Synthesis and biological evaluation of substituted α- and β-2,3-dihydrofuran naphthoquinones as potent anticandidal agents†
Cristina Pessoa Veloso
Freire
a,
Sabrina Baptista
Ferreira
b,
Nivea Suely Melo
de Oliveira
a,
Ani Beatriz Jackisch
Matsuura
c,
Ivson Lelis
Gama
b,
Fernando de C.
da Silva
b,
Maria Cecília B. V.
de Souza
b,
Emerson Silva
Lima
a and
Vitor Francisco
Ferreira
*b
aFaculdade de Ciências Farmacêuticas, Universidade Federal do Amazonas, 69010-300, Manaus, AM, Brazil
bDepartamento de Química Orgânica, Instituto de Química, Universidade Federal Fluminense, Campus do Valonguinho, CEG, 24020-150, Niterói, RJ, Brazil. E-mail: cegvito@vm.uff.br; Fax: +55 21 26292362; Tel: +55 21 26292345
cCentro de Pesquisas Leônidas Maria Deane, Fundação Oswaldo Cruz–FIOCRUZ, Manaus, AM, Brazil
First published on 4th August 2010
Abstract
We report herein, the synthesis and antifungal activity of substituted α- and β-dihydrofuran naphthoquinones. These compounds were prepared from readily available lawsone and olefins in the presence of cerium(IV) ammonium nitrate (CAN) and were then evaluated against the following six strains of Candida (C.): C. albicans, C. krusei, C. parapsilosis, C. kefyr, C. tropicalis and C. dubliniensis. In addition to exhibiting low cytotoxicity, the furan naphthoquinones proved to be active against these fungi, indicating that they are important scaffolds and potential novel antifungal agents.
1. Introduction
The recent rise of fungal infections may be primarily attributed to the increasing numbers of immunocompromised patients, including those with human immunodeficiency virus infection, cancer patients undergoing chemotherapy and organ transplant recipients taking immunosuppressive drugs.1 Fluconazole and Itraconazole are the most commonly used therapeutics for fungal infections in clinical settings; however, they have major weaknesses regarding their spectra, potency, safety and pharmacokinetic properties. Additionally, the emergence of strains that are resistant to existing antifungal agents, such as Candida, is becoming a significant problem.2,3 Thus, the development of novel, effective antifungal agents is strongly needed.4–6Naphthoquinones represent an important class of biologically active molecules that are widespread in nature.7 Interest in these substances has been intensified in recent years due to their wide range of biological activities. Many natural and synthetic naphthoquinones are known to be potent antitumor,8,9 molluscicidal,10 leischmanicidal,11 anti-inflammatory,12 tripanocidal,13 antibacterial14 and antitubercular15 agents. A number of studies have also demonstrated that substituted naphthoquinone derivatives show a particularly marked activity against fungi.16–22 Recently, our group reported the syntheses of novel dihydrofuran naphthoquinone compounds that show great potential in pharmacological applications.23,24
The present study was focused on the synthesis of α- and β-2,3-dihydrofuran naphthoquinones and their evaluation against six strains of Candida, isolated from the oral cavity of patients with removable dentures, and an ATCC (American Type Culture Collection) reference strain, C. albicans.
2. Material and methods
2.1. Chemistry
The preparation of α- and β-2,3-dihydrofuran naphthoquinones, 1a–i and 2a–i, respectively, were carried out in one step, following an improved synthetic protocol that was previously reported.25,22 Briefly, the furan naphthoquinones were obtained by oxidative [3 + 2] cycloaddition of 2-hydroxy-1,4-naphthoquinone (Lawsone, 3) to the alkene, mediated by cerium(IV) ammonium nitrate (CAN) (Scheme 1). All the compounds were obtained in good yields (10–86% of isolated α and β products) and were fully characterized by proton and carbon nuclear magnetic resonance spectroscopy (1H NMR and 13C NMR, respectively), infrared spectroscopy (IR) and elemental analysis.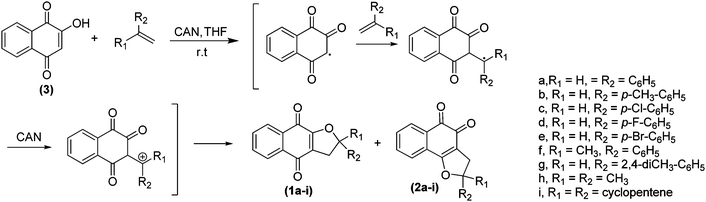 | ||
| Scheme 1 Synthetic route used for the preparation of α- and β-furan naphthoquinones, 1a–i and 2a–i, respectively. | ||
2.2 In vitro antifungal activities
The antifungal activities of 1a–i and 2a–i were assessed against C. albicans, C. krusei, C. parapsilosis, C. kefyr, C. tropicalis and C. dubliniensis, isolated from the oral cavity of patients with removable dentures, by a diffusion technique and minimum inhibitory concentration (MIC) assay. Activity against an ATCC reference strain of C. albicans (90028) was also assessed to ensure result reproducibility.263. Results and discussion
The α- and β-2,3-dihydropyran naphthoquinones were obtained in good chemical yields (10–86% of isolated α and β products) with a high degree of purity. When a solution of 2-hidroxy-1,4-naphthoquinone (3) and alkene in THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was treated with a solution of CAN (2.3 eq.) in THF (room temperature, 3 h), two products, α- and β-2,3-dihydropyran naphthoquinone were generated (Scheme 1). It is noteworthy that all the products were formed regioselectively, with respect to the double bond of the alkene. Products were isolated by silica gel column chromatography (Table 1).
1) was treated with a solution of CAN (2.3 eq.) in THF (room temperature, 3 h), two products, α- and β-2,3-dihydropyran naphthoquinone were generated (Scheme 1). It is noteworthy that all the products were formed regioselectively, with respect to the double bond of the alkene. Products were isolated by silica gel column chromatography (Table 1).
| Entry | R1 | R2 | 1 (%) | 2 (%) |
|---|---|---|---|---|
| 1 | H | C6H5 | 1a (70) | 2a (28) |
| 2 | H | p-CH3–C6H5 | 1b (75) | 2b (13) |
| 3 | H | p-Cl–C6H5 | 1c (85) | 2c (12) |
| 4 | H | p-F–C6H5 | 1d (65) | 2d (12) |
| 5 | H | p-Br–C6H5 | 1e (45) | 2e (10) |
| 6 | CH3 | C6H5 | 1f (60) | 2f (32) |
| 7 | H | 2,4-diCH3-C6H5 | 1g (75) | 2g (20) |
| 8 | CH3 | CH3 | 1h (86) | 2h (12) |
| 9 | — | Cyclopentadiene | 1i (15) | 2i (30) |
All compound structures were confirmed by IR spectral data, 1D and 2D NMR techniques and by elemental analysis. The compounds 1a–c, 1f, 1h, 1i, 2a–c, 2f, 2h and 2i25,27 have been previously described in literature, and our spectroscopic data are consistent with those reported. In general, the IR spectra showed the disappearance of an intense absorption band, referring to the stretching vibrations of O–H from the starting material to the products. All spectra displayed strong carbonyl absorption between 1670–1702 cm−1. Additionally, all proton signals (1H NMR spectra) were assigned to peaks on the basis of their chemical shifts, multiplicities and coupling constants. For the α- derivatives, the 1H NMR spectra primarily displayed the signal of the hydrogen bonded to the ortho carbon of the chromogenic ring as a doublet of doublets between 5.15–6.30 ppm. The hydrogen signals of the CH2 located in the furan ring were assigned in the region of 2.00 to 3.67 ppm as a doublet of doublets. The assignment of α or β-furan naphthoquinones were established based on the analysis of the aromatic hydrogen signal region. The aromatic hydrogens in β-dihydrofuran naphthoquinones are differentiated into four signals, while in the α-dihydrofuran naphthoquinones, there are only two 1H signals due to the symmetric aromatic ring. In general, the 13C NMR spectra of naphthoquinones, 1a–i and 2a–i were easily distinguished by the signals between 79.2–80.0 ppm and 81.0–82.0 ppm, respectively, due to substituted carbon of the chromogenic ring.
The first technique employed to evaluate the antifungal activity of compounds 1a–i and 2a–i was diffusion in solid medium through a hole. In these studies, substances that influence halo formation of 9–14 mm, 14–17 mm or >17 mm are considered to be moderate, active, or very active antifungal agents, respectively. In this regard, all of our naphthoquinones have lower activity than itraconazole and some of them have higher activity than fluconazole. Compounds 1a, 1h and 1i demonstrate significant inhibitory activity on C. albicans, with inhibitory zones of 21.26, 16.97 and 16.62 mm, respectively, as shown in Table 2. Compound 1h, 1i and 2i also show good activity profile against C. dubliniensis.28
| Compound | C. albicans | C. tropicalis | C. kefyr | C. parapsilosis | C. krusei | C. dubliniensis |
|---|---|---|---|---|---|---|
| 1a | 16.97 ± 4.02 | 7.7 ± 2.4 | 1.96 ± 3.4 | 6.88 ± 1.15 | 8.29 ± 2.55 | 4.3 ± 1.00 |
| 1b | 10.43 ± 2.62 | 7.28 ± 2.18 | 4.27 ± 3.71 | 0 | 4.94 ± 1.15 | 2.75 ± 0.92 |
| 1c | 1.5 ± 1.10 | 2.49 ± 0.07 | 5. 27 ± 4.66 | 0 | 6.14 ± 1.00 | 0 |
| 1d | 10.34 ± 2.95 | 3.31 ± 1.35 | 7.28 ± 1.14 | 3. 86 ± 4.59 | 9.12 ± 2.32 | 2.66 ± 1.26 |
| 1e | 1.39 ± 0.40 | 0 | 0 | 9.18 ± 2.10 | 7.50 ± 2.36 | 0.49 ± 0.38 |
| 1f | 16.14 ± 4.25 | 3.83 ± 2.14 | 7.42 ± 0.98 | 0 | 7.05 ± 1.32 | 4.63 ± 1.20 |
| 1g | 1.38 ± 1.02 | 6.42 ± 1.84 | 0 | 0 | 9.87 ± 1.88 | 2.02 ± 0.33 |
| 1h | 21.26 ± 0.87 | 5.63 ± 2.76 | 18.44 ± 0.77 | 9.29 ± 0.62 | 14.63 ± 1.42 | 15.23 ± 1.45 |
| 1i | 16.62 ± 1.99 | 7.8 ± 1.77 | 9.3 ± 1.24 | 0 | 10.76 ± 3.50 | 16.60 ± 0.59 |
| 2a | 7.37 ± 1.74 | 5.7 ± 0.65 | 10.84 ± 3.14 | 7.22 ± 1.13 | 11.99 ± 2.43 | 11.95 ± 0.48 |
| 2b | 0.39 ± 0.78 | 2.26 ± 2.08 | 0 | 0 | 5.77 ± 0.53 | 6.44 ± 1.02 |
| 2c | 1.5 ± 1.3 | 2.21 ± 0.83 | 1.96 ± 3.4 | 6.11 ± 0.82 | 5.65 ± 1.41 | 9.01 ± 0.10 |
| 2d | 1.37 ± 0.47 | 8.59 ± 1.53 | 8.96 ± 1.06 | 8.12 ± 1.23 | 9.8 ± 1.36 | 1.70 ± 1.28 |
| 2e | 6.17 ± 1.64 | 0 | 8.68 ± 6.77 | 7.75 ± 0.51 | 11.28 ± 2.59 | 9.23 ± 0.78 |
| 2f | 8.41 ± 0.4 | 3.48 ± 0.79 | 10.08 ± 0.54 | 7.34 ± 1.01 | 8.52 ± 1.92 | 12.88 ± 0.55 |
| 2g | 2.93 ± 0.71 | 7.51 ± 2.84 | 9.28 ± 0.59 | 9 ± 0.05 | 10.83 ± 1.91 | 4.19 ± 0.05 |
| 2h | 1.64 ± 0.71 | 4.78 ± 0.47 | 8.27 ± 1.16 | 7.41 ± 1.17 | 9.01 ± 2.54 | 0.10 ± 0.21 |
| 2i | 5.85 ± 0.60 | 7.19 ± 1.57 | 10.70 ± 0.86 | 6.14 ± 0.80 | 8.80 ± 1.40 | 18.53 ± 1.36 |
| Itraconazole | 23.06 ± 0 | 23.63 ± 2.03 | 30.88 ± 2.20 | 26.01 ± 0.93 | 30.34 ± 4.32 | 19.45 ± 1.21 |
| Fluconazole | 12.99 ± 2.75 | 14.97 ± 3.56 | 21.23 ± 0.83 | 10.38 ± 0.95 | 9.64 ± 0.91 | 12.84 ± 1.05 |
The second technique used to determine the antifungal activity of furan naphthoquinones was a microdilution quantitative assay in which fungi cultures were exposed to antifungal agents for 24 h. The percentage of growth reduction was then measured by visual inspection and the analysis of the turbidity of the samples in different concentrations. The standard developed by the National Committee for Clinical Laboratory Standards (NCCLS)29 defines the MIC as the lowest antimicrobial agent concentration that inhibits visible growth of a microorganism in the midst of a dilution made in liquid medium. The MIC values of all substances tested, as well as the major antifungal standards are listed in Table 3. The results revealed that substituted α-furan naphthoquinones exhibit greater antifungal activity than β-compounds. There was marked variability among the different strains, with respect to their susceptibility to the test compounds, indicating that there are biological factor(s) affecting strain/species and drug bioactivity. For C. albicans, compound 1h appeared to display the broadest antifungal activity, exhibiting a MIC value of 0.54 μM, as compared with 4.42 μM for Itraconazole and 1.63 μM Fluconazole. Molecule li showed another interesting MIC value, 2.09 μM for C. albicans. Only compounds 1h (MIC = 3.42 μM), 1i (MIC = 3.27 μM) and 2i (MIC = 3.27 μM) demonstrated promising antifungal activity for C. tropicalis in comparison with antifungal drug fluconazole (313.44 μM). Analysis of the results for strains C. kefyr and C. krusei revealed that substituted β-dihydrofuran naphthoquinones showed high antifungal activity, highlighting compounds 2a, 2b, 2c and 2e. For C. dubliniensis, compounds 1i (MIC = 3.27 μM), 2b (MIC = 2.69 μM) and 2e (MIC = 2.19 μM) displayed low antifungal activity as compared with the standard drugs used. Strain C. parapsilosis and ATCC 90028 appeared to be the least sensitive to almost all of the compounds; only 2e (MIC = 8.69 μM) demonstrated minimal antifungal activity for C. parapsilosis.
| Compound | C. albicans | C. tropicalis | C. kefyr | C. parapsilosis | C. krusei | C. dubliniensis | ATCC 90028 |
|---|---|---|---|---|---|---|---|
| 1a | 11.31 | 45.24 | 90.48 | 90.48 | 90.48 | 14.92 | 90.48 |
| 1b | 43.10 | 43.10 | 86.20 | 43.10 | 86.20 | 43.10 | 86.20 |
| 1c | 10.05 | 40.22 | 80.45 | 40.22 | 80.45 | 40.22 | 80.45 |
| 1d | 21.23 | 42.47 | 84.95 | 42.47 | 84.95 | 42.47 | 84.95 |
| 1e | 35.19 | 35.19 | 70.38 | 35.19 | 70.38 | 70.38 | 70.38 |
| 1f | 45.24 | 45.24 | 90.48 | 45.24 | 172.22 | 45.24 | 45.24 |
| 1g | 41.07 | 41.07 | 82.14 | 82.14 | 82.14 | 41.07 | 82.14 |
| 1h | 0.54 | 3.42 | 27.38 | 109.53 | 13.41 | 13.69 | 54.76 |
| 1i | 2.09 | 3.27 | 52.46 | 13.11 | 12.85 | 3.27 | 13.11 |
| 2a | 45.24 | 11.31 | 5.65 | 22.62 | 2.82 | 5.65 | 90.48 |
| 2b | 10.77 | 43.10 | 5.39 | 10.77 | 5.39 | 2.69 | 10.77 |
| 2c | 40.22 | 20.11 | 5.02 | 10.05 | 40.22 | 40.22 | 80.45 |
| 2d | 42.47 | 42.47 | 42.47 | 42.47 | 10.61 | 42.47 | 84.95 |
| 2e | 35.19 | 8.79 | 4.39 | 8.79 | 4.39 | 2.19 | 70.38 |
| 2f | 45.24 | 11.31 | 11.31 | 45.24 | 22.62 | 5.65 | 45.24 |
| 2g | 82.14 | 41.07 | 41.07 | 10.26 | 5.13 | 41.07 | 82.14 |
| 2h | 54.76 | 54.76 | 109.53 | 27.38 | 109.53 | 6.84 | 109.53 |
| 2i | 6.55 | 3.27 | 26.23 | 13.11 | 13.11 | 6.55 | 13.11 |
| Itraconazole | 4.42 | 0.17 | 0.08 | 0.02 | 0.70 | 0.02 | 0.17 |
| Fluconazole | 1.63 | 313.44 | 1.63 | 1.63 | 104.48 | 1.63 | 1.63 |
Additional toxicity tests, hemolytic activity in the blood of mice and cytotoxicity against NIH3T3 murine fibroblast culture using fluorescent Alamar Blue assay, were performed for all compounds (see ESI†). The studied compounds showed no significant hemolytic activity or cytotoxicity at concentrations of 50 μg mL−1 and 12.5 μg mL−1. These results indicated that the tested compounds do not disrupt the cellular membrane or present unspecific cytotoxicity.
In conclusion, we have synthesized a series of substituted α- and β-dihydrofuran naphthoquinones, 1a–i and 2a–i, and evaluated them as antifungal agents against six strains of Candida. The results indicated that 1h was more active than commercially available drugs, itraconazole and fluconazole against C. albicans; 1i also demonstrated good antifungal activity. Compounds 1h, 1i and 2i exhibited a promising antifungal activity against strains C. tropicalis. C. kefyr and C. krusei were sensitive to compounds 2a, 2b, 2c and 2e. When compared with the standard drugs used, 1i, 2b, and 2e showed discrete antifungal activity against C. dubliniensis. Strains C. parapsilosis and ATCC 90028 were the least sensitive to the naphthoquinones. Only compound 2e demonstrated some antifungal activity against C. parapsilosis. Overall, some of the α-furan naphthoquinones exhibited potent antifungal activity, with no hemolytic activity or cytotoxic effects. These compounds are promising agents since only a few naphthoquinones are described in the literature as having antifungal activity.
Acknowledgements
The authors thank FAPERJ, CAPES and CNPq for providing fellowship and grant funding. V.F.F. and E.S.L. are members of the INCT-Redox in Biomedicinal-Redoxoma (MCT/CNPq).References
- D. A. Enoch, H. A. Ludlam and N. M. Brown, J. Med. Microbiol., 2006, 55, 809–818 CrossRef CAS.
- M. M. Canuto and F. G. Rodero, Lancet Infect. Dis., 2002, 2, 550–563 CrossRef CAS.
- J. B. Anderson, Nat. Rev. Microbiol., 2005, 3, 547–556 Search PubMed.
- J. H. Rex, M. G. Rinaldi and M. A. Pfaller, Antimicrob. Agents Chemother., 1995, 39, 1–8 CAS.
- S. Sternberg, Science, 1994, 266, 1632–1634 CrossRef CAS.
- K. S. Shin, S. Lee and B. Cha, Plant Pathol. J., 2007, 23, 113–115 Search PubMed.
- S. B. Ferreira, F. C. da Silva, A. C. Pinto, D. T. G. Gonzaga and V. F. Ferreira, J. Heterocycl. Chem., 2009, 46, 1080–1097 CrossRef CAS.
- E. N. Silva Jr, M. C. B. V. Souza, A. V. Pinto, M. C. Pinto, M. O. O. Goulart, F. W. A. Barros, C. Pessoa, L. V. Costa-Lotufo, R. C. Montenegro, M. O. Moraes and V. F. Ferreira, Bioorg. Med. Chem., 2007, 15, 7035–7041 CrossRef.
- E. N. Silva Jr, C. F. Deus, B. C. Cavalcanti, C. Pessoa, L. V. Costa-Lotufo, R. C. Montenegro, M. O. Moraes, M. C. F. R. Pinto, C. A. Simone, V. F. Ferreira, M. O. F. Goulart, C. K. Z. Andrade and A. V. Pinto, J. Med. Chem., 2010, 53, 504–508 CrossRef.
- A. F. Santos, P. A. L. Ferraz, F. C. de Abreu, E. Chiari, M. O. F. Goulart and A. E. G. Sant'Ana, Planta Med., 2001, 67, 92–95 CrossRef CAS.
- M. J. Teixeira, Y. M. de Almeida, J. R. Viana, J. G. Holanda, T. P. Rodrigues, J. R. C. Prata, I. V. B. Coelho, V. S. Rao and M. M. L. Pompeu, Phytother. Res., 2001, 15, 44–48 CrossRef CAS.
- E. R. Almeida, A. A. S. Filho, E. R. dos Santos and C. A. C. Lopes, J. Ethnopharmacol., 1990, 29, 239–245 CrossRef.
- E. N. da Silva Jr, R. F. Menna-Barreto, M. C. Pinto, R. S. Silva, D. V. Teixeira, M. C. B. V. Souza, C. A. De Simone, S. L. De Castro, V. F. Ferreira and A. V. Pinto, Eur. J. Med. Chem., 2008, 43, 1774–1780 CrossRef.
- A. P. Neves, C. C. Barbosa, S. J. Greco, M. D. Vargas, L. C. Visentin, C. B. Pinheiro, A. S. Mangrich, J. P. Barbosa and G. L. Costa, J. Braz. Chem. Soc., 2009, 20 Search PubMed.
- S. B. Ferreira, F. C. da Silva, F. A. F. M. Bezerra, M. C. S. Lourenço, C. R. Kaiser, A. C. Pinto and V. F. Ferreira, Arch. Pharm., 2010, 343, 81–90 CAS.
- K. T. Vishnu, K. M. Hardesh and K. S. Praveen, Eur. J. Med. Chem., 2009, 8, 3130–3137.
- A. Riffel, L. F. Medina, V. Stefani, R. C. Santos, D. Bizani and A. Brandelli, Brazilian J. Med. Biol. Res., 2002, 35, 811–818 Search PubMed.
- B. H. Kim, J. Yoo, S. H. Park, J. K. Jung, H. Cho and Y. Chung, Arch. Pharmacal Res., 2006, 29, 123–130 Search PubMed.
- M. M. M. Santos, N. Faria, J. Iley, S. J. Coles, M. B. Hursthouse, M. L. Martins and R. Moreira, Bioorg. Med. Chem. Lett., 2010, 20, 193–195 CrossRef CAS.
- C. S. Medeiros, N. T. Pontes-Filho, C. A. Camara, J. V. Lima-Filho, P. C. Oliveira, S. A. Lemos, A. G. F. Leal, J. O. C. Brandao and R. P. Neves, Braz. J. Med. Biol. Res., 2010, 43 Search PubMed.
- V. K. Tandon, H. K. Maurya, N. N. Mishra and P. K. Shukla, Eur. J. Med. Chem., 2009, 44, 3130–3137 CrossRef CAS.
- K. O. Eyong, P. S. Kumar, V. Kuete, G. N. Folefoc, E. A. Nkengfack and S. Baskaran, Bioorg. Med. Chem. Lett., 2008, 18, 5387–5390 CrossRef CAS.
- E. N. Silva Jr, C. F. Deus, B. C. Cavalcanti, C. Pessoa, L. V. Costa-Lotufo, R. C. Montenegro, M. O. Moraes, M. C. F. R. Pinto, C. A. Simone and V. F. Ferreira, J. Med. Chem., 2010, 53, 504–508 CrossRef.
- E. N. Silva Jr, M. C. B. V. Souza, M. C. Fernandes, R. F. S. Menna-Barreto, M. C. F. R. Pinto, F. A. Lopes, C. A. Simone, C. K. Z. Andrade, A. V. Pinto, V. F. Ferreira and S. L. Castro, Bioorg. Med. Chem., 2008, 16, 5030–5038 CrossRef.
- V. Nair, P. M. Treesa, D. Maliakal and N. Rath, Tetrahedron, 2001, 57, 7705–7710 CrossRef CAS.
- The National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard, M27-A2. 2002, 22, 1–30 Search PubMed.
- K. Kobayashi, A. Sasaki, H. Takeuchi and H. Suginome, J. Chem. Soc., Perkin Trans. 1, 1992, 115–21 RSC.
- M. C. C. Ayres, M. S. Brandão, G. M. Vieira Jr, J. C. A. S. Menor, H. B. Silva, M. J. S. Soares and M. H. Chaves, Rev. Brasil. Farmacog., 2008, 18, 90–97 Search PubMed.
- http://www.clsi.org/ .
Footnote |
| † Electronic supplementary information (ESI) available: Materials, methods, preparation and spectroscopic data of the naphthoquinones derivatives; Potential hemolytic of compounds tested; Toxicity test in NIH3T3 Cell Culture; Antifungal tests. See DOI: 10.1039/c0md00074d |
| This journal is © The Royal Society of Chemistry 2010 |
