DOI:
10.1039/C0MD00014K
(Concise Article)
Med. Chem. Commun., 2010,
1, 61-72
Synthesis and selective inhibition of human monoamine oxidases of a large scaffold of (4,5-substituted-thiazol-2-yl)hydrazones†
Received
18th February 2010
, Accepted 21st March 2010
First published on
28th April 2010
Abstract
A large class of (4,5-substituted-thiazol-2-yl)hydrazone derivatives (1–66) was synthesized in good yield (82–99%) and characterized by elemental analysis and 1H NMR studies. The compounds were assayed for their in vitro human monoamine oxidase inhibitory activity and selectivity. Most of them showed IC50 values in the micromolar range. Our aim was to evaluate the importance of a bulky group at C2, C4, and C5 positions of the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundthiazole nucleus in modulating the biological activity of this scaffold.
1 Introduction
Monoamine oxidases (MAOs; EC 1.4.3.4) are flavoenzymes which exist in two isoforms, MAO-A and MAO-B, sharing about 70% amino acid identity. They are identified by separated encoding genes and can be distinguished on the basis of tissue distribution, inhibitor sensitivity, and substrate selectivity. They catalyze the oxidative deamination of endogenous and exogenous monoamines, playing a significant role in the control of intracellular concentration of monoaminergic neurotransmitters or neuromodulators such as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound5-hydroxytryptamine (5-HT), COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddopamine (DA), COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundnorepinephrine (NA), and dietary amines. In particular, hMAO-A is present in catecholaminergic neurons, placenta, and fibroblasts; it preferentially deaminates 5-HT and NA and is irreversibly inhibited by low concentrations of clorgyline. Instead, hMAO-B is concentrated in hystaminergic and serotoninergic neurons, glial cells, and lymphocytes; it prefers substrates like phenylethylamines and is selectively inhibited by R-(−)-deprenyl. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDopamine and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundtyramine are common substrates of both isoforms.1–3 By inhibiting MAO, the degradation of these monoamines is blocked, leading to an increase in their availability for physiological functions. Indeed, the rapid degradation of these molecules ensures the proper functioning of synaptic neurotransmission and is critically important for the regulation of emotion and other brain functions. The monoaminergic hypothesis of depression, in fact, postulates that the lack of adequate concentrations of monoamines (NA, 5-HT, and DA) in the synaptic cleft could be the cause of the abnormal behaviors that characterize this pathology. The enzymatic isoform which is principally involved in the metabolism of such neurotransmitters is hMAO-A. On the basis of this assumption, the medical treatment of depression makes use of a large number of hMAO-A inhibitors (MAO-I). These molecules, in fact, are not only able to increase the concentration of NA and 5-HT in the synaptic cleft, but they even reduce the oxidative stress caused by the metabolism of such neurotransmitters and, in chronic administration, they can determine an increase of expression of neurotrophic factors (BDNF) whose action is essential to guarantee neuronal plasticity and survival.
Furthermore, the observation that hMAO-B levels in humans increase 4–5-fold on aging presents a rationale for the involvement of hMAO-B in age-related neurological disorders such as Parkinson's and Alzheimer's disease. Increased hMAO-B activity would be expected to diminish COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddopamine concentration and to release catalytic reaction products (H2O2). Dopamine has been involved in R-synuclein aggregation implicated in the etiology of Parkinson's disease, while high levels of H2O2 in the cell promote apoptotic signaling events and the development of Parkinson's disease. Inhibition of hMAO-B allows a sort of neuroprotection against this bioactivation and treatment of pre-Parkinson's patients with selective hMAO-B inhibitors has been shown to be effective in reducing the development of this neurodegeneration.4 As hMAO-A inhibitors are used for the treatment of mental disorders, particularly depression, and hMAO-B inhibitors are used as coadjuvants for the treatment of Parkinson's and Alzheimer's disease, the aim of researchers is to synthesize selective inhibitors that would permit control over this enzymatic activity.5–7
In previous studies conducted by our research group,8–10 numerous substituted thiazolylhydrazones have been investigated as hMAO inhibitors. In this paper, our interest is to point out the influence of steric hindrance on the inhibitory activity and selectivity of a wide series of new 2-thiazolylhydrazones on which the portion interacting with the active site of the enzymes (substituents at C4 and C5 of the thiazole nucleus) was opportunely modified on the basis of the information we extrapolated from the interaction pattern among these isoforms and their inhibitors.11,12 From molecular modelling studies previously reported by us,8,9 the corresponding residues Ile335 (hMAO-A) and Tyr326 (hMAO-B) appeared relevant to explain the molecular recognition of this scaffold, because they oriented the substituent in C4 of the inhibitor in the catalytic site. In particular, in the B isoform Tyr326, due to its consistent steric effects, induced the inhibitor to interact with the FAD. Conversely, the hMAO-A Ile335, characterized by a lower hindrance with respect to the corresponding hMAO-B residue, allowed the ligand to assume multiple poses into the binding cleft with higher enthalpic and entropic penalties. In addition, the presence of a methyl group at C5 of the thiazole has not been evaluated before. Finally, the choice of different aliphatic, cycloaliphatic, heterocyclic, and bicyclic moieties on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group (C2 of the thiazole) allowed us to better comprehend the steric and electronic influence on the enzyme-inhibitor interaction and to modulate the chemical structure of these derivatives.
N group (C2 of the thiazole) allowed us to better comprehend the steric and electronic influence on the enzyme-inhibitor interaction and to modulate the chemical structure of these derivatives.
2 Results and discussion
4,5-Substituted-2-thiazolylhydrazone derivatives (1–66) were synthesized in high yields (82–99%) as reported in our previous communications.8–10 Different carbonyl compounds reacted directly with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundthiosemicarbazide in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-propanol with catalytic amounts of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundacetic acid, and the obtained thiosemicarbazones were subsequently converted into 4,5-substituted-2-thiazolylhydrazones by reaction with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundα-bromo acetylnaphthalene or COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundα-bromo propiophenone in the same solvent (Hantzsch reaction) (Scheme 1).
All synthesized products were purified by washing them with petroleum ether and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddiethyl ether and, if required, by chromatography (SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane) before characterization by 1H NMR and elemental analysis. Moreover, the presence of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond can give rise to isomeric geometry E/Z. The 1H NMR (in CDCl3) spectra analysis revealed that the E isomer was more favoured and stable than the Z-configuration. The amounts of both conformers were measured by area integration of the signal relative to the CH3 protons (area ratio of proton signals E
N double bond can give rise to isomeric geometry E/Z. The 1H NMR (in CDCl3) spectra analysis revealed that the E isomer was more favoured and stable than the Z-configuration. The amounts of both conformers were measured by area integration of the signal relative to the CH3 protons (area ratio of proton signals E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z was generally 6
Z was generally 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The low-field signal was assigned to the E isomer, since it is widely accepted in thiosemicarbazone derivatives.13 Our choice, as reaction medium, of a polar alcoholic solvent appeared to be preferred to obtain the E-configuration and limit the interconversion according to the results of our previous theoretical and chromatographic study for similar compounds.14
1). The low-field signal was assigned to the E isomer, since it is widely accepted in thiosemicarbazone derivatives.13 Our choice, as reaction medium, of a polar alcoholic solvent appeared to be preferred to obtain the E-configuration and limit the interconversion according to the results of our previous theoretical and chromatographic study for similar compounds.14
The biological activity of the test drugs (assayed as E/Z mixture) on hMAOs was investigated by measuring their effects on the production of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhydrogen peroxide (H2O2) from p-tyramine (a common substrate for both hMAO-A and hMAO-B), using the Amplex Red MAO assay kit and microsomal MAO isoforms prepared from insect cells (BTI-TN-5B1-4) infected with recombinant baculovirus containing cDNA inserts for hMAO-A or hMAO-B. The production of H2O2 catalyzed by MAO isoforms can be detected using COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound10-acetyl-3,7-dihydroxyphenoxazine, a non-fluorescent and highly sensitive probe, that reacts with H2O2 in the presence of horseradish peroxidase to produce a fluorescent product, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundresorufin. In this study hMAO activity was evaluated using the above method following the general procedure previously described by us.15 The test drugs (new compounds and reference inhibitors) themselves were unable to react directly with the Amplex Red reagent, which indicates that these drugs do not interfere with the measurements. On the other hand, in our experiments and under our experimental conditions, the control activity of hMAO-A and hMAO-B, using p-tyramine, was 165 ± 2 pmol of p-tyramine oxidized to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundp-hydroxyphenylacetaldehyde/min (n = 20). Most tested drugs concentration-dependently inhibited this enzymatic control activity.
Structure–activity relationships (SARs) were inferred from data of enzymatic experiments reported in Table 1, 2, and 3. All tested compounds inhibited hMAOs at micromolar concentrations with ratio values ranging from 0.043 (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound55) to >16.67 (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound45). All compounds bearing a COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundnaphthalene moiety on C4 of the thiazole ring (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1–9, 19–27, and 37–50), to which for practical reasons we will refer as “first series”, possessed a greater inhibitory activity on hMAO-A than on hMAO-B. This evidence could be justified on the basis of the detailed informations that PDB provides about the active site of both isoforms: the active site of hMAO-A is characterized by a single large hydrophobic cavity which adapts itself to the naphthalene moiety better than the two small hydrophobic cavities of hMAO-B.11,12 In addition, compounds bearing a less bulky substituent (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundbenzene) on C4 of the thiazole ring and a CH3 group on C5 (10–18, 28–36, 51–66), the “second series”, were characterized by a decrease of selectivity towards MAO-A. Moreover, these compounds were not as active as the former ones, probably because of the effect of steric hindrance of a methyl group on C5 of the thiazole. Molecular modelling studies reported for similar compounds,3 in fact, proved that this region of the thiazole moiety was involved in the interaction between inhibitor and FAD cofactor which was placed inside the active site of the enzyme and was responsible of its oxidative activity. Therefore small changes of substituent dimension in this position were scarcely tolerated.
Table 1 Structures and biological activity of aliphatic derivatives 1–18a
Table 2 Structures and biological activity of cycloaliphatic derivatives 19–36a
Table 3 Structures and biological activity of heterocyclic derivatives 37–66 and reference drugsa
More interesting information about activity and selectivity of thiazole derivatives on hMAOs could be gathered from the analysis of the influence that different substituents on the hydrazonic nitrogen (C2 of the thiazole nucleus) had on biological data of both series. Aliphatic moieties (1–18) showed that hMAO inhibitory activity and selectivity were largely influenced by the chain length, in particular when even the C4 position was substituted with bulky functional groups (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1–9). Elongation of the alkyl chain produced a slight reduction of hMAO-A inhibition, but an increase of selectivity towards hMAO-A (derivatives COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7 and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound9 with ratio 6.67 and >6.25 respectively) could be determined, while the presence of a double bond at the end of the aliphatic chain (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6) endowed the compound with discrete hMAO-A selectivity (IC50 hMAO-A = 2.45 ± 0.12 μM and ratio 2.86). Instead, when the C4 position was substituted with benzene (10–18), the elongation of the chain led to only slight differences in activity (with the only exception of compound COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound15) or selectivity.
On the other hand, carbocyclic derivatives (19–36) showed a surprising coherent behavior in both series: as well as the aliphatic ones, they lost activity when the ring dimension increased: the most active compounds, in fact, were the smallest ones (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclopentane derivatives COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound19 and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound28 with IC50 hMAO-A = 1.21 ± 0.06 μM and IC50 hMAO-A = 0.55 ± 0.02 μM, respectively). The introduction of a methyl group on the aliphatic ring (20–21, 23–25, 29–30, 32–34) caused a reduction of activity in comparison to the corresponding non-substituted compounds (compare COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound19 to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound20, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound22 to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound23, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound28 to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound29, and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound31 to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound32). However, it is easily noticeable that the presence of a methyl group on C2′ of the aliphatic ring, especially in the first series, influenced activity more than the same substitution on C3′ or C4′ (compare COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound20 to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound21 and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound23 to 24–25), while, in the second series, the decrease of activity on hMAO-A due to steric effects on the hydrazonic carbon caused an inversion of selectivity in compounds COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound29 and 32–36, which, on the contrary, maintained a good inhibitory activity on hMAO-B because of the less bulky substituent on C4 of the thiazole.
The anti-hMAO activity and selectivity associated with the presence of a heterocyclic moiety (37–66) on the hydrazonic nitrogen was also influenced by steric hindrance. The bulkiest compounds of the naphthalene derivatives (45–50), in fact, displayed both low inhibitory activity or the best selectivity toward hMAO-A (compound COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound45, IC50 hMAO-A = 5.92 ± 0.13 μM and ratio >16.67). Conversely, compounds bearing a smaller heterocycle in that position (37–43) were quite potent but less selective hMAO-A inhibitors.
Regarding compounds 51–66, on which the naphthalene moiety was substituted with a phenyl ring and a methyl group was introduced at C5 of the thiazole nucleus, a surprising inversion of selectivity towards hMAO-B (compound COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound55, IC50 hMAO-A = 6.52 ± 0.21 μM, IC50 hMAO-B = 0.28 ± 0.01 μM, and ratio 0.043) was observed, confirming the hypothesis that the decrease of steric hindrance also on the C2 (hydrazone substituent) of the thiazole ring determines a reduction of selectivity toward hMAO-A which, in the case of heterocyclic derivatives at the hydrazonic nitrogen, resulted in a dramatic change of activity.
3 Experimental
Starting materials and reagents were obtained from commercial suppliers and were used without purification. Melting points (mp) were determined by the capillary method on an FP62 apparatus (Mettler-Toledo) and are uncorrected. 1H NMR spectra were recorded at 400 MHz on a Bruker spectrometer using DMSO-d6 or CDCl3 as solvent. Chemical shifts are expressed as δ units (ppm) relative to TMS. Coupling constants J are expressed in hertz (Hz). Elemental analyses for C, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, and N were determined with a Perkin-Elmer 240 B microanalyzer and the analytical results were ≥ 95% purity for all compounds. All reactions were monitored by TLC performed on 0.2 mm thick silica gel plates (60 F254 Merck). Preparative flash column chromatography was carried out on silica gel (230–400 mesh, G60 Merck). Organic solutions were dried over anhydrous COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsodium sulfate. Concentration and evaporation of the solvent after reaction or extraction was carried out on a rotary evaporator (Büchi Rotavapor) operating at reduced pressure.
3.1 General procedure for the synthesis of derivatives 1–66
The appropriate carbonyl compound, aldehyde or ketone, (1 g, 50 mmol) was dissolved in 100 mL of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-propanol and stirred with an equimolar quantity of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundthiosemicarbazide for 24 h at room temperature with catalytic amounts of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundacetic acid. Within 30 min the starting reaction mixture turned into a suspension and the precipitated thiosemicarbazone was filtered, washed with a suitable solvent (petroleum ether, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddiethyl ether), and dried. The structures of the synthesized intermediates were confirmed by 1H NMR. Equimolar amounts of the prepared thiosemicarbazone (25 mmol), α-bromo-COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundacetylnaphthalene (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1–9, 19–27, and 37–50) or α-bromopropiophenone (10–18, 28–36, and 51–66) both suspended in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-propanol, were reacted at room temperature under magnetic stirring for 2 h. The obtained powder was filtered on Gooch filter, washed with adequate solvents, and dried under vacuum to give compounds 1–66 in high yield. All the yields are on an isolated basis.
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(4-(Naphthalen-2-yl)thiazol-2-yl)-2-(propan-2-ylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1).
(1.05 g, 99%); ivory solid; mp 222–223 °C; (Found: C, 68.32; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.38; N, 14.94. C16H15N3S requires C, 68.30; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.37; N, 14.93%); δH(400 MHz, CDCl3, Me4Si) 2.14 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3), 2.25 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3), 6.81 (1 H, s, C(5)H-thiazole), 7.56 (2 H, m, naphthalene), 7.69 (1 H, s, naphthalene), 7.86 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.46 (1 H, s, naphthalene), 12.46 (1 H, br s, NH, D2O exch).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(Butan-2-ylidene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2).
(0.99 g, 99%); ivory solid; mp 180–181 °C; (Found: C, 69.10; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.81; N, 14.23. C17H17N3S requires C, 69.12; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.80; N, 14.22%); δH(400 MHz, CDCl3, Me4Si) 1.16 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3), 1.25 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3), 2.56 (2 H, m, CH2), 6.69 (1 H, s, C(5)H-thiazole), 7.53 (2 H, m, naphthalene), 7.72 (1 H, m, naphthalene), 7.90 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.53 (1 H, s, naphthalene), 12.35 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(4-(Naphthalen-2-yl)thiazol-2-yl)-2-(pentan-2-ylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3).
(0.99 g, 99%); cream solid; mp 181–184 °C; (Found: C, 69.89; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.18; N, 13.59. C18H19N3S requires C, 69.87; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.19; N, 13.58%); δH(400 MHz, DMSO-d6) 0.98 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3), 1.66 (2 H, m, CH2), 2.15 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3), 2.37 (2 H, m, CH2), 6.70 (1 H, s, C(5)H-thiazole), 7.47 (2 H, m, naphthalene), 7.68 (1 H, m, naphthalene), 7.84 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.40 (1 H, s, naphthalene), 12.29 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(4-(Naphthalen-2-yl)thiazol-2-yl)-2-(pentan-3-ylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound4).
(0.90 g, 97%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 1/1); cream solid; mp 196–198 °C; (Found: C, 69.88; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.18; N, 13.59. C18H19N3S requires C, 69.87; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.19; N, 13.58%); δH(400 MHz, DMSO-d6) 1.16 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3), 1.25 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3), 2.42 (2 H, m, CH2), 2.56 (2 H, m, CH2), 6.69 (1 H, s, C(5)H-thiazole), 7.52 (2 H, m, naphthalene), 7.72 (1 H, m, naphthalene), 7.90 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.53 (1 H, s, naphthalene), 12.35 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(4-(Naphthalen-2-yl)thiazol-2-yl)-2-(4-methylpentan-2-ylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound5).
(0.96 g, 99%); yellow solid; mp 119–120 °C; (Found: C, 70.54; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.55; N, 12.98. C19H21N3S requires C, 70.55; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.54; N, 12.99%); δH(400 MHz, CDCl3, Me4Si) 0.95 (6 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, d, J 6.6 Hz, 2 × CH3), 2.05 (1 H, m, J 6.6 Hz, J 7.2 Hz, CH(CH3)2), 2.15 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 2.26 (2 H, d, J 7.2 Hz, CH2), 6.81 (1 H, s, C(5)H-thiazole), 7.55 (2 H, m, naphthalene), 7.57 (1 H, m, naphthalene), 7.78 (3 COMPOUND LINKS
), 2.26 (2 H, d, J 7.2 Hz, CH2), 6.81 (1 H, s, C(5)H-thiazole), 7.55 (2 H, m, naphthalene), 7.57 (1 H, m, naphthalene), 7.78 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.27 (1 H, s, naphthalene), 12.51 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(Hex-5-en-2-ylidene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6).
(0.83 g, 88%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 1/1); light yellow solid; mp 195–197 °C; (Found: C, 70.97; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.97; N, 13.06. C19H19N3S requires C, 70.99; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.96; N, 13.07%); δH(400 MHz, CDCl3, Me4Si) 2.20 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.38 (2 H, m, CH2CH
N), 2.38 (2 H, m, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 2.50 (2 H, m, CH2C
), 2.50 (2 H, m, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 5.07 (2 H, m, CH2
N), 5.07 (2 H, m, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 5.83 (1 H, m, CH
), 5.83 (1 H, m, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.81 (1 H, s, C(5)H-thiazole), 7.55 (2 H, m, naphthalene), 7.71 (1 H, m, naphthalene), 7.92 (3 COMPOUND LINKS
), 6.81 (1 H, s, C(5)H-thiazole), 7.55 (2 H, m, naphthalene), 7.71 (1 H, m, naphthalene), 7.92 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.27 (1 H, s, naphthalene), 12.43 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(Heptan-2-ylidene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7).
(0.87 g, 99%) green–yellow solid; mp 133–135 °C; (Found: C, 71.20; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.86; N, 12.44. C20H23N3S requires C, 71.18; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.87; N, 12.45%); δH(400 MHz, DMSO-d6) 0.91 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3CH2), 1.34 (2 H, m, CH2), 1.57 (2 H, m, CH2), 1.62 (2 H, m, CH2), 2.19 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.37 (2 H, m, CH2C
N), 2.37 (2 H, m, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 6.69 (1 H, s, C(5)H-thiazole), 7.54 (2 H, m, naphthalene), 7.76 (1 H, m, naphthalene), 7.97 (3 COMPOUND LINKS
N), 6.69 (1 H, s, C(5)H-thiazole), 7.54 (2 H, m, naphthalene), 7.76 (1 H, m, naphthalene), 7.97 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.67 (1 H, s, naphthalene), 12.30 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(Heptan-3-ylidene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound8).
(0.82 g, 93%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 1/1); camel solid; mp 170–172 °C; (Found: C, 71.17; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.88; N, 12.46. C20H23N3S requires C, 71.18; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.87; N, 12.45%); δH(400 MHz, CDCl3, Me4Si) 0.98 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3CH2), 1.15 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1.56 (2 H, m, CH3CH2C
N), 1.56 (2 H, m, CH3CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1–61 (2 H, m, CH2C
N), 1–61 (2 H, m, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.41 (2 H, m, CH2), 2.54 (2 H, m, CH2), 6.80 (1 H, s, C(5)H-thiazole), 7.54 (2 H, m, naphthalene), 7.71 (1 H, m, naphthalene), 7.83 (3 COMPOUND LINKS
N), 2.41 (2 H, m, CH2), 2.54 (2 H, m, CH2), 6.80 (1 H, s, C(5)H-thiazole), 7.54 (2 H, m, naphthalene), 7.71 (1 H, m, naphthalene), 7.83 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.28 (1 H, s, naphthalene), 12.52 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(4-(Naphthalen-2-yl)thiazol-2-yl)-2-(octan-2-ylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound9).
(0.73 g, 84%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 1/1); camel solid; mp 145–146 °C; (Found: C, 71.74; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.18; N, 11.96. C21H25N3S requires C, 71.75; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.17; N, 11.95%); δH(400 MHz, CDCl3, Me4Si) 0.91 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3CH2), 1.32 (2 H, m, CH2), 1.36 (2 H, m, CH2), 1.39 (2 H, m, CH2), 1.59 (2H, m, CH2), 2.20 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.37 (2 H, m, CH2C
N), 2.37 (2 H, m, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 6.81 (1 H, s, C(5)H-thiazole), 7.55 (2 H, m, naphthalene), 7.74 (1 H, m, naphthalene), 7.93 (3 COMPOUND LINKS
N), 6.81 (1 H, s, C(5)H-thiazole), 7.55 (2 H, m, naphthalene), 7.74 (1 H, m, naphthalene), 7.93 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.29 (1 H, s, naphthalene), 12.63 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(propan-2-ylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound10).
(0.91 g, 94%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 1/1); white solid; mp 235–237 °C; (Found: C, 63.65; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.15; N, 17.14. C13H15N3S requires C, 63.64; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.16; N, 17.13%); δH(400 MHz, CDCl3, Me4Si) 1.97 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3), 1.99 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3), 2.33 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.38 (1 H, m, phenyl), 7.54 (2 H, m, phenyl), 7.59 (2 H, m, phenyl), 12.46 (1 H, br s, NH, D2O exch).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(Butan-2-ylidene)-2-(5-methyl-4-phenylthiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound11).
(0.87 g, 99%); sand solid; mp 180–183 °C; (Found: C, 64.84; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.60; N, 16.21. C14H17N3S requires C, 64.83; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.61; N, 16.20%); δH(400 MHz, CDCl3, Me4Si) 1.15 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3), 1.25 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.56 (2 H, m, CH2), 6.69 (3 COMPOUND LINKS
N), 2.56 (2 H, m, CH2), 6.69 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.39 (1 H, m, phenyl), 7.53 (2 H, m, phenyl), 7.62 (2 H, m, phenyl), 12.35 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(pentan-2-ylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound12).
(0.81 g, 99%); orange solid; mp 163–165 °C; (Found: C, 65.88; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.01; N, 15.38. C15H19N3S requires C, 65.90; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.00; N, 15.37%); δH(400 MHz, CDCl3, Me4Si) 0.98 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3), 1.66 (2 H, m, CH2), 2.15 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.37 (2 H, m, CH2), 6.70 (3 COMPOUND LINKS
N), 2.37 (2 H, m, CH2), 6.70 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.83 (1 H, m, phenyl), 7.86 (2 H, m, phenyl), 7.87 (2 H, m, phenyl), 12.29 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(pentan-3-ylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound13).
(0.67 g, 82%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 1/1); sand solid; mp 135–137 °C; (Found: C, 65.89; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.99; N, 15.37. C15H19N3S requires C, 65.90; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.00; N, 15.37%); δH(400 MHz, CDCl3, Me4Si) 1.17 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3), 1.24 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3), 2.42 (2 H, m, CH2), 2.57 (2 H, m, CH2), 6.69 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.41 (1 H, m, phenyl), 7.51 (2 H, m, phenyl), 7.56 (2 H, m, phenyl), 12.35 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(4-methylpentan-2-ylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound14).
(0.76 g, 99%); sand solid; mp 150–155 °C; (Found: C, 66.87; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.35; N, 14.63. C16H21N3S requires C, 66.86; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.36; N, 14.62%); δH(400 MHz, CDCl3, Me4Si) 0.95 (6 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, 2 × CH3), 1.92 (1 H, m, CH(CH3)2), 2.15 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.26 (2 H, m, CH2), 6.81 (3 COMPOUND LINKS
N), 2.26 (2 H, m, CH2), 6.81 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.28 (1 H, m, phenyl), 7.47 (2 H, m, phenyl), 7.54 (2 H, m, phenyl), 12.51 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(Hex-5-en-2-ylidene)-2-(5-methyl-4-phenylthiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound15).
(0.82 g, 99%); camel solid; mp 132–140 °C; (Found: C, 67.32; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.72; N, 14.71. C16H19N3S requires C, 67.33; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.71; N, 14.72%); δH(400 MHz, CDCl3, Me4Si) 1.96 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 2.32 (2 H, m, CH2CH
), 2.32 (2 H, m, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 2.49 (2 H, m, CH2C
), 2.49 (2 H, m, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 5.09 (2 H, m, CH2
N), 5.09 (2 H, m, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 5.83 (1 H, m, CH
), 5.83 (1 H, m, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.81 (3 COMPOUND LINKS
), 6.81 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.39 (5 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, Ar), 12.43 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(Heptan-2-ylidene)-2-(5-methyl-4-phenylthiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound16).
(0.79 g, 99%); ivory solid; mp 143–145 °C; (Found: C, 67.75; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.68; N, 13.95. C17H23N3S requires C, 67.73; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.69; N, 13.94%); δH(400 MHz, CDCl3, Me4Si) 0.92 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3CH2), 1.35 (2 H, m, CH2), 1.57 (2 H, m, CH2), 1.62 (2 H, m, CH2), 2.19 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.37 (2 H, m, CH2C
N), 2.37 (2 H, m, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 6.69 (3 COMPOUND LINKS
N), 6.69 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.42 (1 H, m, phenyl), 7.47 (2 H, m, phenyl), 7.57 (2 H, m, phenyl), 12.30 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(Heptan-3-ylidene)-2-(5-methyl-4-phenylthiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound17).
(0.66 g, 84%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 1/1); light pink solid; mp 75–77 °C; (Found: C, 67.74; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.69; N, 13.95. C17H23N3S requires C, 67.73; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.69; N, 13.94%); δH(400 MHz, CDCl3, Me4Si) 0.99 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3CH2), 1.15 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1.56 (2 H, m, CH2), 1.61 (2 H, m, CH2), 2.41 (2 H, m, CH3CH2C
N), 1.56 (2 H, m, CH2), 1.61 (2 H, m, CH2), 2.41 (2 H, m, CH3CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.54 (2 H, m, CH2C
N), 2.54 (2 H, m, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 6.80 (3 COMPOUND LINKS
N), 6.80 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.43 (1 H, m, phenyl), 7.48 (2 H, m, phenyl), 7.55 (2 H, m, phenyl), 12.52 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(octan-2-ylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound18).
(0.93 g, 98%); light brown solid; mp 117–119 °C; (Found: C, 68.51; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 8.00; N, 13.33. C17H23N3S requires C, 68.53; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.99; N, 13.32%); δH(400 MHz, CDCl3, Me4Si) 0.92 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3CH2), 1.34 (2 H, m, CH2), 1.40 (2 H, m, CH2), 1.45 (2 H, m, CH2), 1.59 (2 H, m, CH2), 2.20 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.37 (2 H, m, CH2C
N), 2.37 (2 H, m, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 6.81 (3 COMPOUND LINKS
N), 6.81 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.55 (1 H, m, phenyl), 7.60 (2 H, m, phenyl), 7.64 (2 H, m, phenyl), 12.63 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-Cyclopentylidene-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound19).
(0.97 g, 99%); pink solid; mp 212–213 °C; (Found: C, 70.34; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.58; N, 13.66. C18H17N3S requires C, 70.33; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.57; N, 13.67%); δH(400 MHz, DMSO-d6) 1.86 (2 H, m, CH2-cyclopentyl), 1.95 (2 H, m, CH2-cyclopentyl), 2.54 (2 H, m, CH2-cyclopentyl), 2.66 (2 H, m, CH2-cyclopentyl), 6.67 (1 H, s, C(5)H-thiazole), 7.45 (2 H, m, naphthalene), 7.67 (1 H, m, naphthalene), 7.85 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.44 (1 H, s, naphthalene), 12.28 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(2-Methylcyclopentylidene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound20).
(0.89 g, 99%); yellow solid; mp 170–171 °C; (Found: C, 70.97; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.97; N, 13.06. C19H19N3S requires C, 70.99; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.96; N, 13.07%); δH(400 MHz, DMSO-d6) 1.20 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3), 1.22 (1 H, m, CH3CH-cyclopentyl), 1.61 (2 H, m, CH2-cyclopentyl), 1.90 (2 H, m, CH2-cyclopentyl), 2.77 (2 H, m, CH2-cyclopentyl), 6.68 (1 H, s, C(5)H-thiazole), 7.54, (2 H, m, naphthalene), 7.74 (1 H, m, naphthalene), 7.95 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.49 (1 H, s, naphthalene), 12.28 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(3-Methylcyclopentylidene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound21).
(0.86 g, 93%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 1/1); yellow solid; mp 205–207 °C; (Found: C, 70.98; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.95; N, 13.08. C19H19N3S requires C, 70.99; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.96; N, 13.07%); δH(400 MHz, DMSO-d6) 1.02 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3), 1.13 (1 H, m, CH3CH-cyclopentyl), 1.90 (2 H, m, CH2-cyclopentyl), 2.38 (2 H, m, CH2-cyclopentyl), 2.66 (2 H, m, CH2-cyclopentyl), 6.68 (1 H, s, C(5)H-thiazole), 7.39 (2 H, m, naphthalene), 7.55 (1 H, m, naphthalene), 7.76 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.33 (1 H, s, naphthalene), 10.70 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-Cyclohexyliden-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound22).
(0.92 g, 99%); yellow solid; mp 195–196 °C; (Found: C, 70.97; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.95; N, 13.06. C19H19N3S requires C, 70.99; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.96; N, 13.07%); δH(400 MHz, CDCl3, Me4Si) 1.59 (2 H, m, CH2-cyclohexyl), 1.64 (2 H, m, CH2-cyclohexyl), 1.69 (2 H, m, CH2-cyclohexyl), 1.77 (2 H, m, CH2-cyclohexyl), 2.59 (2 H, m, CH2-cyclohexyl), 6.68 (1 H, s, C(5)H-thiazole), 7.72 (2 H, m, naphthalene), 7.88 (1 H, m, naphthalene), 7.95 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.50 (1 H, s, naphthalene), 12.28 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(2-Methylcyclohexylidene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound23).
(0.76 g, 87%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 1/1); sand solid; mp 161–162 °C; (Found: C, 71.63; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.32; N, 12.54. C20H21N3S requires C, 71.61; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.31; N, 12.53%); δH(400 MHz, CDCl3, Me4Si) 1.16 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3CH-cyclohexyl), 1.32 (1 H, m, CH3CH-cyclohexyl), 1.60 (2 H, m, CH2-cyclohexyl), 1.83 (2 H, m, CH2-cyclohexyl), 2.00 (2 H, m, CH2-cyclohexyl), 2.19 (2 H, m, CH2-cyclohexyl), 6.80 (1 H, s, C(5)H-thiazole), 7.55 (2 H, m, naphthalene), 7.73 (1 H, m, naphthalene), 7.85 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.27 (1 H, s, naphthalene), 12.53 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(3-Methylcyclohexylidene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound24).
(0.75 g, 86%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 1/1); sand solid; mp 185–186 °C; (Found: C, 71.60; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.30; N, 12.54. C20H21N3S requires C, 71.61; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.31; N, 12.53%); δH(400 MHz, CDCl3, Me4Si) 1.02 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3CH-cyclohexyl), 1.10 (2 H, m, CH2-cyclohexyl), 1.60 (1 H, m, CH3CH-cyclohexyl), 1.68 (2 H, m, CH2-cyclohexyl), 2.36 (2 H, m, CH2-cyclohexyl), 3.19 (2 H, m, CH2-cyclohexyl), 6.67 (1 H, s, C(5)H-thiazole), 7.27 (2 H, m, naphthalene), 7.43 (1 H, naphthalene), 7.64 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, naphthalene), 8.15 (1 H, s, naphthalene), 12.28 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(4-Methylcyclohexylidene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound25).
(0.89 g, 99%); sand solid; mp 186–187 °C; (Found: C, 71.62; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.30; N, 12.52. C20H21N3S requires C, 71.61; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.31; N, 12.53%); δH(400 MHz, CDCl3, Me4Si) 1.23 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3CH-cyclohexyl), 1.87 (1 H, m, CH3CH-cyclohexyl), 1.97 (2 H, m, CH2-cyclohexyl), 2.42 (2 H, m, CH2-cyclohexyl), 2.61 (2 H, m, CH2-cyclohexyl), 3.09 (2 H, m, CH2-cyclohexyl), 6.80 (1 H, s, C(5)H-thiazole), 7.54 (2 H, m, naphthalene), 7.73 (1 H, m, naphthalene), 7.96 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.25 (1 H, s, naphthalene), 12.10 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-Cycloheptylidene-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound26).
(0.90 g, 99%); sand solid; mp 198–199 °C; (Found: C, 71.63; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.30; N, 12.54. C20H21N3S requires C, 71.61; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.31; N, 12.53%); δH(400 MHz, DMSO-d6) 1.59 (2 H, m, CH2-cycloheptyl), 1.63 (2 H, m, CH2-cycloheptyl), 1.66 (2 H, m, CH2-cycloheptyl), 1.72 (2 H, m, CH2-cycloheptyl), 2.84 (2 H, m, CH2-cycloheptyl), 2.89 (2 H, m, CH2-cycloheptyl), 7.26 (1 H, s, C(5)H-thiazole), 7.65 (2 H, m, naphthalene), 7.74 (1 H, m, naphthalene), 7.85 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.41 (1 H, s, naphthalene), 12.30 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-Cyclooctylidene-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound27).
(0.75 g, 85%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 1/1); sand solid; mp 169–170 °C; (Found: C, 72.15; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.64; N, 12.01. C21H23N3S requires C, 72.17; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.63; N, 12.02%); δH(400 MHz, CDCl3, Me4Si) 1.27 (2 H, m, CH2-cyclooctyl), 1.59 (2 H, m, CH2-cyclooctyl), 1.72 (2 H, m, CH2-cyclooctyl), 1.78 (2 H, m, CH2-cyclooctyl), 2.09 (2 H, m, CH2-cyclooctyl), 2.44 (2 H, m, CH2-cyclooctyl), 2.58 (2 H, m, CH2-cyclooctyl), 6.81 (1 H, s, C(5)H-thiazole), 7.54 (2 H, m, naphthalene), 7.80 (1 H, m, naphthalene), 8.08 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.39 (1 H, s, naphthalene), 12.33 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-Cyclopentylidene-2-(5-methyl-4-phenylthiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound28).
(0.95 g, 99%); white solid; mp 203–205 °C; (Found: C, 66.38; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.30; N, 15.49. C15H17N3S requires C, 66.39; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.31; N, 15.48%); δH(400 MHz, DMSO-d6) 1.80 (2 H, m, CH2-cyclopentyl), 1.91 (2 H, m, CH2-cyclopentyl), 2.39 (2 H, m, CH2-cyclopentyl), 2.57 (2 H, m, CH2-cyclopentyl), 6.67 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.32 (1 H, m, phenyl), 7.47 (2 H, m, phenyl), 7.57 (2 H, m, phenyl), 12.28 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(2-methylcyclopentylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound29).
(0.82 g, 99%); sand solid; mp 163–165 °C; (Found: C, 67.35; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.72; N, 14.72. C16H19N3S requires C, 67.33; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.71; N, 14.72%); δH(400 MHz, DMSO-d6) 1.22 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3CH-cyclopentyl), 1.26 (1 H, m, CH3CH-cyclopentyl), 1.56 (2 H, m, CH2-cyclopentyl), 2.39 (2 H, m, CH2-cyclopentyl), 2.78 (2 H, m, CH2-cyclopentyl), 6.65 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.38 (1 H, m, phenyl), 7.42 (2 H, m, phenyl), 7.50 (2 H, m, phenyl), 12.28 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(3-methylcyclopentylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound30).
(0.82 g, 99%); yellow ochre solid; mp 181–183 °C; (Found: C, 67.34; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.71; N, 14.73. C16H19N3S requires C, 67.33; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.71; N, 14.72%); δH(400 MHz, DMSO-d6) 1.16 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3CH-cyclopentyl), 1.22 (1 H, m, CH3CH-cyclopentyl), 1.94 (2 H, m, CH2-cyclopentyl), 2.33 (2 H, m, CH2-cyclopentyl), 2.66 (2 H, m, CH2-cyclopentyl), 6.71 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.41 (1 H, m, phenyl), 7.46 (2 H, m, phenyl), 7.59 (2 H, m, phenyl), 10.70 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-Cyclohexylidene-2-(5-methyl-4-phenylthiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound31).
(0.82 g, 99%); yellow ochre solid; mp 195–196 °C; (Found: C, 67.34; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.72; N, 14.71. C16H19N3S requires C, 67.33; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.71; N, 14.72%); δH(400 MHz, CDCl3, Me4Si) 1.52 (2 H, m, CH2-cyclohexyl), 1.67 (2 H, m, CH2-cyclohexyl), 1.75 (2 H, m, CH2-cyclohexyl), 1.83 (2 H, m, CH2-cyclohexyl), 2.58 (2 H, m, CH2-cyclohexyl), 6.78 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.28 (1 H, m, phenyl), 7.35 (2 H, m, phenyl), 7.49 (2 H, m, phenyl), 12.28 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(2-methylcyclohexylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound32).
(0.68 g, 84%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 1/1); ivory solid; mp 155–157 °C; (Found: C, 68.20; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.08; N, 14.02. C17H21N3S requires C, 68.19; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.07; N, 14.03%); δH(400 MHz, CDCl3, Me4Si) 1.20 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3CH-cyclohexyl), 1.36 (1 H, m, CH3CH-cyclohexyl), 1.68 (2 H, m, CH2-cyclohexyl), 1.76 (2 H, m, CH2-cyclohexyl), 1.92 (2 H, m, CH2-cyclohexyl), 2.56 (2 H, m, CH2-cyclohexyl), 6.77 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.32 (1 H, m, phenyl), 7.44 (2 H, m, phenyl), 7.52 (2 H, m, phenyl), 12.53 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(3-methylcyclohexylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound33).
(0.79 g, 99%); light brown solid; mp 159–162 °C; (Found: C, 68.21; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.07; N, 14.02. C17H21N3S requires C, 68.19; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.07; N, 14.03%); δH(400 MHz, CDCl3, Me4Si) 1.08 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3CH-cyclohexyl), 1.24 (2 H, m, CH2-cyclohexyl), 1.32 (1 H, m, CH3CH-cyclohexyl), 1.66 (2 H, m, CH2-cyclohexyl), 1.72 (2 H, m, CH2-cyclohexyl), 2.39 (2 H, m, CH2-cyclohexyl), 3.02 (2 H, m, CH2-cyclohexyl), 6.78 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.29 (1 H, m, phenyl), 7.39 (2 H, m, phenyl), 7.54 (2 H, m, phenyl), 12.28 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(4-methylcyclohexylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound34).
(0.80 g, 99%); light brown solid; mp 158–160 °C; (Found: C, 68.21; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.08; N, 14.04. C17H21N3S requires C, 68.19; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.07; N, 14.03%); δH(400 MHz, CDCl3, Me4Si) 1.19 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, CH3CH-cyclohexyl), 1.74 (1 H, m, CH3CH-cyclohexyl), 1.90 (2 H, m, CH2-cyclohexyl), 2.28 (2 H, m, CH2-cyclohexyl), 2.64 (2 H, m, CH2-cyclohexyl), 3.00 (2 H, m, CH2-cyclohexyl), 6.81 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.32 (1 H, m, phenyl), 7.48 (2 H, m, phenyl), 7.53 (2 H, m, phenyl), 12.10 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-Cycloheptylidene-2-(5-methyl-4-phenylthiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound35).
(0.77 g, 99%); ivory solid; mp 175–177 °C; (Found: C, 68.20; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.06; N, 14.03. C17H21N3S requires C, 68.19; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.07; N, 14.03%); δH(400 MHz, CDCl3, Me4Si) 1.50 (2 H, m, CH2-cycloheptyl), 1.61 (2 H, m, CH2-cycloheptyl), 1.70 (2 H, m, CH2-cycloheptyl), 1.76 (2 H, m, CH2-cycloheptyl), 2.84 (2 H, m, CH2-cycloheptyl), 2.90 (2 H, m, CH2-cycloheptyl), 6.83 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 12.30 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-Cyclooctylidene-2-(5-methyl-4-phenylthiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound36).
(0.64 g, 82%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 1/1); white solid; mp 135–137 °C; (Found: C, 68.98; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.41; N, 13.42. C18H23N3S requires C, 68.97; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.40; N, 13.41%); δH(400 MHz, CDCl3, Me4Si) 1.49 (2 H, m, CH2-cyclooctyl), 1.56 (2 H, m, CH2-cyclooctyl), 1.68 (2 H, m, CH2-cyclooctyl), 1.81 (2 H, m, CH2-cyclooctyl), 1.95 (2 H, m, CH2-cyclooctyl), 2.28 (2 H, m, CH2-cyclooctyl), 2.49 (2 H, m, CH2-cyclooctyl), 6.81 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.35 (1 H, m, phenyl), 7.44 (2 H, m, phenyl) 7.52 (2 H, m, phenyl), 12.33 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(Furan-2-ylmethylene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound37).
(0.89 g, 99%); camel solid; mp 249–250 °C; (Found: C, 67.70; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.11; N, 13.15. C18H13N3OS requires C, 67.69; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.10; N, 13.16%); δH(400 MHz, CDCl3, Me4Si) 7.73 (1 H, m, C(3)H-furan), 7.78 (1 H, m, C(4)H-furan), 7.90 (1 H, C(5)H-thiazole), 7.46 (2 H, m, naphthalene), 7.65 (1 H, m, naphthalene), 7.79 (1 H, m, C(5)H-furan), 7.81 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 7.92 (1 H, s, CH = N), 8.46 (1 H, s, naphthalene), 11.95 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(1-(Furan-2-yl)ethylidene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound38).
(0.77 g, 85%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 3/1); camel solid; mp 245–247 °C; (Found: C, 68.46; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.54; N, 12.61. C19H15N3OS requires C, 68.45; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.53; N, 12.60%); δH(400 MHz, CDCl3, Me4Si) 2.23 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 6.61 (1 H, m, C(3)H-furan), 6.76 (1 H, m, C(4)H-furan), 7.31 (1 H, s, C(5)H-thiazole), 7.52 (2 H, m, naphthalene), 7.59 (1 H, m, C(5)H-furan), 7.68 (1 H, m, naphthalene), 7.76 (3 COMPOUND LINKS
N), 6.61 (1 H, m, C(3)H-furan), 6.76 (1 H, m, C(4)H-furan), 7.31 (1 H, s, C(5)H-thiazole), 7.52 (2 H, m, naphthalene), 7.59 (1 H, m, C(5)H-furan), 7.68 (1 H, m, naphthalene), 7.76 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.46 (1 H, s, naphthalene), 11.28 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(4-(Naphthalen-2-yl)thiazol-2-yl)-2-(thiophen-2-ylmethylene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound39).
(0.87 g, 99%); yellow ochre solid; mp 244–245 °C; (Found: C, 64.43; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 3.92; N, 12.54. C18H13N3S2 requires C, 64.45; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 3.91; N, 12.53%); δH(400 MHz, DMSO-d6) 6.80 (1 H, s, C(5)H-thiazole), 7.09 (1 H, m, C(3)H-thiophene), 7.31 (1 H, m, C(5)H-thiophene), 7.57 (1 H, m, C(4)H-thiophene), 7.64 (2 H, m, naphthalene), 7.72 (1 H, m, naphthalene), 7.89 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.22 (1 H, s, CH = N), 8.64 (1 H, s, naphthalene), 12.22 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(1-Cyclohexylethylidene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound41).
(0.86 g, 86%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 3/1); ivory solid; mp 208–210 °C; (Found: C, 72.19; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.64; N, 12.03. C21H23N3S requires C, 72.17; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 6.63; N, 12.02%); δH(400 MHz, CDCl3, Me4Si) 1.60 (2 H, m, CH2-cyclohexyl), 1.64 (2 H, m, CH2-cyclohexyl), 1.69 (2 H, m, CH2-cyclohexyl), 2.34 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.60 (2 H, m, CH2-cyclohexyl), 2.83 (2 H, m, CH2-cyclohexyl), 6.68 (1 H, s, C(5)H-thiazole), 7.74 (2 H, m, naphthalene), 7.88 (1 H, m, naphthalene), 7.96 (3 COMPOUND LINKS
N), 2.60 (2 H, m, CH2-cyclohexyl), 2.83 (2 H, m, CH2-cyclohexyl), 6.68 (1 H, s, C(5)H-thiazole), 7.74 (2 H, m, naphthalene), 7.88 (1 H, m, naphthalene), 7.96 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.70 (1 H, s, naphthalene), 12.31 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(4-(Naphthalen-2-yl)thiazol-2-yl)-2-(pyridin-3-ylmethylene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound43).
(0.85 g, 99%); chrome yellow solid; mp 210–211 °C; (Found: C, 69.09; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.28; N, 16.97. C19H14N4S requires C, 69.07; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.27; N, 16.96%); δH(400 MHz, DMSO-d6) 7.54 (2 H, m, naphthalene), 7.58 (1 H, m, C(2)H-pyridine), 7.86 (1 H, m, naphthalene), 8.05 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.15 (1 H, s, CH = N), 8.38 (1 H, s, C(5)H-thiazole) 8.49 (1 H, m, C(6)H-pyridine), 8.55 (1 H, s, naphthalene), 8.76 (1 H, m, C(4)H-pyridine), 8.99 (1 H, m, C(5)H-pyridine), 11.36 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(4-(Naphthalen-2-yl)thiazol-2-yl)-2-(pyridin-4-ylmethylene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound44).
(0.85 g, 99%); orange solid; mp 250–252 °C; (Found: C, 69.09; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.28; N, 16.97. C19H14N4S requires C, 69.07; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.27; N, 16.96%); δH(400 MHz, DMSO-d6) 7.40 (1 H, s, C(5)H-thiazole), 7.45 (1 H, m, C(3)H-pyridine), 7.58 (1 H, m, C(5)H-pyridine), 7.73 (2 H, m, naphthalene), 7.82 (1 H, m, naphthalene), 8.12 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.15 (1 H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.26 (1 H, s, naphthalene), 8.39 (1 H, m, C(2)H-pyridine), 8.82 (1 H, m, C(6)H-pyridine), 13.10 (1 H, br s, NH, D2O exch.).
N), 8.26 (1 H, s, naphthalene), 8.39 (1 H, m, C(2)H-pyridine), 8.82 (1 H, m, C(6)H-pyridine), 13.10 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(Benzodioxol-5-ylmethylene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound45).
(0.85 g, 99%); salmon solid; mp 244–245 °C; (Found: C, 67.56; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.06; N, 11.24. C21H15N3O2S requires C, 67.54; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.05; N, 11.25%); δH(400 MHz, DMSO-d6) 6.06 (2 H, s, OCH2O), 6.90 (1 H, s, C(5)H-thiazole), 7.16 (1 H, m, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundbenzodioxole), 7.27 (2 H, m, benzodioxole), 7.46 (2 H, m, naphthalene), 7.52 (1 H, m, naphthalene), 7.66 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 7.98 (1 H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.55 (1 H, s, naphthalene), 12.19 (1 H, br s, NH, D2O exch.).
N), 8.55 (1 H, s, naphthalene), 12.19 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-((1H-Indol-3-yl)methylene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound46).
(0.78 g, 85%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 5/1); light pink solid; mp 258–259 °C; (Found: C, 71.70; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.37; N, 15.20. C22H16N4S requires C, 71.71; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.38; N, 15.21%); δH(400 MHz, DMSO-d6) 7.10 (1 H, s, C(5)H-thiazole), 7.32 (1 H, m, C(6)H-indole), 7.43 (1 H, m, C(7)H-indole), 7.55 (2 H, m, naphthalene), 7.64 (1 H, m, naphthalene), 7.77 (1 H, s, C(2)H-indole), 7.83 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.23 (1 H, s, C(4)H-indole), 8.28 (1 H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.55 (1 H, s, naphthalene), 11.55 (1 H, br s, NH, D2O exch.), 12.15 (1 H, br s, NH, D2O exch.).
N), 8.55 (1 H, s, naphthalene), 11.55 (1 H, br s, NH, D2O exch.), 12.15 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(Naphthalen-1-ylmethylene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound47).
(0.71 g, 86%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 3/1); orange solid; mp 198–199 °C; (Found: C, 75.95; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.51; N, 11.06. C24H17N3S requires C, 75.96; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.52; N, 11.07%); δH(400 MHz, DMSO-d6) 6.98 (1 H, s, C(5)H-thiazole), 7.34 (4 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 7.57 (2 H, m, naphthalene), 7.97 (6 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.33 (1 H, s, naphthalene), 8.36 (1 H, s, naphthalene), 8.91 (1 H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 11.45 (1 H, br s, NH, D2O exch.).
N), 11.45 (1 H, br s, NH, D2O exch.).
1-(1-(2H-2-Oxo-chromen-3-yl)ethylidene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (49).
(0.77 g, 99%); yellow solid; mp 210–211 °C; (Found: C, 70.03; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.17; N, 10.20. C24H17N3O2S requires C, 70.05; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.16; N, 10.21%); δH(400 MHz, DMSO-d6) 2.27 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 7.05 (1 H, s, C(5)H-thiazole), 7.34 (2 H, m, naphthalene), 7.44 (1 H, m, C(6)H-chromene), 7.48 (1 H, m, C(8)H-chromene) 7.56 (1 H, m, C(7)H-chromene), 7.66 (1 H, m, naphthalene), 7.76 (1 H, m, C(5)H-chromene), 7.94 (3 COMPOUND LINKS
N), 7.05 (1 H, s, C(5)H-thiazole), 7.34 (2 H, m, naphthalene), 7.44 (1 H, m, C(6)H-chromene), 7.48 (1 H, m, C(8)H-chromene) 7.56 (1 H, m, C(7)H-chromene), 7.66 (1 H, m, naphthalene), 7.76 (1 H, m, C(5)H-chromene), 7.94 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.16 (1 H, s, naphthalene), 8.64 (1 H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 11.40 (1 H, br s, NH, D2O exch.).
N), 11.40 (1 H, br s, NH, D2O exch.).
1-(1-(Ferrocen-1-yl)ethylidene)-2-(4-(naphthalen-2-yl)thiazol-2-yl)hydrazine (50).
(0.70 g, 94%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 5/1); brown solid; mp 185–186 °C; (Found: C, 66.21; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.12; N, 9.28. C25H23FeN3S requires C, 66.23; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.11; N, 9.27%); δH(400 MHz, DMSO-d6) 2.48 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 4.20 (5 COMPOUND LINKS
N), 4.20 (5 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, ferrocene), 4.48 (2 H, m, ferrocene), 4.70 (2 H, m, ferrocene), 6.82 (1 H, s, C(5)H-thiazole), 7.56 (2 H, m, naphthalene), 7.73 (1 H, m, naphthalene), 7.86 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.29 (1 H, s, naphthalene), 12.40 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(1-(Furan-2-yl)ethylidene)-2-(5-methyl-4-phenylthiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound52).
(0.75 g, 85%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 3/1); ivory solid; mp 225–227 °C; (Found: C, 64.63; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.09; N, 14.14. C16H15N3OS requires C, 64.62; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.08; N, 14.13%); δH(400 MHz, DMSO-d6) 2.17 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.33 (3 COMPOUND LINKS
N), 2.33 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 6.72 (1 H, m, C(3)H-furan), 6.98 (1 H, m, C(4)H-furan), 7.22 (1 H, m, phenyl), 7.38 (2 H, m, phenyl), 7.56 (1 H, m, C(5)H-furan), 7.63 (2 H, m, phenyl), 7.65 (1 H, m, C(5)H-furan), 11.28 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(1-(thiophen-2-yl)ethylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound54).
(0.76 g, 97%); light orange solid; mp 210–212 °C; (Found: C, 61.30; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.81; N, 13.42. C16H15N3S2 requires C, 61.31; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.82; N, 13.41%); δH(400 MHz, DMSO-d6) 2.33 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.37 (3 COMPOUND LINKS
N), 2.37 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.12 (1 H, m, C(3)H-thiophene), 7.31 (1 H, m, C(5)H-thiophene), 7.37 (1 H, m, phenyl), 7.40 (1 H, m, C(4)H-thiophene), 7.58 (2 H, m, phenyl), 7.87 (2 H, m, phenyl), 11.40 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(1-(thiazol-2-yl)ethylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound55).
(0.78 g, 99%); salmon solid; mp 250–251 °C; (Found: C, 57.31; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.50; N, 17.83. C15H14N4S2 requires C, 57.30; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.49; N, 17.82%); δH(400 MHz, DMSO-d6) 2.28 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.39 (3 COMPOUND LINKS
N), 2.39 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.42 (1 H, m, phenyl), 7.59 (2 H, m, phenyl), 7.64 (2 H, m, phenyl), 7.74 (1 H, m, C(5)H-thiazole), 8.51 (1 H, m, C(4)H-thiazole), 12.31 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(1-Cyclohexylethylidene)-2-(5-methyl-4-phenylthiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound56).
(0.78 g, 99%); light yellow solid; mp 175–177 °C; (Found: C, 68.95; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.41; N, 13.42. C18H23N3S requires C, 68.97; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 7.40; N, 13.41%); δH(400 MHz, DMSO-d6) 1.97 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.87 (2 H, m, CH2-cyclohexyl), 2.96 (2 H, m, CH2-cyclohexyl), 3.16 (2 H, m, CH2-cyclohexyl), 3.66 (2 H, m, CH2-cyclohexyl), 7.58 (1 H, m, phenyl), 7.79 (2 H, m, phenyl), 7.97 (2 H, m, phenyl), 12.10 (1 H, br s, NH, D2O exch.).
N), 2.87 (2 H, m, CH2-cyclohexyl), 2.96 (2 H, m, CH2-cyclohexyl), 3.16 (2 H, m, CH2-cyclohexyl), 3.66 (2 H, m, CH2-cyclohexyl), 7.58 (1 H, m, phenyl), 7.79 (2 H, m, phenyl), 7.97 (2 H, m, phenyl), 12.10 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(1-phenylethylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound57).
(0.79 g, 99%); green–yellow solid; mp 144–146 °C; (Found: C, 70.34; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.58; N, 13.68. C18H17N3S requires C, 70.33; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.57; N, 13.67%); δH(400 MHz, CDCl3, Me4Si) 2.21 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.39 (3 COMPOUND LINKS
N), 2.39 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.33 (2 H, m, phenyl), 7.45 (2 H, m, phenyl), 7.52 (1 H, m, phenyl), 7.68 (1 H, m, phenyl), 7.76 (1 H, m, phenyl), 7.93 (2 H, m, phenyl), 8.02 (2 H, m, phenyl), 11.39 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(1-(pyridin-2-yl)ethylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound58).
(0.65 g, 82%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 3/1); orange solid; mp 215–220 °C; (Found: C, 66.23; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.22; N, 18.16. C17H16N4S requires C, 66.21; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.23; N, 18.17%); δH(400 MHz, DMSO-d6) 2.27 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.30 (1 H, m, phenyl), 7.38 (2 H, m, phenyl), 7.52 (2 H, m, phenyl), 7.61 (2 H, m, C(5)H-pyridine), 7.67 (2 H, m, C(4)H-pyridine), 8.17 (1 H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.40 (1 H, m, C(3)H-pyridine), 8.46 (1 H, m, C(6)H-pyridine), 11.36 (1 H, br s, NH, D2O exch.).
N), 8.40 (1 H, m, C(3)H-pyridine), 8.46 (1 H, m, C(6)H-pyridine), 11.36 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(pyridin-4-ylmethylene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound60).
(0.64 g, 82%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 3/1); red solid; mp 220–223 °C; (Found: C, 65.29; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.79; N, 19.02. C16H14N4S requires C, 65.28; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.79; N, 19.03%); δH(400 MHz, DMSO-d6) 2.19 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.16 (1 H, m, phenyl), 7.28 (2 H, m, phenyl), 7.36 (2 H, m, phenyl), 7.54 (1 H, m, C(3)H-pyridine), 7.61 (1 H, m, C(5)H-pyridine), 7.98 (1 H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.30 (1 H, m, C(2)H-pyridine), 8.35 (1 H, m, C(6)H-pyridine), 12.19 (1 H, br s, NH, D2O exch.).
N), 8.30 (1 H, m, C(2)H-pyridine), 8.35 (1 H, m, C(6)H-pyridine), 12.19 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(Benzodioxol-5-ylmethylene)-2-(5-methyl-4-phenylthiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound61).
(0.66 g, 99%); ivory solid; mp 240–242 °C; (Found: C, 64.10; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.47; N, 12.46. C18H15N3O2S requires C, 64.08; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.48; N, 12.45%); δH(400 MHz, DMSO-d6) 2.25 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 6.31 (2 H, s, OCH2O), 7.22 (1 H, m, benzodioxole), 7.29 (1 H, m, phenyl), 7.39 (2 H, m, benzodioxole), 7.56 (2 H, m, phenyl), 7.96 (2 H, m, phenyl), 7.98 (1 H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 12.50 (1 H, br s, NH, D2O exch.).
N), 12.50 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-((1H-Indol-3-yl)methylene)-2-(5-methyl-4-phenylthiazol-2-yl)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound62).
(0.65 g, 86%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 5/1); sand solid; mp 198–200 °C; (Found: C, 68.66; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.86; N, 16.85. C19H16N4S requires C, 68.65; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.85; N, 16.85%); δH(400 MHz, DMSO-d6) 2.22 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.29 (1 H, m, phenyl), 7.36 (1 H, m, C(6)H-indole), 7.47 (1 H, m, C(7)H-indole), 7.52 (2 H, m, phenyl), 7.59 (2 H, m, phenyl), 7.83 (1 H, m, C(2)H-indole), 7.98 (1 H, s, C(2)H-indole), 8.23 (1 H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 11.55 (1 H, br s, NH, D2O exch.), 12.80 (1 H, br s, NH, D2O exch.).
N), 11.55 (1 H, br s, NH, D2O exch.), 12.80 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(naphthalen-1-ylmethylene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound63).
(0.71 g, 99%); ivory solid; mp 192–194 °C; (Found: C, 73.45; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.98; N, 12.22. C21H17N3S requires C, 73.44; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.99; N, 12.23%); δH(400 MHz, DMSO-d6) 2.16 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.43 (1 H, m, phenyl), 7.58 (2 H, m, naphthalene), 7.60 (2 H, m, phenyl), 7.64 (2 H, m, phenyl), 7.72 (1 H, m, naphthalene), 7.98 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 8.21 (1 H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 11.45 (1 H, br s, NH, D2O exch.).
N), 11.45 (1 H, br s, NH, D2O exch.).
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1-(5-Methyl-4-phenylthiazol-2-yl)-2-(1-(naphthalen-2-yl)ethylidene)hydrazine (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound64).
(0.62 g, 88%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 3/1); ivory solid; mp 200–201 °C; (Found: C, 73.91; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.35; N, 11.76. C22H19N3S requires C, 73.92; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.36; N, 11.75%); δH(400 MHz, DMSO-d6) 2.27 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.42 (3 COMPOUND LINKS
N), 2.42 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.28 (1 H, m, phenyl), 7.36 (2 H, m, naphthalene), 7.42 (2 H, m, phenyl), 7.49 (2 H, m, phenyl), 7.71 (1 H, m, naphthalene), 7.88 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, m, naphthalene), 11.30 (1 H, br s, NH, D2O exch.).
1-(5-Methyl-4-phenylthiazol-2-yl)-2-(1-(2H-2-oxo-chromen-3-yl)ethylidene)hydrazine (65).
(0.68 g, 90%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 3/1); light yellow solid; mp 215–218 °C; (Found: C, 67.20; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.56; N, 11.20. C21H17N3O2S requires C, 67.18; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 4.56; N, 11.19%); δH(400 MHz, DMSO-d6) 2.27 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.31 (3 COMPOUND LINKS
N), 2.31 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 7.33 (1 H, m, phenyl), 7.39 (2 H, m, phenyl), 7.45 (2 H, m, phenyl), 7.52 (1 H, m, C(6)H-chromene), 7.56 (1 H, m, C(8)H-chromene), 7.67 (1 H, m, C(7)H-chromene), 7.94 (1 H, m, C(5)H-chromene), 8.31 (1 H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 11.40 (1 H, br s, NH, D2O exch.).
N), 11.40 (1 H, br s, NH, D2O exch.).
1-(Ferrocen-1-yl)-2-(5-methyl-4-phenylthiazol-2-yl)ethylidene)hydrazine (66).
(0.62 g, 90%, purified by chromatography: SiO2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane 5/1); brown solid; mp 175–176 °C; (Found: C, 63.63; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.09; N, 10.11. C22H21FeN3S requires C, 63.62; COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, 5.10; N, 10.12%); δH(400 MHz, DMSO-d6) 2.21 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.48 (3 COMPOUND LINKS
N), 2.48 (3 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, CH3-thiazole), 4.20 (5 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH, s, ferrocene), 4.39 (2 H, m, ferrocene), 4.52 (2 H, m, ferrocene), 7.37 (1 H, m, phenyl), 7.45 (2 H, m, phenyl), 7.51 (2 H, m, phenyl), 12.40 (1 H, br s, NH, D2O exch.).
3.2 Determination of hMAO isoform activity.
The effects of the test compounds on the enzymatic activity of hMAO isoform were evaluated by a fluorimetric method. Briefly, 0.1 mL of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsodium phosphate buffer (0.05 M, pH 7.4) containing different concentrations of the test drugs (new compounds or reference inhibitors) and adequate amounts of recombinant hMAO-A or hMAO-B required and adjusted to obtain in our experimental conditions the same reaction velocity, i.e., to oxidize (in the control group) 165 pmol of p-tyramine/min (hMAO-A: 1.1 μg protein; specific activity: 150 nmol of p-tyramine oxidized to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundp-hydroxyphenylacetaldehyde/min/mg protein; hMAO-B: 7.5 μg protein; specific activity: 22 nmol of p-tyramine transformed/min/mg protein) were incubated for 15 min at 37 °C in a flat-black-bottom 96-well microtest plate (BD Biosciences, Franklin Lakes, NJ, USA) placed in the dark multimode microplate reader chamber. After this incubation period, the reaction was started by adding (final concentrations) 200 μM Amplex Red reagent, 1 U/mL horseradish peroxidase and 1 mM p-tyramine (final saturating concentration). The production of H2O2 and, consequently, of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundresorufin was quantified at 37 °C in a multidetection microplate fluorescence reader (Fluostar Optima, BMG Labtech GmbH, Offenburg, Germany) based on the fluorescence generated (excitation, 545 nm, emission, 590 nm) over a 15 min period, in which the fluorescence increased linearly. Control experiments were carried out simultaneously by replacing the test drugs (new compounds and reference inhibitors) with appropriate dilutions of the vehicles. In addition, the possible capacity of the above test drugs to modify the fluorescence generated in the reaction mixture due to non-enzymatic inhibition (e.g., for directly reacting with Amplex Red reagent) was determined by adding these drugs to solutions containing only the Amplex Red reagent in a COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsodium phosphate buffer. The specific fluorescence emission (used to obtain the final results) was calculated after subtraction of the background activity, which was determined from vials containing all components except the hMAO isoforms, which were replaced by a COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsodium phosphate buffer solution. In our experimental conditions, this background activity was practically negligible.
4 Conclusion
These findings increase our confidence in the 2-thiazolylhydrazone model and stimulate us to continue our investigations in designing more potent and selective analogues. The results obtained in this study indicate that some of the compounds tested may have interesting therapeutic potential as original chemical models (templates) for the design and subsequent development of new drugs useful for improving the pharmacological treatment of major depressive disorders. Finally, in Scheme 2, we have collected all SAR studies which could be drawn about this scaffold.8–10
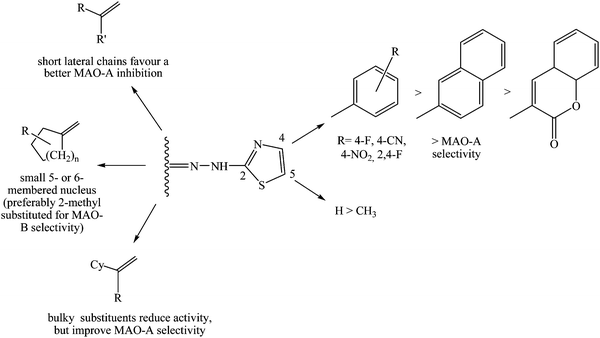 |
| | Scheme 2 SAR studies of MAO inhibition by 2-thiazolylhydrazone scaffold. | |
Bibliographic references and notes
- D. E. Edmondson, A. Mattevi, C. Binda, M. Li and F. Hubálek, Curr. Med. Chem., 2004, 11, 1983–1993 CAS
 .
.
- D. E. Edmondson, L. DeColibus, C. Binda, M. Li and A. Mattevi, J. Neural Transm., 2007, 114, 703–705 CrossRef CAS
 .
.
- D. E. Edmondson, C. Binda, J. Wang, A. K. Upadhayay and A. Mattevi, Biochemistry, 2009, 48, 4220–4230 CrossRef CAS
 .
.
-
(a) P. Riederer, L. Lachenmayer and G. Laux, Curr. Med. Chem., 2004, 11, 2033–2043 CAS
 ;
(b) M. C. Carreiras and J. L. Marco, Curr. Pharm. Des., 2004, 10, 3167–3175 CrossRef CAS
;
(b) M. C. Carreiras and J. L. Marco, Curr. Pharm. Des., 2004, 10, 3167–3175 CrossRef CAS  ;
(c) D. R. Guay, Am. J. Geriatr. Pharmacother, 2006, 4, 330–346 Search PubMed
;
(c) D. R. Guay, Am. J. Geriatr. Pharmacother, 2006, 4, 330–346 Search PubMed  .
.
- M. B. H. Youdim, D. E. Edmondson and K. F. Tipton, Nat. Rev. Neurosci., 2006, 7, 295–309 CrossRef CAS
 .
.
- M. Bortolato, K. Chen and J. C. Shih, Adv. Drug Delivery Rev., 2008, 60, 1527–1533 CrossRef CAS
 .
.
- A. Bolasco, R. Fioravanti and S. Carradori, Expert Opin. Ther. Pat., 2005, 15, 1763–1782 Search PubMed
 .
.
- F. Chimenti, E. Maccioni, D. Secci, A. Bolasco, P. Chimenti, A. Granese, O. Befani, P. Turini, S. Alcaro, F. Ortuso, M. C. Cardia and S. Distinto, J. Med. Chem., 2007, 50, 707–712 CrossRef CAS
 .
.
- F. Chimenti, E. Maccioni, D. Secci, A. Bolasco, P. Chimenti, A. Granese, S. Carradori, S. Alcaro, F. Ortuso, M. Yáñez, F. Orallo, R. Cirilli, R. Ferretti and F. La Torre, J. Med. Chem., 2008, 51, 4874 CrossRef CAS
 .
.
- F. Chimenti, S. Carradori, D. Secci, A. Bolasco, P. Chimenti, A. Granese and B. Bizzarri, J. Heterocycl. Chem., 2009, 46, 575–578 CrossRef CAS
 .
.
- S. Y. Son, J. Ma, Y. Kondou, M. Yoshimura, E. Yamashita and T. Tsukihara, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 5739–5744 CrossRef CAS
 . Data deposition: http://www.pdb.org (PDB ID code 2Z5X).
. Data deposition: http://www.pdb.org (PDB ID code 2Z5X).
- F. Hubálek, C. Binda, A. Khalil, M. Li, A. Mattevi, N. Castagnoli and D. E. Edmondson, J. Biol. Chem., 2005, 280, 15761–15766 CrossRef CAS
 . Data deposition: http://www.pdb.org (PDB ID code 2BK3).
. Data deposition: http://www.pdb.org (PDB ID code 2BK3).
- R. Benassi, A. Benedetti, F. Taddei, R. Cappelletti, D. Nardi and A. Tajana, Org. Magn. Reson., 1982, 20, 26–30 CrossRef CAS
 .
.
- R. Cirilli, R. Ferretti, F. La Torre, D. Secci, A. Bolasco, S. Carradori and M. Pierini, J. Chromatogr., A, 2007, 1172, 160–169 CrossRef CAS
 .
.
- M. Yáñez, N. Fraiz, E. Cano and F. Orallo, Biochem. Biophys. Res. Commun., 2006, 344, 688–695 CrossRef CAS
 .
.
- M. S. Solanki and J. P. Trivedi, J. Indian Chem. Soc., 1972, 49, 37–39 CAS
 .
.
-
D. S. Goldfarb, US 2009163545 A1 20090625, 2009.
Footnote |
| † Electronic supplementary information (ESI) available: Pharmacological studies. See DOI: 10.1039/c0md00014k |
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group (C2 of the thiazole) allowed us to better comprehend the steric and electronic influence on the enzyme-inhibitor interaction and to modulate the chemical structure of these derivatives.
N group (C2 of the thiazole) allowed us to better comprehend the steric and electronic influence on the enzyme-inhibitor interaction and to modulate the chemical structure of these derivatives.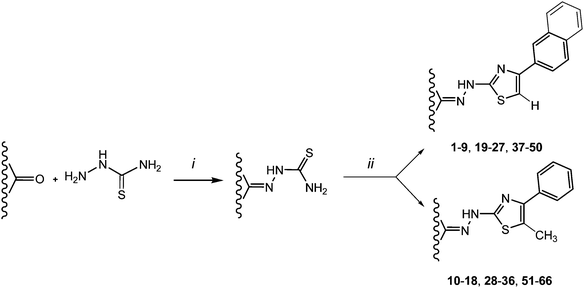
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond can give rise to isomeric geometry E/Z. The 1H NMR (in CDCl3) spectra analysis revealed that the E isomer was more favoured and stable than the Z-configuration. The amounts of both conformers were measured by area integration of the signal relative to the CH3 protons (area ratio of proton signals E
N double bond can give rise to isomeric geometry E/Z. The 1H NMR (in CDCl3) spectra analysis revealed that the E isomer was more favoured and stable than the Z-configuration. The amounts of both conformers were measured by area integration of the signal relative to the CH3 protons (area ratio of proton signals E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z was generally 6
Z was generally 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The low-field signal was assigned to the E isomer, since it is widely accepted in thiosemicarbazone derivatives.13 Our choice, as reaction medium, of a polar alcoholic solvent appeared to be preferred to obtain the E-configuration and limit the interconversion according to the results of our previous theoretical and chromatographic study for similar compounds.14
1). The low-field signal was assigned to the E isomer, since it is widely accepted in thiosemicarbazone derivatives.13 Our choice, as reaction medium, of a polar alcoholic solvent appeared to be preferred to obtain the E-configuration and limit the interconversion according to the results of our previous theoretical and chromatographic study for similar compounds.14![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 2.26 (2 H, d, J 7.2 Hz, CH2), 6.81 (1 H, s, C(5)H-thiazole), 7.55 (2 H, m, naphthalene), 7.57 (1 H, m, naphthalene), 7.78 (3 H, m, naphthalene), 8.27 (1 H, s, naphthalene), 12.51 (1 H, br s, NH, D2O exch.).
), 2.26 (2 H, d, J 7.2 Hz, CH2), 6.81 (1 H, s, C(5)H-thiazole), 7.55 (2 H, m, naphthalene), 7.57 (1 H, m, naphthalene), 7.78 (3 H, m, naphthalene), 8.27 (1 H, s, naphthalene), 12.51 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.38 (2 H, m, CH2CH
N), 2.38 (2 H, m, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 2.50 (2 H, m, CH2C
), 2.50 (2 H, m, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 5.07 (2 H, m, CH2
N), 5.07 (2 H, m, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 5.83 (1 H, m, CH
), 5.83 (1 H, m, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.81 (1 H, s, C(5)H-thiazole), 7.55 (2 H, m, naphthalene), 7.71 (1 H, m, naphthalene), 7.92 (3 H, m, naphthalene), 8.27 (1 H, s, naphthalene), 12.43 (1 H, br s, NH, D2O exch.).
), 6.81 (1 H, s, C(5)H-thiazole), 7.55 (2 H, m, naphthalene), 7.71 (1 H, m, naphthalene), 7.92 (3 H, m, naphthalene), 8.27 (1 H, s, naphthalene), 12.43 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.37 (2 H, m, CH2C
N), 2.37 (2 H, m, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 6.69 (1 H, s, C(5)H-thiazole), 7.54 (2 H, m, naphthalene), 7.76 (1 H, m, naphthalene), 7.97 (3 H, m, naphthalene), 8.67 (1 H, s, naphthalene), 12.30 (1 H, br s, NH, D2O exch.).
N), 6.69 (1 H, s, C(5)H-thiazole), 7.54 (2 H, m, naphthalene), 7.76 (1 H, m, naphthalene), 7.97 (3 H, m, naphthalene), 8.67 (1 H, s, naphthalene), 12.30 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1.56 (2 H, m, CH3CH2C
N), 1.56 (2 H, m, CH3CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1–61 (2 H, m, CH2C
N), 1–61 (2 H, m, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.41 (2 H, m, CH2), 2.54 (2 H, m, CH2), 6.80 (1 H, s, C(5)H-thiazole), 7.54 (2 H, m, naphthalene), 7.71 (1 H, m, naphthalene), 7.83 (3 H, m, naphthalene), 8.28 (1 H, s, naphthalene), 12.52 (1 H, br s, NH, D2O exch.).
N), 2.41 (2 H, m, CH2), 2.54 (2 H, m, CH2), 6.80 (1 H, s, C(5)H-thiazole), 7.54 (2 H, m, naphthalene), 7.71 (1 H, m, naphthalene), 7.83 (3 H, m, naphthalene), 8.28 (1 H, s, naphthalene), 12.52 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.37 (2 H, m, CH2C
N), 2.37 (2 H, m, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 6.81 (1 H, s, C(5)H-thiazole), 7.55 (2 H, m, naphthalene), 7.74 (1 H, m, naphthalene), 7.93 (3 H, m, naphthalene), 8.29 (1 H, s, naphthalene), 12.63 (1 H, br s, NH, D2O exch.).
N), 6.81 (1 H, s, C(5)H-thiazole), 7.55 (2 H, m, naphthalene), 7.74 (1 H, m, naphthalene), 7.93 (3 H, m, naphthalene), 8.29 (1 H, s, naphthalene), 12.63 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.56 (2 H, m, CH2), 6.69 (3 H, s, CH3-thiazole), 7.39 (1 H, m, phenyl), 7.53 (2 H, m, phenyl), 7.62 (2 H, m, phenyl), 12.35 (1 H, br s, NH, D2O exch.).
N), 2.56 (2 H, m, CH2), 6.69 (3 H, s, CH3-thiazole), 7.39 (1 H, m, phenyl), 7.53 (2 H, m, phenyl), 7.62 (2 H, m, phenyl), 12.35 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.37 (2 H, m, CH2), 6.70 (3 H, s, CH3-thiazole), 7.83 (1 H, m, phenyl), 7.86 (2 H, m, phenyl), 7.87 (2 H, m, phenyl), 12.29 (1 H, br s, NH, D2O exch.).
N), 2.37 (2 H, m, CH2), 6.70 (3 H, s, CH3-thiazole), 7.83 (1 H, m, phenyl), 7.86 (2 H, m, phenyl), 7.87 (2 H, m, phenyl), 12.29 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.26 (2 H, m, CH2), 6.81 (3 H, s, CH3-thiazole), 7.28 (1 H, m, phenyl), 7.47 (2 H, m, phenyl), 7.54 (2 H, m, phenyl), 12.51 (1 H, br s, NH, D2O exch.).
N), 2.26 (2 H, m, CH2), 6.81 (3 H, s, CH3-thiazole), 7.28 (1 H, m, phenyl), 7.47 (2 H, m, phenyl), 7.54 (2 H, m, phenyl), 12.51 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 2.32 (2 H, m, CH2CH
), 2.32 (2 H, m, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 2.49 (2 H, m, CH2C
), 2.49 (2 H, m, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 5.09 (2 H, m, CH2
N), 5.09 (2 H, m, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 5.83 (1 H, m, CH
), 5.83 (1 H, m, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.81 (3 H, s, CH3-thiazole), 7.39 (5 H, m, Ar), 12.43 (1 H, br s, NH, D2O exch.).
), 6.81 (3 H, s, CH3-thiazole), 7.39 (5 H, m, Ar), 12.43 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.37 (2 H, m, CH2C
N), 2.37 (2 H, m, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 6.69 (3 H, s, CH3-thiazole), 7.42 (1 H, m, phenyl), 7.47 (2 H, m, phenyl), 7.57 (2 H, m, phenyl), 12.30 (1 H, br s, NH, D2O exch.).
N), 6.69 (3 H, s, CH3-thiazole), 7.42 (1 H, m, phenyl), 7.47 (2 H, m, phenyl), 7.57 (2 H, m, phenyl), 12.30 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1.56 (2 H, m, CH2), 1.61 (2 H, m, CH2), 2.41 (2 H, m, CH3CH2C
N), 1.56 (2 H, m, CH2), 1.61 (2 H, m, CH2), 2.41 (2 H, m, CH3CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.54 (2 H, m, CH2C
N), 2.54 (2 H, m, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 6.80 (3 H, s, CH3-thiazole), 7.43 (1 H, m, phenyl), 7.48 (2 H, m, phenyl), 7.55 (2 H, m, phenyl), 12.52 (1 H, br s, NH, D2O exch.).
N), 6.80 (3 H, s, CH3-thiazole), 7.43 (1 H, m, phenyl), 7.48 (2 H, m, phenyl), 7.55 (2 H, m, phenyl), 12.52 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.37 (2 H, m, CH2C
N), 2.37 (2 H, m, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 6.81 (3 H, s, CH3-thiazole), 7.55 (1 H, m, phenyl), 7.60 (2 H, m, phenyl), 7.64 (2 H, m, phenyl), 12.63 (1 H, br s, NH, D2O exch.).
N), 6.81 (3 H, s, CH3-thiazole), 7.55 (1 H, m, phenyl), 7.60 (2 H, m, phenyl), 7.64 (2 H, m, phenyl), 12.63 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 6.61 (1 H, m, C(3)H-furan), 6.76 (1 H, m, C(4)H-furan), 7.31 (1 H, s, C(5)H-thiazole), 7.52 (2 H, m, naphthalene), 7.59 (1 H, m, C(5)H-furan), 7.68 (1 H, m, naphthalene), 7.76 (3 H, m, naphthalene), 8.46 (1 H, s, naphthalene), 11.28 (1 H, br s, NH, D2O exch.).
N), 6.61 (1 H, m, C(3)H-furan), 6.76 (1 H, m, C(4)H-furan), 7.31 (1 H, s, C(5)H-thiazole), 7.52 (2 H, m, naphthalene), 7.59 (1 H, m, C(5)H-furan), 7.68 (1 H, m, naphthalene), 7.76 (3 H, m, naphthalene), 8.46 (1 H, s, naphthalene), 11.28 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.60 (2 H, m, CH2-cyclohexyl), 2.83 (2 H, m, CH2-cyclohexyl), 6.68 (1 H, s, C(5)H-thiazole), 7.74 (2 H, m, naphthalene), 7.88 (1 H, m, naphthalene), 7.96 (3 H, m, naphthalene), 8.70 (1 H, s, naphthalene), 12.31 (1 H, br s, NH, D2O exch.).
N), 2.60 (2 H, m, CH2-cyclohexyl), 2.83 (2 H, m, CH2-cyclohexyl), 6.68 (1 H, s, C(5)H-thiazole), 7.74 (2 H, m, naphthalene), 7.88 (1 H, m, naphthalene), 7.96 (3 H, m, naphthalene), 8.70 (1 H, s, naphthalene), 12.31 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.26 (1 H, s, naphthalene), 8.39 (1 H, m, C(2)H-pyridine), 8.82 (1 H, m, C(6)H-pyridine), 13.10 (1 H, br s, NH, D2O exch.).
N), 8.26 (1 H, s, naphthalene), 8.39 (1 H, m, C(2)H-pyridine), 8.82 (1 H, m, C(6)H-pyridine), 13.10 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.55 (1 H, s, naphthalene), 12.19 (1 H, br s, NH, D2O exch.).
N), 8.55 (1 H, s, naphthalene), 12.19 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.55 (1 H, s, naphthalene), 11.55 (1 H, br s, NH, D2O exch.), 12.15 (1 H, br s, NH, D2O exch.).
N), 8.55 (1 H, s, naphthalene), 11.55 (1 H, br s, NH, D2O exch.), 12.15 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 11.45 (1 H, br s, NH, D2O exch.).
N), 11.45 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 7.05 (1 H, s, C(5)H-thiazole), 7.34 (2 H, m, naphthalene), 7.44 (1 H, m, C(6)H-chromene), 7.48 (1 H, m, C(8)H-chromene) 7.56 (1 H, m, C(7)H-chromene), 7.66 (1 H, m, naphthalene), 7.76 (1 H, m, C(5)H-chromene), 7.94 (3 H, m, naphthalene), 8.16 (1 H, s, naphthalene), 8.64 (1 H, s, CH
N), 7.05 (1 H, s, C(5)H-thiazole), 7.34 (2 H, m, naphthalene), 7.44 (1 H, m, C(6)H-chromene), 7.48 (1 H, m, C(8)H-chromene) 7.56 (1 H, m, C(7)H-chromene), 7.66 (1 H, m, naphthalene), 7.76 (1 H, m, C(5)H-chromene), 7.94 (3 H, m, naphthalene), 8.16 (1 H, s, naphthalene), 8.64 (1 H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 11.40 (1 H, br s, NH, D2O exch.).
N), 11.40 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 4.20 (5 H, s, ferrocene), 4.48 (2 H, m, ferrocene), 4.70 (2 H, m, ferrocene), 6.82 (1 H, s, C(5)H-thiazole), 7.56 (2 H, m, naphthalene), 7.73 (1 H, m, naphthalene), 7.86 (3 H, m, naphthalene), 8.29 (1 H, s, naphthalene), 12.40 (1 H, br s, NH, D2O exch.).
N), 4.20 (5 H, s, ferrocene), 4.48 (2 H, m, ferrocene), 4.70 (2 H, m, ferrocene), 6.82 (1 H, s, C(5)H-thiazole), 7.56 (2 H, m, naphthalene), 7.73 (1 H, m, naphthalene), 7.86 (3 H, m, naphthalene), 8.29 (1 H, s, naphthalene), 12.40 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.33 (3 H, s, CH3-thiazole), 6.72 (1 H, m, C(3)H-furan), 6.98 (1 H, m, C(4)H-furan), 7.22 (1 H, m, phenyl), 7.38 (2 H, m, phenyl), 7.56 (1 H, m, C(5)H-furan), 7.63 (2 H, m, phenyl), 7.65 (1 H, m, C(5)H-furan), 11.28 (1 H, br s, NH, D2O exch.).
N), 2.33 (3 H, s, CH3-thiazole), 6.72 (1 H, m, C(3)H-furan), 6.98 (1 H, m, C(4)H-furan), 7.22 (1 H, m, phenyl), 7.38 (2 H, m, phenyl), 7.56 (1 H, m, C(5)H-furan), 7.63 (2 H, m, phenyl), 7.65 (1 H, m, C(5)H-furan), 11.28 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.37 (3 H, s, CH3-thiazole), 7.12 (1 H, m, C(3)H-thiophene), 7.31 (1 H, m, C(5)H-thiophene), 7.37 (1 H, m, phenyl), 7.40 (1 H, m, C(4)H-thiophene), 7.58 (2 H, m, phenyl), 7.87 (2 H, m, phenyl), 11.40 (1 H, br s, NH, D2O exch.).
N), 2.37 (3 H, s, CH3-thiazole), 7.12 (1 H, m, C(3)H-thiophene), 7.31 (1 H, m, C(5)H-thiophene), 7.37 (1 H, m, phenyl), 7.40 (1 H, m, C(4)H-thiophene), 7.58 (2 H, m, phenyl), 7.87 (2 H, m, phenyl), 11.40 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.39 (3 H, s, CH3-thiazole), 7.42 (1 H, m, phenyl), 7.59 (2 H, m, phenyl), 7.64 (2 H, m, phenyl), 7.74 (1 H, m, C(5)H-thiazole), 8.51 (1 H, m, C(4)H-thiazole), 12.31 (1 H, br s, NH, D2O exch.).
N), 2.39 (3 H, s, CH3-thiazole), 7.42 (1 H, m, phenyl), 7.59 (2 H, m, phenyl), 7.64 (2 H, m, phenyl), 7.74 (1 H, m, C(5)H-thiazole), 8.51 (1 H, m, C(4)H-thiazole), 12.31 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.87 (2 H, m, CH2-cyclohexyl), 2.96 (2 H, m, CH2-cyclohexyl), 3.16 (2 H, m, CH2-cyclohexyl), 3.66 (2 H, m, CH2-cyclohexyl), 7.58 (1 H, m, phenyl), 7.79 (2 H, m, phenyl), 7.97 (2 H, m, phenyl), 12.10 (1 H, br s, NH, D2O exch.).
N), 2.87 (2 H, m, CH2-cyclohexyl), 2.96 (2 H, m, CH2-cyclohexyl), 3.16 (2 H, m, CH2-cyclohexyl), 3.66 (2 H, m, CH2-cyclohexyl), 7.58 (1 H, m, phenyl), 7.79 (2 H, m, phenyl), 7.97 (2 H, m, phenyl), 12.10 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.39 (3 H, s, CH3-thiazole), 7.33 (2 H, m, phenyl), 7.45 (2 H, m, phenyl), 7.52 (1 H, m, phenyl), 7.68 (1 H, m, phenyl), 7.76 (1 H, m, phenyl), 7.93 (2 H, m, phenyl), 8.02 (2 H, m, phenyl), 11.39 (1 H, br s, NH, D2O exch.).
N), 2.39 (3 H, s, CH3-thiazole), 7.33 (2 H, m, phenyl), 7.45 (2 H, m, phenyl), 7.52 (1 H, m, phenyl), 7.68 (1 H, m, phenyl), 7.76 (1 H, m, phenyl), 7.93 (2 H, m, phenyl), 8.02 (2 H, m, phenyl), 11.39 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.40 (1 H, m, C(3)H-pyridine), 8.46 (1 H, m, C(6)H-pyridine), 11.36 (1 H, br s, NH, D2O exch.).
N), 8.40 (1 H, m, C(3)H-pyridine), 8.46 (1 H, m, C(6)H-pyridine), 11.36 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.30 (1 H, m, C(2)H-pyridine), 8.35 (1 H, m, C(6)H-pyridine), 12.19 (1 H, br s, NH, D2O exch.).
N), 8.30 (1 H, m, C(2)H-pyridine), 8.35 (1 H, m, C(6)H-pyridine), 12.19 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 12.50 (1 H, br s, NH, D2O exch.).
N), 12.50 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 11.55 (1 H, br s, NH, D2O exch.), 12.80 (1 H, br s, NH, D2O exch.).
N), 11.55 (1 H, br s, NH, D2O exch.), 12.80 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 11.45 (1 H, br s, NH, D2O exch.).
N), 11.45 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.42 (3 H, s, CH3-thiazole), 7.28 (1 H, m, phenyl), 7.36 (2 H, m, naphthalene), 7.42 (2 H, m, phenyl), 7.49 (2 H, m, phenyl), 7.71 (1 H, m, naphthalene), 7.88 (3 H, m, naphthalene), 11.30 (1 H, br s, NH, D2O exch.).
N), 2.42 (3 H, s, CH3-thiazole), 7.28 (1 H, m, phenyl), 7.36 (2 H, m, naphthalene), 7.42 (2 H, m, phenyl), 7.49 (2 H, m, phenyl), 7.71 (1 H, m, naphthalene), 7.88 (3 H, m, naphthalene), 11.30 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.31 (3 H, s, CH3-thiazole), 7.33 (1 H, m, phenyl), 7.39 (2 H, m, phenyl), 7.45 (2 H, m, phenyl), 7.52 (1 H, m, C(6)H-chromene), 7.56 (1 H, m, C(8)H-chromene), 7.67 (1 H, m, C(7)H-chromene), 7.94 (1 H, m, C(5)H-chromene), 8.31 (1 H, s, CH
N), 2.31 (3 H, s, CH3-thiazole), 7.33 (1 H, m, phenyl), 7.39 (2 H, m, phenyl), 7.45 (2 H, m, phenyl), 7.52 (1 H, m, C(6)H-chromene), 7.56 (1 H, m, C(8)H-chromene), 7.67 (1 H, m, C(7)H-chromene), 7.94 (1 H, m, C(5)H-chromene), 8.31 (1 H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 11.40 (1 H, br s, NH, D2O exch.).
N), 11.40 (1 H, br s, NH, D2O exch.).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.48 (3 H, s, CH3-thiazole), 4.20 (5 H, s, ferrocene), 4.39 (2 H, m, ferrocene), 4.52 (2 H, m, ferrocene), 7.37 (1 H, m, phenyl), 7.45 (2 H, m, phenyl), 7.51 (2 H, m, phenyl), 12.40 (1 H, br s, NH, D2O exch.).
N), 2.48 (3 H, s, CH3-thiazole), 4.20 (5 H, s, ferrocene), 4.39 (2 H, m, ferrocene), 4.52 (2 H, m, ferrocene), 7.37 (1 H, m, phenyl), 7.45 (2 H, m, phenyl), 7.51 (2 H, m, phenyl), 12.40 (1 H, br s, NH, D2O exch.).

.
.
.
; (b) M. C. Carreiras and J. L. Marco, Curr. Pharm. Des., 2004, 10, 3167–3175 CrossRef CAS
; (c) D. R. Guay, Am. J. Geriatr. Pharmacother, 2006, 4, 330–346 Search PubMed
.
.
.
.
.
.
.
. Data deposition: http://www.pdb.org (PDB ID code 2Z5X).
. Data deposition: http://www.pdb.org (PDB ID code 2BK3).
.
.
.
.