Fluorocarbon oligonucleotide conjugates for nucleic acids delivery†
Guilhem
Godeau
ab,
Hélène
Arnion
ab,
Christophe
Brun
ab,
Cathy
Staedel
ab and
Philippe
Barthélémy
*ab
aUniversité de Bordeaux, 146 rue Léo Saignat, 33076 Bordeaux Cedex, France. E-mail: philippe.barthelemy@inserm.fr; Fax: +33 5 5757 1015; Tel: +33 5 5757 4853
bINSERM U869, 146 rue Léo Saignat, 33076 Bordeaux Cedex, France
First published on 4th June 2010
Abstract
Here we describe an automated synthetic pathway for the synthesis of fluorocarbon oligonucleotide conjugates (FONs) featuring fluorocarbon hydrophobic and lipophobic moieties. The presence of highly fluorinated chains allows the delivery of nucleic acids into human cells.
As drug candidates, polyanionic oligonucleotides (ONs) must be capable of crossing the cell membrane. To overcome this barrier, several approaches involving cationic lipophilic carriers and/or polymers have been developed.1 Alternatively, lipid hybrid ON-conjugates have been synthesized with the aim of improving cellular uptake and antisense activity.2 Conjugation of ONs with hydrophobic lipid moieties via covalent bonds has provided one of the most popular means for modulating their physicochemical and biological properties.3
Recently, we reported that lipid conjugation of oligonucleotides (LONs) increased the cellular uptake of 17mer ONs.4 The encouraging biological results observed with such derivatives prompted us to further investigate the potential of new ON conjugated analogues containing a fluorocarbon chain (Scheme 1).
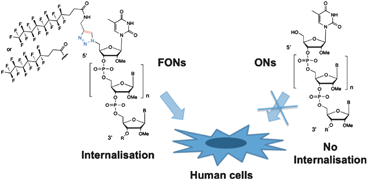 | ||
| Scheme 1 Conjugation of fluorocarbon chains (FONs) allows the delivery of oligonucleotides into human cells. | ||
We hypothesized that the nature of the hydrophobic segment could influence cellular uptake. Indeed, puzzling questions are emerging from the ON conjugate internalization, for example how the nature of the hydrophobic moiety conjugated to the ONs can affect their cellular internalization. To address this issue, highly fluorinated chains were conjugated to 17mer ONs. An appealing part of perfluorinated or semi fluorinated chains lies in their intrinsic properties of being simultaneously highly hydrophobic and lipophobic, as well as chemically and biologically stable.5 Based upon these characteristics, fluorocarbon conjugates would feature different biodistributions. For example, their lipophobic character would avoid their accumulation in lipid membranes or environment, which could be of biological interest in the case of biological targets located in a non-lipidic environment. Numerous examples of fluorocarbon amphiphiles6 have been reported in particular for biomedical applications.7
Herein we report the first example of fluorocarbon oligonucleotide conjugates (FONs) allowing the delivery of ONs into human cells (Scheme 1). In this work we present the synthesis, and the biological properties of two FONs featuring C8F17 and C6F13 chains (compounds 5a and 5b, Fig. 1).
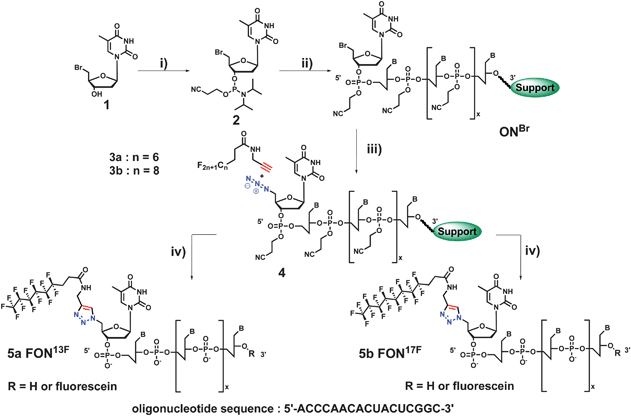 | ||
| Fig. 1 Post synthetic route to FONs (5a and 5b). i) 1.5 eq of 2-cyanoethyl-N,N-diisopropylchlorophosphite, 2 eq of DIEA, DCM, rt, 4h; ii) automated synthesis with tetrazole; iii) 50 eq of sodium iodide, 50 eq of sodium azide, DMF, 70 °C, 80 min; iv) 50 eq of copper sulfate, 50 eq of sodium ascorbate, t-BuOH/H2O, mw, 65 °C (100 W), 35 min. | ||
Different strategies were reported to covalently link ONs with biomolecules including the use of the Staudinger reaction8 or the formation of an oxime bond.9 Alternatively, a “click chemistry reaction”10 was adapted to the synthesis of ON-conjugates.11 For example, the 1,3-dipolar Huisgen's reaction12 allows the covalent linkage of oligonucleotides with a large range of molecular structures such as peptides, sugars13 or probes.14 Accordingly, the click coupling reaction provides an efficient means for the synthesis of new therapeutic bio-conjugated oligonucleotides.15 Amphiphilic structures derived from ONs are an illustration of the successful conjugation of oligonucleotides with biomolecules.1d Notably, these hybrid systems can be designed for either therapeutic applications16 or encoding lipid membrane.17
In the case of LONs previously reported,4 the click coupling reaction was used to synthesize the nucleoside–lipid phosphoramidite building blocks, which were then incorporated during the 3′ to 5′ elongation at the 5′ extremity. Herein, we covalently conjugated C8F17 and C6F13 propargylated chains directly to a 5′-azido 17mer oligonucleotide (Fig. 1b). Interestingly, this post synthetic approach is amenable to a large diversity of propargylated building blocks, such as for example hydrophobic segments featuring functional groups (i.e. hydroxyls, amides, etc.) without using any protecting group.
The synthesis of FONs, via a “post synthetic” approach, required the preparation of a 5′-bromophosphoramidite building block. 5′-bromo-5′-deoxythymidine-3′-phosphoramidite was synthesized in three steps (intermediate 2, Fig. 1, see ESI for details) from thymidine. Thymidine bromination was realized in two steps using first methanesulfonyl chloride in pyridine at room temperature and then potassium bromide in N,N-dimethylformamide (DMF) at 80 °C. Following this procedure, 5′-bromo-5′-deoxythymidine 1 was isolated without purification in 65% yield from thymidine. Next, compound 1 was converted into phosphoramidite in one step by treatment with 2-cyanoethyl-N,N-diisopropylchlorophosphoramidite in dichloromethane (DCM) at room temperature. After purification on silica gel the expected phosphoramidite 2 was isolated in 70% yield.
The 5′-bromo-5′-deoxythymidine-3′-phosphoramidite 2 was further coupled to a 17mer oligonucleotide using a solid support synthesis via a 3′ to 5′ elongation approach (Fig. 1). The resulting 5′-bromo-ON 3 was not isolated and was directly reacted with sodium iodide and sodium azide at 70 °C for 80 min in DMF.18 This reaction provided azido derivative 4 in 50% yield. Yield was monitored after treatment of a small part of the CPG-ON beads by ammonia providing the ONs, which were analyzed by HPLC and MALDI-TOF MS.
The expected FONs 5a and 5b derived from 2H,2H,3H,3H-perfluorononanamide (C6F13 chain) and 2H,2H,3H,3H-perfluoroundecanamide respectively (C8F17 chain), were obtained via a 1,3-dipolar cycloaddition involving the azido 4 ON intermediate and the propargylated fluorocarbon chains 3a or 3b. Theses reactions were performed on the protected ON 4 at 65 °C in the presence of copper sulfate and sodium ascorbate. After 35 min of reaction, the supported oligonucleotides were washed with an EDTA saturated aqueous solution to remove copper salts. Deprotection of cyanoethyl protected ONs by a NH4OH solution for 6 h at 55 °C afforded crude FONs, which were the purified on a C4 reverse phase. As previously described for lipidic conjugates,8 the presence of a hydrophobic part induced a dramatic impact on the retention time of FONs. The post synthetic approach provided FONs 5a and 5b in 10 and 15%, respectively of total yields, including the 18 steps of ON synthesis, azidation, microwave assisted click-reaction and purification. Notably, the post-synthetic conjugation of fluorocarbon moieties to ON, which simplifies the access to ON conjugates, is amenable to a large diversity of hydrophobic moieties.
The ON sequence selected was a 17mer 2′-O-methylribonucleotide antisense complementary to the IIId sub-domain of the Hepatitis C Virus RNA IRES.19
An important objective of this study is to use the fluorinated conjugates for the delivery of oligonucleotide sequences. Non-toxic fluorinated chains that can act as a hydrophobic carrier for the delivery of ONs would be of interest for both in vitro and in vivo applications. To validate our approach we used fluorescence microscopy (see Fig. ESI 7 and 8) and flow cytometry to determine whether the FON conjugates penetrated cultured eukaryotic cells without using any transfecting reagents. Three different human cell lines, including hepatic Huh7, gastric epithelial NCI-N87 and embryonic kidney HEK293 cells were incubated in the presence of serum with 0.5 μM of either both 3′-fluorescein-labelled C8F17 and C6F13 FONs, or unconjugated ONs for 24 h at 37 °C (Fig. 2a). Examination of the images collected by confocal microscopy experiments confirms that FON17F are internalized in the cytoplasm of all the human cells investigated (Fig. 2b and Figure ESI 7). Similarly to hydrocarbon LONC18 (analogue featuring a C18 chain), FON17F are internalized in roughly 100% of cells for the three cell lines investigated, whereas unconjugated ONs do not penetrate into the cells in these conditions. With 40% fluorescent cells for Huh7 and NCI-N87, FON13F is less internalized, indicating that the hydrophobic character is an important parameter for the cellular internalization. Note that the unconjugated labelled4 ON17mer3′F* did not penetrate cells after 24 h of incubation in the same conditions, indicating that efficient uptake requires either lipid or fluorinated chain conjugation of the ONs (Fig. 2a).
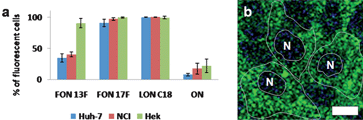 | ||
| Fig. 2 Cellular internalization of FONs. a) Percentage of fluorescent cells (Huh7 blue, NCI red and Hek green) incubated in the presence of 0.5 μM of FON13F, FON17F, LONC18 or unconjugated ON 17mer. Incubation for 24 h at 37 °C. b) Internalization of FON17F 3′ fluorescein (500 nM) after 24 h of incubation (Huh-7 cells, scale = 10 μm). | ||
In order to evaluate the internalization kinetics of FONs, Huh7 cells were incubated for either 4 or 24 h in the presence of 0.5 μM of FONs, LONC18 or unconjugated ONs. As shown in Fig. 3a LONs are internalized more rapidly than both FONs. Almost 100% of cells are fluorescent after 4 h of incubation, whereas only 20% and 40% cells were fluorescent with FON13F and FON17F, respectively. Interestingly, the cellular uptake of both FON13F and FON17F was dramatically decreased at 4 °C (Fig. 3b). These results suggest that similarly to the lipid conjugates (LONs), FONs enter the cells via an energy dependent mechanism, rather than by passive diffusion. The cytotoxicity of FON17F was also evaluated by assessing cell viability after incubation for 5 days of growing Huh7 cells with increasing concentrations of FON17F. Results shown in Fig. ESI9 demonstrate that FONs were not toxic up to 2 μM.
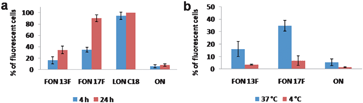 | ||
| Fig. 3 a) Percentage of fluorescent cells (Huh7) incubated in the presence of 0.5 μM of FON13F, FON17F, LONC18 and unconjugated ON 17mer. Incubation for 4 or 24 h at 37 °C. b) Percentage of fluorescent cells (Huh7) incubated in the presence of 0.5 μM of FON13F, FON17F and unconjugated ON 17mer. Incubation for 4 h at either 4 or 37 °C. | ||
In conclusion, this work presents for the first time a new family of fluorocarbon oligonucleotide conjugates (FONs) featuring either C8F17 or C6F13 hydrophobic and lipophobic chains. To synthesize the fluorocarbon–oligonucleotide conjugates, a straightforward automated synthetic pathway involving a “post synthetic” grafting of the fluorocarbon chains was developed. The conjugation of a fluorocarbon moiety allows the cellular uptake of the ONs as measured by fluorescence microscopy and flow cytometry. Similarly to LONs, toxicity of FONs was negligible and cellular internalization of FONs was efficient and not affected by the presence of serum. The results collected in this study indicate that fluorocarbon conjugation may be used as a new tool for the delivery of oligonucleotides for intracellular targets, such as antisense sequences, aptamers or siRNA.
The authors acknowledge financial support from the Army Research Office (P.B.), the Region Aquitaine (G.G.) and the French National Agency (ANR) in the frame of its program in Nanosciences and Nanotechnologies (NANAN project no. ANR-08-NANO-028).
Notes and references
- (a) K. Mukherjee, J. Bhattacharyya, J. Sen, R. Sistla and A. Chaudhuri, J. Med. Chem., 2008, 51, 1967 CrossRef CAS; (b) S. D. Li and L. Huang, Gene Ther., 2006, 13, 1313–1319 CrossRef CAS; (c) A. W. Fraley, B. Pons, D. Dalkara, G. Nullans, J.-P. Behr and G. Zuber, J. Am. Chem. Soc., 2006, 128, 10763–10771 CrossRef CAS; (d) A. Gissot, M. Camplo, M. W. Grinstaff and P. Barthélémy, Org. Biomol. Chem., 2008, 6, 1324–1333 RSC.
- M. Manoharan, K. L. Tivel, T. P. Condon, L. K. Andrade, I. Barber-Peoch, G. Inamati, S. Shah, V. Mohan, M. J. Graham, F. C. Bennett, S. T. Crooke and P. D. Cook, Nucleosides, Nucleotides Nucleic Acids, 1997, 16, 1129–1138 CAS.
- (a) R. L. Letsinger, G. Zhang, D. K. Sun, T. Ikeuchi and P. S. Sarin, Proc. Natl. Acad. Sci. U. S. A., 1989, 86, 6553–6556 CAS.
- G. Godeau, C. Staedel and P. Barthélémy, J. Med. Chem., 2008, 51, 4374–4376 CrossRef CAS.
- (a) D. Matyszewska, K. Tappura, G. Orädd and R. Bilewicz, J. Phys. Chem. B, 2007, 111, 9908–9918 CrossRef CAS; (b) F. Guillod, J. Greiner and J. G. Riess, Biochim. Biophys. Acta, 1996, 1282, 283–292; (c) P. Barthélémy, V. Tomao, J. Selb, Y. Chaudier and B. Pucci, Langmuir, 2002, 18, 2557–2563 CrossRef CAS.
- (a) S. Areva, C. Boissière, D. Grosso, T. Asakawa, C. Sanchez and M. Lindén, Chem. Commun., 2004, 1630–1631 RSC; (b) G. Mathew, S. L. Snyder, P. Terech, C. J. Glinka and R. G. Weiss, J. Am. Chem. Soc., 2003, 125, 10275–10283 CrossRef CAS; (c) P. Barthélémy, Y. Chaudier, V. Tomao and B. Pucci, Fluorocarbon Amphiphiles for Supramolecular Assemblies, in Self Assembly, Robinson, B. H., Ed. IOS Press, Amsterdam, 2003; pp 80–91 Search PubMed.
- (a) M. P. Krafft and J. G. Riess, Chem. Rev., 2009, 109, 1714–1792 CrossRef CAS; (b) C. Richard, P. Chaumet-Riffaud, A. Belland, A. Parat, N. Contino-Pepin, M. Bessodes, D. Scherman, B. Pucci and N. Mignet, Int. J. Pharm., 2009, 379, 301–308 CrossRef CAS; (c) C. Boulanger, C. Di Giorgio, J. Gaucheron and P. Vierling, Bioconjugate Chem., 2004, 15, 901–908 CrossRef CAS.
- H. Chapuis, L. Bui, I. Bestel and P. Barthélémy, Tetrahedron Lett., 2008, 49, 6838–6840 CrossRef CAS.
- E. Defrancq, A. Hoang, F. Vinet and P. Dumy, Bioorg. Med. Chem. Lett., 2003, 13, 2683–2686 CrossRef CAS.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS.
- P. M. E. Gramlich, C. T. Wirges, A. Manetto and T. Carell, Angew. Chem., Int. Ed., 2008, 47, 8350–8358 CrossRef CAS.
- (a) R. Huisgen, 1,3-Dipolar Cycloaddition Chemistry; Wiley, New York, 1984; pp 1–176 Search PubMed; (b) C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef CAS.
- C. Bouillion, A. Meyer, S. Vidal, A. Jochum, Y. Chevolot, J.–P. Cloarec, J.–P. Praly, J.-J. Vasseur and F. Morvan, J. Org. Chem., 2006, 71, 4700–4702 CrossRef CAS.
- (a) P. M. E. Gramlich, S. Warncke, J. Gierlich and T. Carell, Angew. Chem., Int. Ed., 2008, 47, 3442–3444 CrossRef CAS; (b) T. S. Seo, Z. Li, H. Ruparel and J. Ju, J. Org. Chem., 2003, 68, 609–612 CrossRef; (c) F. Seela, V. R. Sirivolu and P. Chittepu, Bioconjugate Chem., 2008, 19, 211–224 CrossRef CAS.
- J.–F. Lutz and Z. Zarafshani, Adv. Drug Delivery Rev., 2008, 60, 958–970 CrossRef CAS.
- R. L. Letsinger, G. Zhang, D. K. Sun, T. Ikeuchi and P. S. Sarin, Proc. Natl. Acad. Sci. U. S. A., 1989, 86, 6553–6556 CAS.
- A. Gissot, C. Di Primo, I. Bestel, G. Giannone, H. Chapuis and P. Barthélémy, Chem. Commun., 2008, 5550–5552 RSC.
- (a) J. Lietard, A. Meyer, J.-J. Vasseur and F. Morvan, Tetrahedron Lett., 2007, 48, 8795–8798 CrossRef CAS; (b) G. P. Miller and E. T. Kool, J. Org. Chem., 2004, 69, 2404–2410 CrossRef CAS.
- B. Tallet-Lopez, L. Aldaz-Carroll, S. Chabas, E. Dausse, C. Staedel and J.-J. Toulmé, Nucleic Acids Res., 2003, 31, 734–742 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR and MS data, CAC curves, additional images of fluorescence and electronic microscopy. See DOI: 10.1039/c0md00054j |
| This journal is © The Royal Society of Chemistry 2010 |
