Photo-degradation of amyloid β by a designed fullerene–sugar hybrid†
Yasunori
Ishida
,
Shuho
Tanimoto
,
Daisuke
Takahashi
and
Kazunobu
Toshima
*
Department of Applied Chemistry, Faculty of Science and Technology, Keio University, 3-14-1 Hiyoshi, Kohoku-ku, Yokohama 223-8522, Japan. E-mail: toshima@applc.keio.ac.jp; Fax: +81-45-566-1576; Tel: +81-45-566-1576
First published on 4th August 2010
Abstract
A purpose-designed fullerene–sugar hybrid effectively inhibited amyloid β peptide aggregation and caused degradation of its monomer and oligomers, which are key compounds in Alzheimer's disease. Degradation was achieved using long-wavelength UV radiation in the absence of any additives and under neutral conditions.
Introduction
Proteins are key players in many biological events, including serious diseases. The development of new, smart methods for selective control of specific protein functions is of considerable importance in the fields of chemistry, biology, and medicine. In this context, the possibility of developing an organic photochemical agent which can degrade proteins upon irradiation with a specific wavelength of light under mild conditions and without any additives (such as metals or reducing agents) has attracted much attention.1 Alzheimer's disease (AD)2 is characterized by neuronal loss and the presence of large numbers of senile plaques, consisting of fibrillar aggregates of 40- and 42-residue amyloid β (Aβ) peptides, in the brain.3 In recent studies, it was proposed that soluble oligomers of the Aβ42 peptide were responsible for synaptic dysfunction in the brains of patients with AD and were the key intermediate neurotoxic species in the pathology of AD.4 One way of suppressing Aβ42 assembly in the brain is through small molecules with a high affinity for,5 or cleaving ability toward,6 Aβ42. In this context, Lee and Kim reported the inhibition of Aβ peptide aggregation by a fullerene due to its strong and specific affinity with the KLVFF region of the Aβ peptide.7 We recently discovered that certain fullerene derivatives degraded not only DNA but also proteins under photo-irradiation.8 Herein, in a significant application of this fundamental result, we report that a purpose-designed and synthesized fullerene–sugar hybrid effectively inhibited Aβ peptide aggregation and degraded Aβ peptide monomer and oligomers under photo-irradiation conditions. To the best of our knowledge, this is the first example of degradation of Aβ by light switching under neutral conditions.9Results and discussion
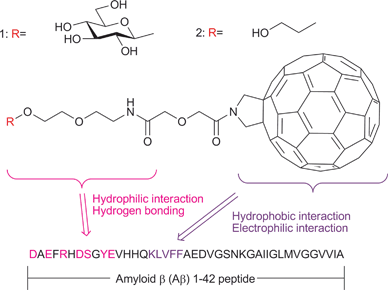
First, we designed and synthesized the novel hybrid molecules 1 and 2, each of which consists of a fullerene attached to a sugar or PEG (poly(ethylene glycol)) derivative (Fig. 1). The design was based on the expectation that the hydrophobic and electrophilic fullerene moiety of the hybrids would show a high affinity for the hydrophobic and electrophilic 16–20 amino acid residue KLVFF in the central part of the Aβ42 peptide. It was also expected that the hydrophilic sugar or PEG segment of the hybrids would enhance the interaction with Aβ42 due to its hydrophilic nature and the formation of one or more hydrogen bonds at the hydrophilic N-terminal of Aβ42. The designed hybrids 1 and 2 were effectively synthesized from known fullerene derivative 310 as outlined in Scheme 1 (also see the ESI†).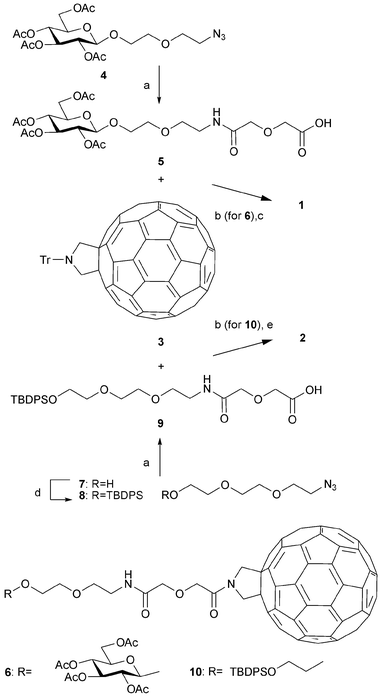 | ||
Scheme 1 Synthesis of fullerene hybrids 1 and 2. a) H2, Pd–C, diglycolic anhydride, THF, rt, 3–6 h, 91 and 60% yields for 5 and 9, respectively; b) (i) TfOH, CH2Cl2, rt, 30 min (ii) EDC, DIEA, rt, 3–4 h, 38 and 18% overall yields for 6 and 10, respectively; c) NaOMe, MeOH–CH2Cl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 0 °C, 4 h, 47%; d) TBDPSCl, imidazole, DMF, rt, 1 h, 86%; e) HF–pyridine, THF, rt, 3 h, 83%. 2), 0 °C, 4 h, 47%; d) TBDPSCl, imidazole, DMF, rt, 1 h, 86%; e) HF–pyridine, THF, rt, 3 h, 83%. | ||
We next examined the binding abilities of 1 and 2 with Aβ42 by observation of the inhibitory effect using a thioflavin T (Th T) fluorescence assay.11 The inhibitory concentration (IC50) was obtained by measuring the Th T fluorescence intensity as a function of the concentration of 1 or 2 while maintaining an Aβ42 concentration of 40 μM. Fig. 2-a shows the plot obtained, which gives IC50 values of 35 and 192 μM for 1 and 2, respectively. These results indicated that the newly designed hybrids had moderate to high affinity with Aβ42, and the affinity of the fullerene–sugar hybrid 1 was stronger than that of the fullerene–PEG hybrid 2.
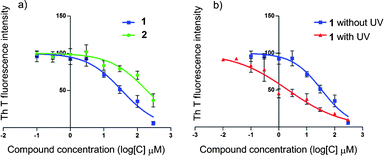 | ||
| Fig. 2 Variation in Th T fluorescence intensity as a function of the concentration of 1 or 2 with and without photo-irradiation. Aβ42 monomer (40 μM) was incubated with each fullerene hybrid (300–0.1 μM) in 10% DMF–Tris-HCl buffer (pH 8.0, 20 mM) at 37 °C for 24 h. After the incubation period, Th T (6.67 μM) was added to each sample. Fluorescence was measured using a Safire (TECAN) microplate reader with excitation and emission wavelengths of 430 nm and 491 nm, respectively. Data were analyzed using GraphPad Prism to obtain IC50 values using log (compound) vs. normalized response-variable slope. a) Dose-response curves showing fractional binding of Th T to Aβ42 fibrils in the presence of fullerene hybrid 1 (blue line) or 2 (green line). b) Dose-response curves showing fractional binding of Th T to Aβ42 fibrils in the presence of fullerene hybrid 1 with (red line) and without (blue line) photo-irradiation (365 nm, 100 W, 10 cm). | ||
Based on these results, we selected 1 for further study, and examined the Aβ42 aggregation-inhibitory ability of 1 at concentrations of 50, 15, 5.0 and 0.15 μM against 5.0 μM Aβ42 monomer12 in 10% DMF–Tris-HCl buffer (pH 8.0, 20 mM) with and without photo-irradiation (365 nm, 100 W). The inhibition of aggregation after incubation of the Aβ42 monomer for 14 h at 25 °C was monitored by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with monoclonal antibody 6E10,12 and the results are shown in Fig. 3-a. Hybrid 1 was found to inhibit Aβ42 aggregation in a dose-dependent manner even without photo-irradiation (lanes 2–4); however, the Aβ42 aggregation-inhibitory ability of 1 under photo-irradiation was higher than without photo-irradiation (lanes 5–7). In addition, it was noteworthy that the Aβ42 monomer disappeared completely on the gel, suggesting that photo-degradation of Aβ42 by 1 had taken place. To determine whether photo-degradation had taken place, we obtained a MALDI-TOF MS profile. Although Aβ42 oligomers were not detected either before or after photo-irradiation in the presence of 1, the MS peak corresponding to Aβ42 monomer disappeared after incubation with 1 under photo-irradiation (see the ESI, Fig. S1†). These results clearly indicated that 1 was capable of degrading Aβ42 monomer upon irradiation with long-wavelength UV light in the absence of other additives. However, at this stage, it was unclear whether the effective inhibition of Aβ42 aggregation by 1 under photo-irradiation resulted from degradation of the Aβ42 monomer only or of both monomer and oligomers (see below).
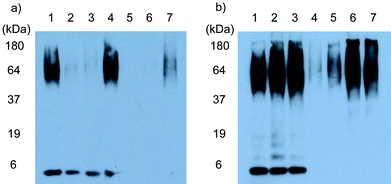 | ||
| Fig. 3 a) Inhibition of Aβ42 aggregation by fullerene hybrid 1. Aβ42 monomer (5.0 μM) was incubated with 1 in 10% DMF–Tris-HCl buffer (pH 8.0, 20 mM) at 25 °C for 2 h with or without photo-irradiation using a UV lamp (365 nm, 100 W) placed 10 cm from the sample, and then further incubated at 25 °C for 14 h. Each sample was analyzed using tricine-SDS-PAGE and immunoblotting with monoclonal antibody 6E10. Lane 1: Aβ42 alone; lanes 2–4: Aβ42 + 1 (concentrations 50, 15 and 5.0 μM, respectively); lanes 5–7: Aβ42 + 1 (concentrations 50, 15, and 5.0 μM, respectively) with photo-irradiation. b) Photo-degradation of Aβ42 by 1. A mixture of Aβ42 monomer and oligomers (5.0 μM) was incubated with 1 in 10% DMF–Tris-HCl buffer (pH 8.0, 20 mM) at 25 °C for 2 h with or without photo-irradiation using a UV lamp (365 nm, 100 W) placed 10 cm from the sample. Each sample was analyzed using tricine-SDS-PAGE and immunoblotting with monoclonal antibody 6E10. Lane 1: Aβ42 alone; lane 2: Aβ42 with photo-irradiation; lane 3: Aβ42 + 1 (50 μM) without photo-irradiation; lanes 4–7: Aβ42 + 1 (concentration 50, 15, 5.0 and 1.5 μM, respectively) with photo-irradiation. | ||
The difference in the Aβ42 aggregation-inhibitory ability of 1 with and without photo-irradiation was also confirmed by Th T fluorescence assay. Fig. 2-b indicates IC50 values of 2 and 35 μM for 1 with and without photo-irradiation, respectively. These results clearly indicate that 1 inhibits Aβ42 aggregation much more efficiently under photo-irradiation.
To determine whether 1 also inhibits the formation of high molecular-weight Aβ42 fibrils, transmission electron microscope (TEM) analysis was used to examine samples of Aβ42 monomer incubated with 1 under photo-irradiation. Comparison of Fig. 4-a and 4-b clearly shows that the formation of high molecular-weight Aβ42 fibrils was effectively prevented by 1 upon incubating the Aβ42 monomer for 7 days at 25 °C under photo-irradiation.
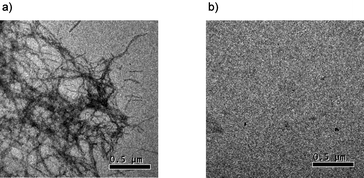 | ||
| Fig. 4 TEM analysis of the prevention of Aβ42 fibril formation by 1. Small aliquots (10 μL) were applied to a grid for TEM analysis. a) Aβ42 monomer (5.0 μM) was incubated in 10% DMF–Tris-HCl buffer (pH 8.0, 20 mM) at 25 °C for 7 days in the absence of 1. b) Aβ42 monomer (5.0 μM) was incubated in 10% DMF–Tris-HCl buffer (pH 8.0, 20 mM) at 25 °C for 2 h in the presence of 1 (50 μM) under irradiation with a UV lamp (365 nm, 100 W) placed at 10 cm from the sample, and then further incubated at 25 °C for 7 days. Images are shown relative to a calibration bar of 0.5 μm. | ||
We also examined the ability of 1 to cause photo-degradation of Aβ42 oligomers using a mixture of Aβ42 monomer and oligomers.12 The results of the photo-degradation experiment, which was monitored by SDS-PAGE and immunoblotting with monoclonal antibody 6E10, are shown in Fig. 3-b. Comparison of lanes 2 and 3 with lane 1 shows that neither photo-irradiation of Aβ42 oligomers in the absence of 1 (lane 2) or treatment of Aβ42 oligomers with 1 but without photo-irradiation (lane 3) resulted in a change in the SDS-PAGE profile. In contrast, lanes 4 and 5 show the disappearance of the SDS-PAGE band corresponding to the Aβ42 oligomers after exposure to 1 and photo-irradiation, which indicates that degradation of Aβ42 oligomers took place. Degradation of the Aβ42 monomer was observed at the same time, as seen in Fig. 3-a. These results show that the fullerene derivative 1 is capable of degrading not only the Aβ42 monomer but also its oligomers upon irradiation with long-wavelength UV light under neutral conditions and in the absence of other additives. Because the degradation of Aβ42 monomer and oligomers by 1 did not take place in the absence of light, it was confirmed that UV light functioned as a trigger to initiate degradation by 1.
In order to investigate the mechanism behind the photo-degradation reaction, an electron paramagnetic resonance (EPR) assay was carried out (see the ESI Fig. S2†). It was found that photo-irradiation of 1 in the presence of the spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) gave products with EPR spectra characteristic of the DMPO-superoxide anion spin adduct DMPO/˙OOH and the DMPO-hydroxyl radical spin adduct DMPO/˙OH, which result from the reactions of DMPO with O2˙− and ˙OH and/or the decay of DMPO/˙OOH.13,14 No peaks corresponding to DMPO/˙OOH or DMPO/˙OH were detected upon treatment of DMPO with 1 without photo-irradiation or upon photo-irradiation of DMPO in the absence of 1. Therefore, the degradation of Aβ42 monomer and oligomers must be due to reactive oxygen species (ROS) produced by photo-excitation of fullerene and O2.15
Conclusions
We have developed a new method for the degradation of Aβ42 monomer and oligomers by photo-irradiation using a fullerene–sugar hybrid under neutral conditions. The results presented here will contribute to the molecular design of novel artificial protein photo-degradation agents. We expect that this method will provide a means of controlling the specific functions of certain proteins.Acknowledgements
This research was supported in part by the High-Tech Research Center Project for Private Universities: Matching Fund Subsidy, 2006–2011, and Scientific Research (B) (No. 20310140) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).Notes and references
- R. Miyake, J. T. Owens, D. Xu, W. M. Jackson and C. F. Meares, J. Am. Chem. Soc., 1999, 121, 7453 CrossRef CAS; G. Plourde, II, A. El-Shafey, F. S. Fouad, A. S. Purohit and G. B. Jones, Bioorg. Med. Chem. Lett., 2002, 12, 2985 CrossRef; F. S. Fouad, J. M. Wright, G. Plourde, II, A. D. Purohit, J. K. Wyatt, A. El-Shafey, G. Hynd, C. F. Crasto, Y. Lin and G. B. Jones, J. Org. Chem., 2005, 70, 9789 CrossRef CAS; A. Suzuki, M. Hasegawa, M. Ishii, S. Matsumura and K. Toshima, Bioorg. Med. Chem. Lett., 2005, 15, 4624 CrossRef CAS; A. Suzuki, K. Tsumura, T. Tsuzuki, S. Matsumura and K. Toshima, Chem. Commun., 2007, 4260 RSC; S. Tanimoto, S. Matsumura and K. Toshima, Chem. Commun., 2008, 3678 RSC.
- J. Hardy and G. A. Higgins, Science, 1992, 256, 184 CrossRef CAS; J. Hardy and D. J. Selkoe, Science, 2002, 297, 353 CrossRef CAS; D. J. Selkoe, Physiol. Rev., 2001, 81, 741 CAS.
- K. Sambamurti, N. H. Greig and D. K. Lahiri, J. Neuromol. Med., 2002, 1, 1 Search PubMed.
- S. A. Gravina, L. B. Ho, C. B. Eckman, K. E. Long, L. Otvos, Jr., L. H. Younkin, N. Suzuki and S. G. Younkin, J. Biol. Chem., 1995, 270, 7013 CrossRef CAS; M. P. Lambert, A. K. Barlow, B. A. Chromy, C. Edwards, R. Freed, M. Liosatos, T. E. Morgan, I. Rozovsky, B. Trommer, K. L. Viola, P. Wals, C. Zhang, C. E. Finch, G. A. Kraft and W. Klein, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 6448 CrossRef CAS; S. Li, S. Hong, N. E. Shepardson, D. M. Walsh, G. M. Shankar and D. Selkoe, Neuron, 2009, 62, 788 CrossRef CAS.
- D. R. Howlett, A. E. Perry, F. Godfrey, J. E. Swatton, K. H. Jennings, C. Spitzfaden, H. Wadsworth, S. J. Wood and R. E. Markwell, Biochem. J., 1999, 340, 283 CrossRef CAS; D. Allsop, G. Gibson, I. K. Martin, S. Moore, S. Turnbull and L. J. Twyman, Bioorg. Med. Chem. Lett., 2001, 11, 255 CrossRef CAS; D. R. Wowlett, A. R. George, D. E. Owen, R. V. Ward and R. E. Markwell, Biochem. J., 1999, 343, 419 CrossRef CAS; F. G. Felice, J. Houzel, J. Garcia-Abreu, P. R. Louzada, Jr., R. C. Afonso, M. N. Meirelles, R. Lent, V. M. Neto and S. T. Ferreira, FASEB J., 2001, 15, 1297; S. J. Wood, L. MacKenizies, B. Malceff, M. R. Hurle and R. Wetzel, J. Biol. Chem., 1996, 271, 4086 CrossRef CAS; F. Yang, G. P. Lim, A. N. Begum, O. J. Ubeda, M. R. Simmons, S. S. Ambegaokar, P. Chen, R. Kayed, C. G. Glabe, S. A. Frautschy and G. M. Cole, J. Biol. Chem., 2005, 280, 5892 CAS; T. Cohen, A. Frydman-Marom, M. Rechter and E. Gazit, Biochemistry, 2006, 45, 4727 CrossRef CAS.
- J. Suh, S. H. Yoo, M. G. Kim, K. Jeong, J. Y. Ahn, M. Kim, P. S. Chae, T. Y. Lee, J. Lee, J. Lee, Y. A. Jang and E. H. Ko, Angew. Chem., Int. Ed., 2007, 46, 7064 CrossRef CAS.
- J. E. Kim and M. Lee, Biochem. Biophys. Res. Commun., 2003, 303, 576 CrossRef CAS.
- S. Tanimoto, S. Sakai, S. Matsumura, D. Takahashi and K. Toshima, Chem. Commun., 2008, 5767 RSC.
- In the period of our study, destruction of amyloid fibrils of a β2-microglobulin fragment using thioflavin T by laser beam irradiation was reported, see: D. Ozawa, H. Yagi, T. Ban, A. Kameda, T. Kawakami, H. Naiki and Y. Goto, J. Biol. Chem., 2009, 284, 1009 Search PubMed . However, we confirmed that Aβ42 monomer and oligomers were not degraded by thioflavin T under the photo-irradiation conditions used in the present study.
- M. Maggini, G. Scorrano and M. Prato, J. Am. Chem. Soc., 1993, 115, 9798 CrossRef CAS.
- H. LeVine, III, Protein Sci., 1993, 2, 404 CAS.
- Aβ42 monomer and oligomers were prepared according to the literature, see: V. Rangachari, B. D. Moore, D. K. Reed, L. K. Sonoda, A. W. Bridges, E. Conboy, D. Hartigan and T. Rosenberry, Biochemistry, 2007, 46, 12451 Search PubMed.
- E. Wertz and B. Bolton, Electron Spin Resonance, McGraw-Hill, New York, 1972 Search PubMed; H. M. Swartz, J. R. Bolton and D. C. Borg, Biological Application of Electron Spin Resonance, Wiley-Interscience, New York, 1972 Search PubMed.
- Y. Yamakoshi, N. Umezawa, A. Ryu, K. Arakane, N. Miyata, Y. Goda, T. Masumizu and T. Nagano, J. Am. Chem. Soc., 2003, 125, 12803 CrossRef CAS.
- B. Halliwell and J. M. C. Gutteridge, Free Radicals in Biology and Medicine, Oxford University Press, Oxford, 1985 Search PubMed; K. J. A. Davies, J. Biol. Chem., 1987, 262, 9895 Search PubMed; M. J. Davies, Biochem. Biophys. Res. Commun., 2003, 305, 761 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experiment procedures for chemical synthesis of fullerene derivatives, ThT binding, inhibition and photo-degradation assay, and MALDI TOF MS, TEM and EPR analyses. See DOI: 10.1039/c0md00075b |
| This journal is © The Royal Society of Chemistry 2010 |