2Cl2, 24 h at r.t.; (v) dry propanone (2 eq. per day), 3 days in refluxing CH3OH with 4 Å molecular sieves; (vi) 5 eq. SnCl2, dropwise conc. HCl, 24 h at r.t., then drop-wise aq. 8 M NaOH to pH 11 followed by extraction with CHCl3; (vii) 1.5 eq. 6-bromo-hexylferrocene, 3.5 h in refluxing Et3N. | ||
Ferrocene (Fc) has been used in the preparation of bioactive organometallic structures, such as Fc-conjugates of peptides and nucleotides.11 Fc-conjugates of clinically relevant drugs have also been described as, for instance, arene isosteres in dopamine receptor ligands12 or organometallic antimalarials derived from 4-aminoquinolines.13 Indeed, the most emblematic example of the contribution of the Fc moiety to the improvement of a drug is that of Ferroquine (FQ), a chloroquine (CQ) isostere with an Fc-based side chain that is 22 times more potent than CQ itself and now under clinical trials.14,15 FQ was found to, like CQ, inhibit hemozoin formation14,15 but, remarkably, it is equally active against CQ-sensitive and CQ-resistant strains of both P. falciparum14,15 and P. vivax.16 This prompted Biot and co-workers to further investigate other mechanisms of action for FQ complementary to a CQ-like interference with hematin polymerization.15,17 These studies allowed important differences to be established between CQ and FQ in terms of basicity and lipophilicity, with FQ being 100 times more lipophilic than CQ at cytosolic pH and to be expectedly 50-fold more concentrated than CQ at the putative acidic pH of the parasite's food vacuole (FV).15 This led to the assumption that FQ properties could be due to a highly efficient: (i) partition into, and/or hydrophobic collapse with, parasitic membrane lipids; (ii) concentration at the lipid site of hemozoin formation; (iii) maintenance of toxic hematin in the aqueous environment, by creating a barrier at the water-lipid interface.15 Additionally, the same research group found that under conditions mimicking the environment of the parasite's FV, FQ undergoes a Fenton-like redox reaction that generates highly reactive hydroxyl radicals known both to be rather pernicious towards membrane unsaturated fatty acids, and to promote chain reactions through peroxidation products.17 Such redox behaviour, which cannot be reproduced by CQ under similar conditions, possibly underlies the distinctive properties of FQ, not only concerning its blood-schizontocidal potency but also, and especially, its apparent lack of propensity to rapidly induce parasite resistance.15–17
The above findings have sprang a number of research studies where Fc was used to enhance the antimalarial activity of fluoroquinolones like ciprofloxacin,18 or to create novel dual-action hybrid drugs with highly promising results.19–22 In this connection, we hypothesized that insertion of the ferrocene core into either PQ or its derivatives might enhance their anti-plasmodial and anti-pneumocystis activities, mainly due to the redox properties of the Fc moiety.
Our previous research has been focused on Imidazoquines, a new class of imidazolidin-4-one peptidomimetic derivatives of PQ that display antimalarial and anti-pneumocystis activity while being resistant to premature metabolic inactivation by oxidative deamination.23–31 Thus, we have prepared a first generation of ferrocene–PQ conjugates (2–5) that we have called the Primacenes, by coupling ferrocenecarboxylic acid to amino groups present in PQ-based scaffolds (Scheme 1). We have also synthesized structure 6, a flexible ferrocene derivative of 8-amino-6-methoxyquinoline (MAQ), the heteroaromatic core of PQ (Scheme 1). To the best of our knowledge, there is no precedent in the literature concerning the preparation and study of any kind of ferrocene–primaquine conjugates. Detailed procedures on compound synthesis, chromatographic and spectroscopic data as well as in vitro biological evaluation against P. falciparum and P. carinii are given in the ESI.†
Table 1 summarizes in vitro data concerning the antimalarial and anti-pneumocystis activity of the first set of nine primacenes prepared. The table also includes data on the cytotoxicity of the four most active compounds against P. carinii, on A549 human lung fibroblast carcinoma and L2 rat lung epithelial cell lines. All assays were run in duplicate.
| Compound | Activity against blood-stage P.falciparum W2a | Activity against P. carinii (72 h) | Cytotoxicity IC50 in μM/μg mL−1 | clogP for model structures 8a–ee | |
|---|---|---|---|---|---|
| IC50 in μM | IC50 in μM/μg mL−1d | A549 cells | L2 cells | ||
| a CQ-resistant strain, against which highly active drugs have IC50s at the low nanomolar range [e.g., IC50 for artemisinin = 6.06 nM]. b Taken from ref. 23 c Taken from ref. 19 d Drug anti-Pneumocystis activity scale: highly active (compounds with an IC50 of <0.010 μg ml−1), very marked (IC50s of 0.011 to 0.099 μg ml−1), marked (IC50s from 0.10 to 0.99 μg ml−1), moderate (IC50s from 1.0 to 9.99 μg ml−1), slight (IC50s from 10.0 to 49.9 μg ml−1), and none (i.e., inactive; IC50s of ≥50 μg ml−1);32 e Calculated using the OSIRIS Property Explorer (http://www.organic-chemistry.org/prog/peo/); ND, not determined. | |||||
| 1 | 3.3b | 1.77 (0.46) | 84.6 (22.0) | 83.9 (21.8) | — |
| 2 | >10 | (>100) | ND | — | |
| 3a | >10 | 28.2 (14.9) | ND | −0.93 | |
| 3b | >10 | 23.1 (13.2) | >175 (>100) | −0.52 | |
| 3c | >10 | 8.33 (4.52) | ND | 0.28 | |
| 3d | 8.33 | 2.69 (1.57) | >171 (>100) | 0.74 | |
| 3e | >10 | 1.15 (0.71) | >162 (>100) | 0.75 | |
| 4 | >10 | 46.8 (27.4) | ND | — | |
| 5 | >10 | 35.5 (20.2) | ND | — | |
| 6 | >10 | 5.76 (2.55) | >226 (>100) | — | |
| 7 | 9.1c | 23 (8.3)c | ND | — | |
An immediately eye-catching observation is that, while ferrocenoylation of PQ and of its derivatives, including of Imidazoquine7 (precursor of Primacene5), is generally detrimental for blood-schizontocidal activity against the CQ-resistant P. falciparum strain W2, the same does not apply to the compounds' anti-Pneumocystis activity. Thus, all tested compounds, except for the parent drug PQ (1) and for the PQ–Leucine–Ferrocene conjugate (3d), had IC50 over 10 μM against P. falciparum. Artemisinin, a potent blood-schizontocidal, was also included in the screening as a comparator, and displayed an IC50 of 6.06 nM. In turn, ferrocenoylation was almost never deleterious for anti-Pneumocystis activity, whose levels depended on the specific compound: while direct ferrocenoylation of PQ, as in 2, deleted the marked activity of the parent drug, the presence of an amino acid residue between the PQ moiety and the ferrocene core (3a–e) preserved activity, which was higher for more lipophilic structures (i.e., derived from amino acids leucine, 3d, and phenylalanine, 3e). Considering clogP values for the series of model amides 8a–e, which reflect the same amino acid variation as on primacenes 3a–e, we find a very good linear correlation between IC50 (3a–e) values and clogP (8a–e) values (Fig. 1).
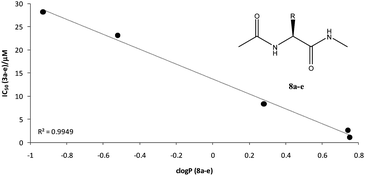 | ||
| Fig. 1 Linear correlation found between IC50 values of Primacenes 3a–e and clogP values estimated (see Table 1) for model amides 8a–e. | ||
Overall, we found that, on a molar basis, primacene 3e was the best anti-Pneumocystis agent of the set, being even more active than PQ. In addition, neither 3e nor the other three most active primacenes (3c, 3d and 6) were cytotoxic against any of the two cell lines used (IC50>100 μg mL−1), in clear contrast with the parent drug (IC50ca. 22 μg mL−1 in both cases).
Finally, it should be mentioned that the distinct performance of primacenes as blood-schizontocidals vs. anti-pneumocystis agents was quite surprising, since our previous findings with PQ derivatives drove us to the assumption that there was a certain degree of correlation between these two types of bioactivity.27,31
At the present stage, we believe that the deletion of blood-schizontocidal activity caused by ferrocen(o)ylation of PQ derivatives or of MAQ is due to the fact that the final primacene structures lack a basic amine. The relevance of such a group has been proposed earlier33 and confirmed by our previous studies with PQ peptide and peptidomimetic (imidazoquine) derivatives.23–31 To check this hypothesis, we are now working on the synthesis and in vitro evaluation of second-generation primacenes bearing basic amino groups.
Concluding remarks
Novel primaquine-ferrocene conjugates, the Primacenes, have been prepared and screened for their anti-Plasmodium (blood-stage parasites) and anti-Pneumocystis activities. Primacenes were seen to be devoid of significant antimalarial action, but one subset was found to be moderately to markedly active against Pneumocystis carinii. This subset included one compound, 3e, that was more active and markedly less cytotoxic than the parent drug, which is an unparalleled finding for PQ-based anti-Pneumocystis agents. Also, to the best of our knowledge, there is no precedent in the literature concerning the study of primaquine-ferrocene conjugates.Acknowledgements
Thanks are due to FCT (Portugal) and FEDER (European Union) for funding this project (ref. PTDC/QUI/65142/2008 and FCOMP-01-0124-FEDER-007418). NV thanks FCT for a Post-Doctoral grant (ref. SFRH/BPD/48345/2008).Notes and references
- N. Vale, R. Moreira and P. Gomes, Eur. J. Med. Chem., 2009, 44, 937 CrossRef CAS and references therein.
- S. A. Readhead, M. T. Cushion, J. K. Frenkel and J. R. Stringer, J. Eukaryotic Microbiol., 2006, 53, 2 Search PubMed.
- D. L. Hanwksworth, Lancet Infect. Dis., 2007, 7, 3 CrossRef.
- C. F. Thomas Jr. and A. H. Limper, Nat. Rev. Microbiol., 2007, 5, 298 Search PubMed.
- G. R. Seage, E. Losina, S. J. Goldie, A. D. Paltiel, A. D. Kimmel and K. A. Freedberg, J. Acquired Immune Defic. Syndr., 2002, 30, 421.
- A. Morris, J. D. Lundgren, H. Masur, P. D. Walzer, D. L. Hanson, T. Frederick, L. Huang, C. B. Beard and J. E. Kaplan, Emerg. Infect. Dis., 2004, 10, 1713 Search PubMed.
- C. F. Thomas Jr. and A. H. Limper, N. Engl. J. Med., 2004, 350, 2487 CrossRef.
- J. C. Peters.on and M. T. Cushion, Curr. Opin. Microbiol., 2005, 8, 393 CrossRef CAS.
- J. A. Fishman, Antimicrob. Agents Chemother., 1998, 42, 995 CAS.
- S. F. Queener, J. Med. Chem., 1995, 38, 4739 CrossRef CAS.
- H.-B. Kraatz, J. Inorg. Organomet. Polym. Mater., 2005, 15, 83 CrossRef CAS.
- D. Huber, H. Hubner and P. Gmeiner, J. Med. Chem., 2009, 52, 6860 CrossRef CAS.
- M. A. L. Blackie, P. Beagley, K. Chibale, C. Clarkson, J. R. Moss and P. J. J. Smith, J. Organomet. Chem., 2003, 688, 144 CrossRef CAS.
- W. Daher, C. Biot, T. Fandeur, H. Jouin, L. Pelinski, E. Viscogliosi, L. Fraisse, B. Pradines, J. Brocard, J. Khalife and D. Dive, Malar. J., 2006, 5, 11 CrossRef and references therein.
- F. Dubar, J. Khalife, J. Brocard, D. Dive and C. Biot, Molecules, 2008, 13, 2900 Search PubMed and references therein.
- M. L. Leimanis, A. Jaidee, K. Sriprawat, S. Kaewpongsri, R. Suwanarusk, M. Barends, A. P. Phyo, B. Russel, L. Renia and F. Nosten, Antimicrob. Agents Chemother., 2010, 54, 2228 CrossRef CAS.
- N. Chavain, H. Vezin, D. Dive, N. Touati, J.-F. Paul, E. Buisine and C. Biot, Mol. Pharmaceutics, 2008, 5, 710 CrossRef CAS.
- F. Dubar, G. Anquetin, B. Pradines, D. Dive, J. Khalife and C. Biot, J. Med. Chem., 2009, 52, 7954 CrossRef CAS.
- F. Bellot, F. Coslédan, L. Vendier, J. Brocard, B. Meunier and A. Robert, J. Med. Chem., 2010, 53, 4103 CrossRef CAS.
- N. Chavain, E. Davioud-Charvet, X. Trivelli, L. Mbeki, M. Rottmann, R. Brun and C. Biot, Bioorg. Med. Chem., 2009, 17, 8048 CrossRef CAS.
- J. Manosroi, K. Rueanto, K. Boonpisuttinant, W. Manosroi, C. Biot, H. Akazawa, T. Akihisa, W. Issarangporn and A. Manosroi, J. Med. Chem., 2010, 53, 3937 CrossRef CAS.
- N. I. Wenzel, N. Chavain, Y. Wang, W. Friebolin, L. Maes, B. Pradines, M. Lanzer, V. Yardley, R. Brun, C. Herold-Mende, C. Biot, K. Tóth and E. Davioud-Charvet, J. Med. Chem., 2010, 53, 3214 CrossRef CAS.
- P. Gomes, M. J. Araújo, M. Rodrigues, N. Vale, Z. Azevedo, J. Iley, P. Chambel, J. Morais and R. Moreira, Tetrahedron, 2004, 60, 5551 CrossRef CAS.
- M. J. Araújo, J. Bom, R. Capela, C. Casimiro, P. Chambel, P. Gomes, J. Iley, F. Lopes, J. Morais, R. Moreira, E. de Oliveira, V. do Rosário and N. Vale, J. Med. Chem., 2005, 48, 888 CrossRef CAS.
- P. Chambel, R. Capela, F. Lopes, J. Iley, J. Morais, L. Gouveia, J. R. B. Gomes, P. Gomes and R. Moreira, Tetrahedron, 2006, 62, 9883 CrossRef CAS.
- R. Ferraz, J. R. B. Gomes, E. de Oliveira, R. Moreira and P. Gomes, J. Org. Chem., 2007, 72, 4189 CrossRef CAS.
- N. Vale, M. S. Collins, J. Gut, R. Ferraz, P. J. Rosenthal, M. T. Cushion, R. Moreira and P. Gomes, Bioorg. Med. Chem. Lett., 2008, 18, 485 CrossRef CAS.
- N. Vale, J. Matos, J. Gut, F. Nogueira, V. do Rosário, P. J. Rosenthal, R. Moreira and P. Gomes, Bioorg. Med. Chem. Lett., 2008, 18, 4150 CrossRef CAS.
- N. Vale, J. Matos, R. Moreira and P. Gomes, Tetrahedron, 2008, 64, 11144 CrossRef CAS.
- N. Vale, L. Gouveia, R. Moreira and P. Gomes, Eur. J. Med. Chem., 2009, 44, 2506 CrossRef CAS.
- N. Vale, M. Prudêncio, C. A. Marques, M. S. Collins, J. Gut, F. Nogueira, J. Matos, P. J. Rosenthal, M. T. Cushion, V. do Rosário, M. M. Mota, R. Moreira and P. Gomes, J. Med. Chem., 2009, 52, 7800 CrossRef CAS.
- M. T. Cushion, F. Chen and N. A. Kloepfer, Antimicrob. Agents Chemother., 1997, 41, 379 CAS.
- M. J. Portela, R. Moreira, E. Valente, L. Constantino, J. Iley, J. Pinto, R. Rosa, P. Cravo and V. do Rosário, Pharm. Res., 1999, 16, 949 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and chromatographic/spectroscopic data of novel compounds. See DOI: 10.1039/c0md00082e |
| This journal is © The Royal Society of Chemistry 2010 |
