Chiral ruthenium polypyridyl complexes as mitochondria-targeted apoptosis inducers†
Tianfeng
Chen
a,
Wen-Jie
Mei
*ab,
Yum-Shing
Wong
c,
Jie
Liu
a,
Yanan
Liu
a,
Huang-Song
Xie
b and
Wen-Jie
Zheng
*a
aDepartment of Chemistry, Jinan University, Guangzhou, 510632, China. E-mail: tzhwj@jnu.edu.cn; Fax: +86-20 -8522-1263; Tel: +86-20-8522-1263
bSchool of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Guangzhou, P.R. China. E-mail: wenjiemei@126.com; Fax: +86-20 -8522-1263; Tel: +86-20-8522-1263
cDepartment of Biology, The Chinese University of Hong Kong, Hong Kong SAR, China. E-mail: yumshingwong@cuhk.edu.hk; Fax: +852- 2603-5745; Tel: +852-2609-6389
First published on 16th June 2010
Abstract
A series of chiral ruthenium polypyridyl complexes have been synthesized and evaluated for their in vitro anticancer activities. Λ-[Ru(bpy)2(o-tFMPIP)]Cl2 · 3H2O was identified as a novel mitochondria-targeted complex, which was able to induce mitochondria-mediated apoptosis in melanoma A375 cells through regulation of Bcl-2 family members and activation of caspases.
The successful application of cisplatin and other platinum complexes as anticancer drugs has stimulated extensive investigations in the field of inorganic medicinal chemistry and the search for alternative transition metal complexes with anticancer activities.1–3 In this respect, ruthenium (Ru) compounds have attracted special attention due to their favorable properties, such as high cytotoxicity against cancer cells, lower toxicity toward healthy tissues, similar ligand exchange kinetics to those of platinum complexes, various oxidation states under physiological conditions and higher coordination number.1,4–7 A number of Ru complexes have shown potential utility in chemotherapy and photodynamic therapy as evidenced by in vitro and in vivo studies, and some of them, such as NAMI-A and KP1019, are currently undergoing clinical trials.8,9 Organoruthenium complexes coordinated by arene ligands also exhibited promising anticancer activities, especially against cisplatin-resistant cancer cells.10–12 However, the molecular mechanisms of the action of Ru complexes remain elusive.
Ru polypyridyl complex is a versatile class of compounds with unique electrochemical and photophysical properties, which have wide applications as oxidation catalysts, photocatalysts, dye sensitizers for solar cells, fabrication of molecular devices, DNA intercalators, and protein binding agents.6,13–15 Until now, a lot of Ru polypyridyl complexes have been synthesized, and some of them have been found cytotoxic against cancer cells.16–19 Due to the importance of chirality in biological systems, recent studies have been devoted to the stereochemistry of Ru polypyridyl complexes and their DNA-binding and photocleavage properties.17,20,21 However, little attention has been paid to the anticancer activities and the mechanisms of different enantiomers of Ru polypyridyl complexes. Therefore, in the present study, a series of chiral Ru polypyridyl complexes, Δ and Λ-[Ru(bpy)2L] (L = p-tFMPIP, 1; m-tFMPIP, 2; o-tFMPIP, 3) (Fig. 1) have been synthesized and their in vitro anticancer activities have been evaluated by comparison with cisplatin. The molecular mechanisms through which the chiral Ru complexes caused cancer cell death were also elucidated.
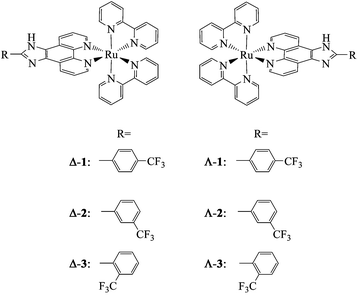 | ||
| Fig. 1 Structures of the chiral Ru polypyridyl complexes. | ||
The antiproliferative activities of the Ru complexes were firstly screened against four human cancer cell lines (melanoma A375, hepatocellular carcinoma HepG2, colorectal adenocarcinoma SW620 and prostate cancer PC-3 cells) and two human normal cell lines (fibroblast HS68 and kidney HK-2 cells) by MTT assay. Table 1 demonstrates the IC50 values of six Ru complexes and cisplatin after 48-h treatment. The results show that four tested cancer cell lines were all susceptible to the chiral Ru polypyridyl complexes. The antiproliferative activities were cell line-specific and affected by the position of –CF3 group in the main ligand of the complexes. Λ-3 exhibited the strongest growth inhibition against cancer cells, with IC50 values at 5.9, 10.0 and 18.9 μM for A375, HepG2 and PC-3 cells, respectively, which are lower than those of cisplatin and other chiral Ru polypyridyl complexes, indicating the higher cytotoxic effects of Λ-3. Despite this potency, Λ-3 was much less toxic toward human normal cells with IC50 values at 18.79 (Hs68 fibroblasts) and 108.52 μM (HK-2 kidney cells), which are significantly higher than those of cisplatin (4.8 and 3.2 μM respectively), indicating the lower cytotoxic effects of Λ-3 on normal cells.
| IC50/μM | ||||||
|---|---|---|---|---|---|---|
| Complexes | A375 | Hep G2 | SW620 | PC-3 | HS68 | HK-2 |
| Δ- 1 | 35.3 ± 4.2 | 19.9 ± 2.5 | 45.2 ± 3.3 | 99.1 ± 7.2 | — | — |
| Λ- 1 | 28.1 ± 3.7 | 11.4 ± 1.3 | 29.9 ± 2.0 | 82.7 ± 5.6 | — | — |
| Δ- 2 | 48.4 ± 6.3 | 16.6 ± 2.3 | 36.6 ± 4.5 | >200 | — | — |
| Λ- 2 | 49.9 ± 8.5 | 19.9 ± 1.7 | 51.0 ± 3.4 | >200 | — | — |
| Δ- 3 | 5.9 ± 1.1 | 10.0 ± 1.2 | 18.9 ± 0.9 | 79.5 ± 8.1 | 18.8 ± 3.9 | 108.5 ± 9.3 |
| Λ- 3 | 17.7 ± 2.6 | 54.8 ± 6.4 | 33.2 ± 2.2 | >200 | — | — |
| Cisplatin | 7.3 ± 0.8 | 13.6 ± 2.0 | 30.0 ± 4.1 | 36.2 ± 2.9 | 1.8 ± 0.7 | 10.3 ± 2.1 |
We next investigated the underlying mechanisms of Λ-3-induced cell death in the most sensitive cell line, melanoma A375 cells. Firstly, we performed flow cytometric analysis to determine whether apoptosis was involved in cell death induced by Λ-3. The results (Fig. 2) reveal that exposure of the A375 cells to different concentrations of Λ-3 for 24 h led to dose-dependent increase in the proportion of apoptotic cells, as reflected by the Sub-G1 cell population. In addition, slight increase in G0/G1phase was also observed in cells exposed to Λ-3. Taken together, these results indicate that cell death induced by Λ-3 is mainly caused by induction of apoptosis.
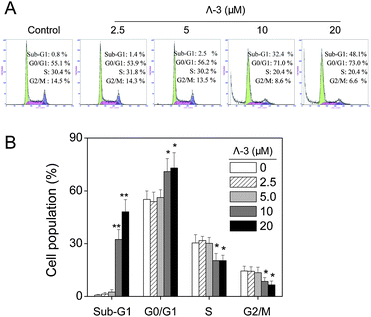 | ||
| Fig. 2 (A) Flow cytometric analysis of A375 cells treated with Λ-3 for 24 h. (B) Quantitative analysis of cell cycle distribution. Within the same phase, significant difference between control and treatment groups is indicated at P < 0.05 (*) or P < 0.01 (**) level. | ||
Mitochondria play a central role in regulating apoptosis. Mitochondrial dysfunction could induce release of apoptogenic factors, such as cytochrome c, Endo-G and AIF, from the mitochondrial inner space into cytosol, thus triggering caspase-dependent and -independent apoptotic pathways.22,23 In this study, experiments were conducted to examine the status of mitochondria in Λ-3-treated A375 cells by real-time living cell microscopy. Using MitoTracker Red CMXRos as a marker of mitochondria, we show that healthy mitochondrial network was extensively interconnected and appeared filamentous extended throughout the cytoplasm (Fig. 3). Treatment of Λ-3 resulted in mitochondrial fragmentation, release of mitochondrial contents, nuclear condensation and cytoplasmic shrinkage. Mitochondrial fragmentation displayed a rapid onset after 1-h treatment, followed by a progressive increase to 24 h (Fig. 3, and ESI† (video)). Loss of mitochondrial membrane potential (ΔΨm) is a crucial step in the process of apoptosis.
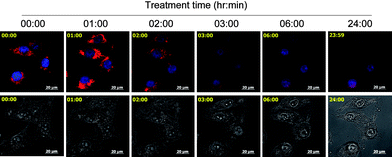 | ||
| Fig. 3 Real-time imaging of the same cells treated with 20 μM Λ-3. Cell morphology was captured by differential internal reflection (DIC) microscope. Mitochondria and nucleuses were visualized by red and blue fluorescence, respectively. The upper panel is the merged images of mitochondria and nucleuses. The lower panel is DIC images. Scale bar: 20 μm. | ||
Therefore, we used a unique cationic dye JC-1 to further examine the role of mitochondria in cell apoptosis. The results show that Λ-3 induced dose- and time-dependent depletion of ΔΨm in A375 cells (Fig. 4A). Significant depletion of ΔΨm could be detected after 1 h of Λ-3 treatment (Fig. 4). These results suggest that mitochondria are early cellular target of Λ-3 in cancer cells.
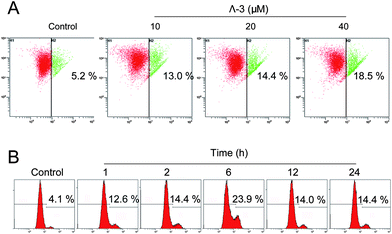 | ||
| Fig. 4 Λ- 3 induces depletion of mitochondrial membrane potential (ΔΨm) in A375 cells. (A) Cells were treated with Λ-3 for 24 h and then analyzed by JC-1 flow cytometry. The number in each dot plot represents the percentage of cells that lost ΔΨm. (B) Cells were treated with 20 μM Λ-3 for various hours. Data are means of three independent experiments. | ||
Bcl-2 family members have been described as key regulators of ΔΨm.24 Pro-survival members associate with the mitochondrial outer membrane and maintain their integrity. In contrast, pro-apoptosis members oligomerize in mitochondrial outer membrane and disrupt its integrity, causing the release of apoptogenic factors into cytosol. As shown in Fig. 5A, treatment of Λ-3 suppressed the expression of pro-survival proteins Bcl-2 and Bcl-xl, and increased the expression level of pro-apoptosis protein Bad. As a result of these changes, the ratios of Bcl-2/Bax and Bcl-xl/Bad reduced significantly, which led to depletion of ΔΨm and release of mitochondrial proteins. Subsequently, Λ-3 induced the activation of initiator caspase-8 and -9 and downstream effector caspase-3 and -7 and cleavage of PARP, and finally resulted in cell apoptosis (Fig. 5B).
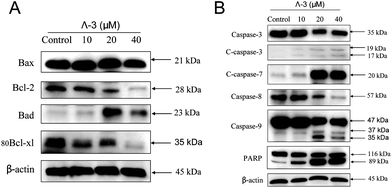 | ||
| Fig. 5 Roles of Bcl-2 and caspase family members in apoptosis induced by Λ-3. Cells were treated with indicated concentrations of Λ-3 for 24 h and the expression levels of the apoptosis-related proteins were examined by Western blotting. | ||
In conclusion, chiral Ru polypyridyl complexes are potent antiproliferative agents that could be less toxic to human normal cells in comparison with cisplatin. Λ-3 represents a novel mitochondria-targeted compound which is able to induce mitochondria-mediated apoptosis in melanoma A375 cells through regulation of Bcl-2 family members and activation of caspases. Based on these results, Λ-3 may be a candidate for further evaluation as a chemotherapeutic agent against human cancers.
We acknowledge the financial support from The Chinese University of Hong Kong IPMBAB Research Fund, Natural Science Foundation of China and Guangdong Province, the Planned Item of Science and Technology of Guangdong Province, the Fundamental Research Funds for the Central Universities, the 211 project grant of Jinan University and the Young Teacher Research Foundation of Guangdong Pharmaceutical University.
Notes and references
- P. C. Bruijnincx and P. J. Sadler, Curr. Opin. Chem. Biol., 2008, 12, 197–206 CrossRef CAS.
- L. Kelland, Nat. Rev. Cancer, 2007, 7, 573–584 CrossRef CAS.
- M. Markman, Expert Opin. Drug Saf., 2003, 2, 597–607 Search PubMed.
- C. S. Allardyce and P. J. Dyson, Platinum Metals Rev., 2001, 45, 62–69 CAS.
- C. Che and J. Huang, Coord. Chem. Rev., 2002, 231, 151–164 CrossRef CAS.
- L. Cosgrave, M. Devocelle, R. J. Forster and T. E. Keyes, Chem. Commun., 2010, 46, 103–105 RSC.
- L. Xu, D. Zhang, M. Deng, M. Zhang and X. Zhou, Chem. Commun., 2010, 46, 743–745 RSC.
- I. Bratsos, S. Jedner, T. Gianferrara and E. Alessio, Chimia, 2007, 61, 692–697 CrossRef CAS.
- X. Meng, M. L. Leyva, M. Jenny, I. Gross, S. Benosman, B. Fricker, S. Harlepp, P. Hebraud, A. Boos, P. Wlosik, P. Bischoff, C. Sirlin, M. Pfeffer, J. P. Loeffler and C. Gaiddon, Cancer Res., 2009, 69, 5458–5466 CrossRef CAS.
- T. Bugarcic, A. Habtemariam, R. J. Deeth, F. P. Fabbiani, S. Parsons and P. J. Sadler, Inorg. Chem., 2009, 48, 9444–9453 CrossRef CAS.
- T. Bugarcic, O. Novakova, A. Halamikova, L. Zerzankova, O. Vrana, J. Kasparkova, A. Habtemariam, S. Parsons, P. J. Sadler and V. Brabec, J. Med. Chem., 2008, 51, 5310–5319 CrossRef CAS.
- S. J. Dougan, A. Habtemariam, S. E. McHale, S. Parsons and P. J. Sadler, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11628–11633 CrossRef CAS.
- V. Marin, E. Holder, R. Hoogenboom and U. S. Schubert, Chem. Soc. Rev., 2007, 36, 618–635 RSC.
- S. Sharma, S. K. Singh and D. S. Pandey, Inorg. Chem., 2008, 47, 1179–1189 CrossRef CAS.
- B. Sun, J. X. Guan, L. Xu, B. L. Yu, L. Jiang, J. F. Kou, L. Wang, X. D. Ding, H. Chao and L. N. Ji, Inorg. Chem., 2009, 48, 4637–4639 CrossRef CAS.
- J. Liu, W. Zheng, S. Shi, C. Tan, J. Chen, K. Zheng and L. Ji, J. Inorg. Biochem., 2008, 102, 193–202 CrossRef CAS.
- W. Mei, N. Wang, Y. Liu, Y. Ma, D. Wang and B. Liang, Transition Met. Chem., 2008, 33, 499–503 CrossRef CAS.
- U. Schatzschneider, J. Niesel, I. Ott, R. Gust, H. Alborzinia and S. Wolfl, ChemMedChem, 2008, 3, 1104–1109 CrossRef CAS.
- O. Zava, S. M. Zakeeruddin, C. Danelon, H. Vogel, M. Gratzel and P. J. Dyson, ChemBioChem, 2009, 10, 1796–1800 CrossRef CAS.
- N. U. Khan, N. Pandya, R. I. Kureshy, S. H. Abdi, S. Agrawal, H. C. Bajaj, J. Pandya and A. Gupte, Spectrochim. Acta, Part A, 2009, 74, 113–119 CrossRef.
- S. Shi, T. M. Yao, X. T. Geng, L. F. Jiang, J. Liu, Q. Y. Yang and L. N. Ji, Chirality, 2009, 21, 276–283 CrossRef CAS.
- T. Chen and Y. S. Wong, Cell. Mol. Life Sci., 2008, 65, 2763–2775 CrossRef CAS.
- T. Chen and Y. S. Wong, Int. J. Biochem. Cell Biol., 2009, 41, 666–676 CrossRef CAS.
- S. Cory and J. M. Adams, Nat. Rev. Cancer, 2002, 2, 647–656 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental details for the synthesis and characterization of chiral Ru polypyridyl complexes, and in vitro cellular studies. See DOI: 10.1039/c0md00060d/ |
| This journal is © The Royal Society of Chemistry 2010 |
