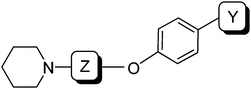
Histamine H3 receptor ligands with a 3-cyclobutoxy motif: a novel and versatile constraint of the classical 3-propoxy linker†
Maikel
Wijtmans
*a,
Frédéric
Denonne
b,
Sylvain
Célanire‡
b,
Michel
Gillard
b,
Saskia
Hulscher
a,
Christel
Delaunoy
b,
Nathalie
Van houtvin
b,
Remko A.
Bakker§
a,
Sabine
Defays
b,
Julien
Gérard
b,
Luc
Grooters
b,
Delphine
Hubert
b,
Henk
Timmerman
a,
Rob
Leurs
a,
Patrice
Talaga
b,
Iwan J. P.
de Esch
a and
Laurent
Provins
*b
aLeiden/Amsterdam Center for Drug Research, Division of Medicinal Chemistry, Faculty of Exact Sciences, VU University Amsterdam, De Boelelaan 1083, 1081 HV Amsterdam, The Netherlands. E-mail: wijtmans@few.vu.nl
bUCB Pharma, UCB NewMedicines, Chemin du Foriest, B-1420 Braine-l'Alleud, Belgium. E-mail: Laurent.Provins@ucb.com
First published on 18th June 2010
Abstract
Antagonists/inverse agonists for the histamine H3 receptor (H3R) are subject to intensive research. Many chemical classes contain a 3-propoxy linker to connect an aromatic moiety and a basic amine. Rigidifying this linker by several moieties has proven successful. However, so far, a 3-cyclobutoxy constraint has not been disclosed in H3R research. Here, we present novel synthetic methodology toward compounds with this functionality. A condensation between piperidine and 1,3-cyclobutanedione followed by a reduction furnishes a versatile cis-3-piperidino-cyclobutanol building block which allows ready access to constrained compounds having a 3-piperidino-cyclobutoxy moiety. Pharmacological studies reveal that this particular rigidification leads to a significant increase in H3R affinity compared to the non-constrained counterpart. In all, the constrained 3-cyclobutoxy linker emerges as a novel, versatile and attractive motif for H3R ligands.
Introduction
The signaling molecule histamine is involved in a plethora of physiological functions. These roles are mediated by four different G protein-coupled histamine receptors, termed H1R–H4R.1 The histamine H3 receptor (H3R) is of high interest because a key role for H3R has been postulated in a remarkably wide variety of disease areas such as cognitive deficits, obesity, narcolepsy and neuralgia.2 Blockage of H3R has been embraced as a potential way to therapeutically combat these diseases and, as a result, the development of H3R antagonists and inverse agonists has been widely pursued during the last decade.2,3An inspection of published and patented antagonists/inverse agonists shows the widespread occurrence of a 3-propoxy linker as part of the general pharmacophore, connecting an aromatic moiety and a basic amine (Fig. 1 and 2A).3,4 In fact, it has been demonstrated that incorporation of a 3-piperidino-propoxy-based unit in known serotonin transporter inhibitors and dopaminergic agents was sufficient to induce considerable H3R affinity.5,6 Structurally varying examples of H3R ligands with the classical 3-propoxy linker (i.e. bearing a 3-aminopropoxy moiety, Fig. 1) include 1,7 JNJ-5207852 (2),8 biphenyls such as A-423579 (3),9 and naphthalenes (4).10 The list also includes the oxazoline and oxazole classes, exemplified by 5 and 6, respectively.11,12 Several research groups have rigidified the 3-propoxy linker using e.g. a piperidine (7, 8),13,14 furan (9),15 pyridine (10)16 or morpholino constraint (11).17
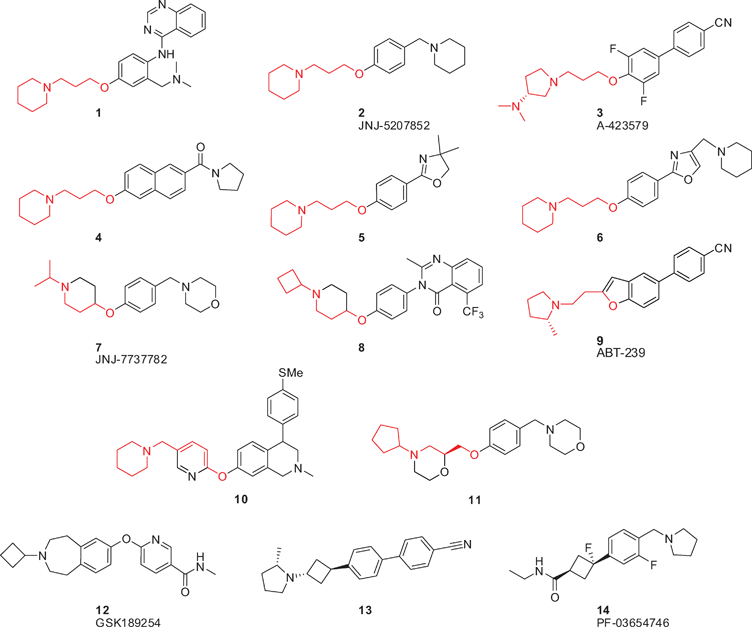 | ||
| Fig. 1 Selected H3R antagonists/inverse agonists. The 3-aminopropoxy unit, be it unconstrained or derivatized to a constrained analogue, is depicted in red. | ||
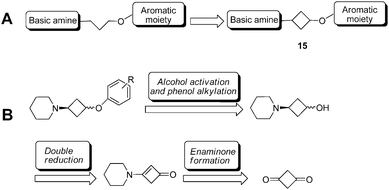 | ||
| Fig. 2 (A) A general pharmacophore for H3R antagonists/inverse agonists showing the classical 3-propoxy linker and its rigidification by means of a cyclobutyl constraint. (B) Retrosynthetic approach. | ||
We were interested in the possibility of rigidifying the 3-propoxy linker by means of a cyclobutyl constraint (Fig. 2A). To us, the resulting 3-cyclobutoxy linker presented several distinct advantages: (1) it is a highly compact cyclo-constraint, yet fully incorporates the three-carbon linker; (2) in contrast to e.g. the piperidine and morpholino constraints (i.e.7, 8, 11), a cyclobutyl group leaves the N-terminus free for attachment of secondary amines, some of which are known to be privileged in H3R antagonists;18 (3) directional fine-tuning can be achieved by the relative stereochemistry of the cyclobutyl substituents. Although the cyclobutyl moiety has been used for some H3R ligands either as a side-chain (e.g.8 and clinical candidate 12, GSK189254)19 or as part of a non-classical linker (e.g.13 and Phase II candidate 14, PF-03654746),20,21 the incorporation of a 3-cyclobutoxy constraint into the classical and widely applied 3-aminopropoxy pharmacophoric element (i.e. to 15) has hitherto not been disclosed. In this article, we report on efficient synthetic methodologies and pharmacological studies related to H3R ligands incorporating this 3-amino-cyclobutoxy unit.
Results and discussion
Chemistry
We selected our recently published oxazoline- and oxazole-scaffolds, exemplified by piperidine-containing ligands 5 and 6,11,12 as the central scaffolds for investigations into 3-cyclobutoxy linkers. Aiming for a late-stage alkylation of the phenolic part by the 3-piperidino-cyclobutyl unit, our initial goal was to achieve the synthesis of cis- and trans-3-piperidino-cyclobutanol. Reports have been disclosed on cis- or trans-3-amino-cyclobutanol22 as well as on cis- or trans-3-dimethylamino-cyclobutanol,23 but both approaches utilize a synthetic strategy in which the cyclobutyl ring is constructed from acyclic precursors and both syntheses amount to at least 6 steps. We decided upon an alternative shorter approach starting from pre-existing cyclobutyl-containing reagents with two functional groups on the 1- and 3-position to allow for attachment of the amine and alcohol units.Interestingly, a search revealed that 1,3-cyclobutanedione existed as its dicyclohexylammonium salt (16).24 Mono-amination of free 1,3-cyclobutanedione to a cyclobutylenaminone has been described by Wasserman et al.25,26 and we postulated this to be an excellent way to selectively install our single amine moiety. A second step involving the reduction27 of the enaminone to the aminoalcohol, a reaction not described in the literature so far for a cyclobutylenaminone, was envisaged (Fig. 2B).
Treatment of salt 16 with 1.1 equiv. of CF3COOH in dioxane (to liberate the parent dione) and subsequent stirring with 1.3 equiv. of piperidine at room temperature for 18 h furnished enaminone 17 in 66% yield (Fig. 3). Compound 17 proved remarkably resistant toward a variety of reductive conditions, including hydrogenation with platinum or palladium catalysts (room temperature or 50 °C) or NaBH4 in acetic acid or methanol (at room temperature). Treatment with LiAlH4 provided some degree of success, but this protocol was plagued by low yields. Gratifyingly, though, we found that 17 can be smoothly reduced by heating it with NaBH4 in EtOH for 12 h (Fig. 3), affording 3-(piperidin-1-yl)cyclobutanol 18 in high yield (78% isolated). The cis-architecture of 18 was established through 2D-NMR (see ESI†). The relative stereochemistry of 18 could be readily inverted by a classical Mitsunobu inversion (Fig. 3). That is, treatment of 18 with diisopropylazodicarboxylate, triphenylphosphine and benzoic acid followed by hydrolysis of intermediate 19 cleanly delivered trans-isomer 20 in 60% yield from 18.
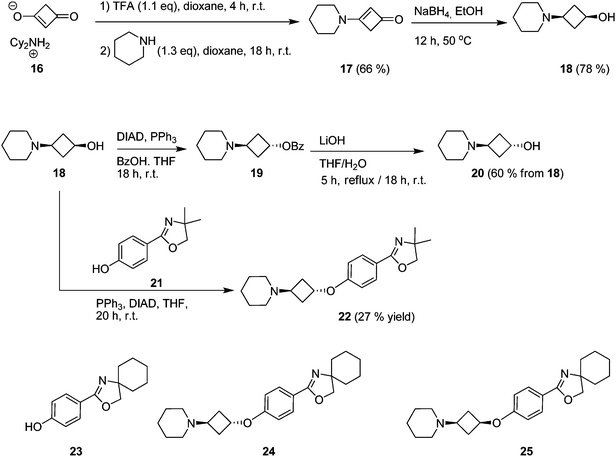 | ||
| Fig. 3 Synthetic routes to cis- and trans-3-piperidino-cyclobutanols 18 and 20 and to compounds 22, 24 and 25. | ||
Having the required 3-piperidino-cyclobutanols 18 and 20 at hand, the stage was set for coupling to the aromatic core of the oxazoline or oxazole part. As outlined earlier, alkylation of the corresponding phenol was the strategy of choice, requiring an activation of the alcohol group of the 3-piperidino-cyclobutanols. Toward this end, two strategies were pursued, both of which are accompanied by inversion at the O-bound carbon. The first approach used Mitsunobu conditions, with which we were able to successfully couple 18 and phenol 2111 to afford the trans-constrained oxazoline ligand 22 in 27% yield (Fig. 3). Analogously, we performed Mitsunobu couplings on spiro-oxazolino-phenol 2311 with both cis- and trans-3-piperidino-cyclobutanol 18 and 20 to give trans- and cis-compounds 24 and 25 in 23% and 18% isolated yield, respectively (Fig. 3).
The second approach toward activation of the alcohol moiety comprised conversion of 18 to versatile building block cis-tosylate 26 by reaction with TsCl in 55% yield. The 3-piperidino-cyclobutyl group was then introduced by displacement of the tosylate by the desired phenolate. Two illustrations of this approach are shown in Fig. 4. Starting phenol 28 was obtained by a condensation between 4-hydroxybenzamide and 1,3-dichloropropan-2-one (to give 27) followed by substitution with piperidine. The phenolate of 28 was then reacted with tosylate 26 to give trans-compound 29. A similar reaction type but with different reaction conditions was applied to phenol 30, affording ester-substituted trans-oxazole 31.
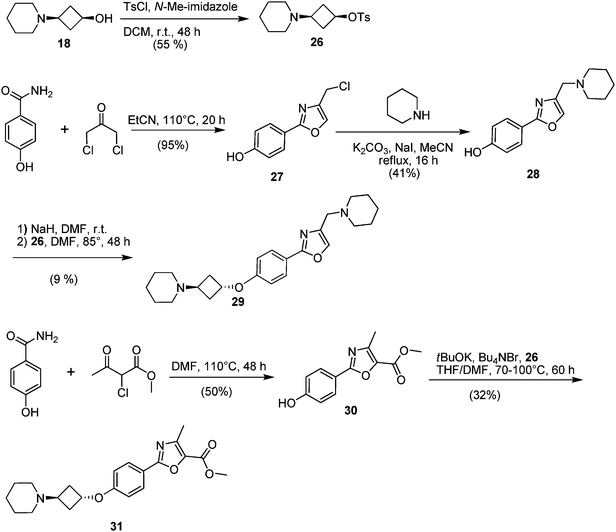 | ||
| Fig. 4 Synthetic routes to compounds 26, 29 and 31. | ||
Pharmacology
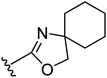
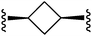
A proof-of-concept comparison between the unconstrained 3-propoxy linker and the linker with either a cis- or trans-3-cyclobutoxy constraint was undertaken on our oxazoline class of H3R ligands (Table 1).11 Comparing the H3R affinities of unconstrained 5 and trans-constrained 22 reveals the beneficial effect of the trans-cyclobutyl lock, since more than a log unit in H3R affinity is gained (22: pKi = 8.1). Likewise, trans-constrained spirocompound 24 displays a 40-fold higher affinity (pKi = 8.7) than the unconstrained derivative 32.11 Interestingly, the cis-constrained analogue 25 has a pKi value of 6.6 and is therefore much less effective than the trans-constrained counterpart 24.28 This observation is, however, limited to that particular example and was not generalized to other cis derivatives, therefore impairing a conclusion on the importance of the stereochemistry of the cyclobutyl constraint. The overall nature of the beneficial effect of the trans-junction was nevertheless further supported by two additional classes of H3 ligands (Table 1). Both are oxazoles,12 but one compound is decorated with a second basic site while the other is not. The compounds with a trans-constrained linker (29, 31) display an increase in affinity by 0.7 and 1.8 log units, respectively, compared to analogues with the unconstrained linker (e.g.6).12 All tested compounds display inverse agonism (Table 1), with trans-compound 22 having a high pEC50inv value of 9.2 as measured by the inhibition of [35S]GTPγS binding to H3R.| Compound |
|
|
pKi ± SD a | pEC50inv ± SD b |
|---|---|---|---|---|
| a Binding affinities (pKi) were assessed by displacement of [3H]-Nα-methylhistamine on membranes from CHO cells stably expressing the human H3R. The results are presented as the mean of two to six experiments. b [35S]GTPγS binding assay was performed on membranes from CHO cells stably expressing the human H3R. The results are presented as the mean of three to six experiments. c Already reported by us.11 d Already reported by us.12 SD = standard deviation. | ||||
| Thioperamide | — | — | 6.8 ± 0.1 | 7.0 ± 0.1 |
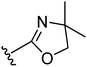
| 5 |
|

|
6.8 ± 0.1 | 7.8 ± 0.1 |
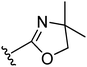
| 22 |
|

|
8.1 ± 0.3 | 9.2 ± 0.1 |
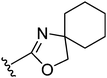
| 32 |
|

|
7.1 ± 0.1 | 8.1 ± 0.1 |
| 24 |
|

|
8.7 ± 0.1 | 8.7 ± 0.1 |
| 25 |
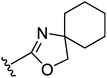
|
|
6.6 ± 0.1 | 7.1 ± 0.1 |
| 6 |
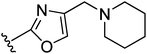
|

|
8.2 ± 0.3 | 8.8 ± 0.2 |
| 29 |
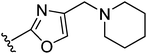
|

|
8.9 ± 0.1 | 8.5 ± 0.1 |
| 31 |
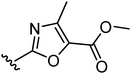
|

|
8.7 ± 0.1 | 8.8 ± 0.1 |
Conclusion
A convenient and short synthesis of cis- and trans-3-piperidino-cyclobutanol is described (two and four steps, respectively). The chemistry consists of a condensation between piperidine and 1,3-cyclobutadione followed by a reduction using NaBH4. The relative stereochemistry of the resulting cis-3-piperidino-cyclobutanol can be inverted using a Mitsunobu inversion. The alcohol building blocks were incorporated into oxazoline- and oxazole-containing H3R ligands as a means to constrain the classical 3-propoxy linker. The presented chemistry routes toward this goal display high versatility and stereochemical fidelity. Compared to their counterparts bearing the 3-propoxy linker, our new trans-3-cyclobutoxy-constrained derivatives consistently afforded a significant increase in H3R affinity. Our results underscore the dramatic effects that constraints can exert on receptor affinity. Further research on the 3-cyclobutoxy linker is warranted and may open new venues in the field of H3R ligands.Acknowledgements
We thank Paul van Waes (Lonza Benelux, Breda, The Netherlands) for a kind initial gift of 1,3-cyclobutanedione dicyclohexylammonium salt. We thank Frans de Kanter (VU University Amsterdam) for assistance with 2D-NMR measurements, Jehan Claessens, Alain Fauconnier and Christelle Derwa (UCB Pharma) for HRMS measurements and purity assessment, and Benoît Mathieu (UCB Pharma) for NMR measurements.References
- L. B. Hough, Mol. Pharmacol., 2001, 59, 415–419 CAS.
- M. Wijtmans, R. Leurs and I. J. P. De Esch, Expert Opin. Invest. Drugs, 2007, 16, 967–985 Search PubMed.
- K. Sander, T. Kottke and H. Stark, Biol. Pharm. Bull., 2008, 31, 2163–2181 CrossRef CAS.
- S. Celanire, M. Wijtmans, P. Talaga, R. Leurs and I. J. P. de Esch, Drug Discovery Today, 2005, 10, 1613–1627 CrossRef CAS.
- Y. von Coburg, T. Kottke, L. Weizel, X. Ligneau and H. Stark, Bioorg. Med. Chem. Lett., 2009, 19, 538–542 CrossRef CAS.
- J. M. Keith, L. A. Gomez, M. A. Letavic, K. S. Ly, J. A. Jablonowski, M. Seierstad, A. J. Barbier, S. J. Wilson, J. D. Boggs, I. C. Fraser, C. Mazur, T. W. Lovenberg and N. I. Carruthers, Bioorg. Med. Chem. Lett., 2007, 17, 702–706 CrossRef CAS.
- O. Roche and R. M. Rodriguez Sarmiento, Bioorg. Med. Chem. Lett., 2007, 17, 3670–3675 CrossRef CAS.
- R. Apodaca, C. A. Dvorak, W. Xiao, A. J. Barbier, J. D. Boggs, S. J. Wilson, T. W. Lovenberg and N. I. Carruthers, J. Med. Chem., 2003, 46, 3938–3944 CrossRef CAS.
- A. A. Hancock, M. S. Diehl, R. Faghih, E. N. Bush, K. M. Krueger, G. Krishna, T. R. Miller, D. M. Wilcox, P. Nguyen, J. K. Pratt, M. D. Cowart, T. A. Esbenshade and P. B. Jacobson, Bas. Clin. Pharmacol. Toxicol., 2004, 95, 144–152 CAS.
- O. Roche, M. Nettekoven, W. Vifian and R. M. Sarmiento, Bioorg. Med. Chem. Lett., 2008, 18, 4377–4379 CrossRef CAS.
- S. Célanire, M. Wijtmans, B. Christophe, P. Collart, I. de Esch, D. Dassesse, C. Delaunoy, F. Denonne, V. Durieu, E. Gelens, M. Gillard, B. Lallemand, Y. Lamberty, F. Lebon, J. M. Nicolas, L. Quere, E. Snip, A. Vanbellinghen, N. Van houtvin, V. Verbois, H. Timmerman, P. Talaga, R. Leurs and L. Provins, ChemMedChem, 2009, 4, 1063–1068 CrossRef CAS.
- F. Denonne, F. Atienzar, S. Célanire, B. Christophe, F. Delannois, C. Delaunoy, M.-L. Delporte, V. Durieu, M. Gillard, B. Lallemand, Y. Lamberty, G. Lorent, A. Vanbellinghen, N. Van houtvin, V. Verbois and L. Provins, ChemMedChem, 2010, 5, 206–212 CrossRef CAS.
- C. A. Dvorak, R. Apodaca, A. J. Barbier, C. W. Berridge, S. J. Wilson, J. D. Boggs, W. Xiao, T. W. Lovenberg and N. I. Carruthers, J. Med. Chem., 2005, 48, 2229–2238 CrossRef CAS.
- T. Nagase, T. Mizutani, E. Sekino, S. Ishikawa, S. Ito, Y. Mitobe, Y. Miyamoto, R. Yoshimoto, T. Tanaka, A. Ishihara, N. Takenaga, S. Tokita and N. Sato, J. Med. Chem., 2008, 51, 6889–6901 CrossRef CAS.
- M. Cowart, J. K. Pratt, A. O. Stewart, Y. L. Bennani, T. A. Esbenshade and A. A. Hancock, Bioorg. Med. Chem. Lett., 2004, 14, 689–693 CrossRef CAS.
- J. M. Keith, A. J. Barbier, S. J. Wilson, K. Miller, J. D. Boggs, I. C. Fraser, C. Mazur, T. W. Lovenberg and N. I. Carruthers, Bioorg. Med. Chem. Lett., 2007, 17, 5325–5329 CrossRef CAS.
- M. A. Letavic, J. M. Keith, K. S. Ly, P. Bonaventure, M. A. Feinstein, B. Lord, K. L. Miller, S. T. Motley, D. Nepomuceno, S. W. Sutton and N. I. Carruthers, Bioorg. Med. Chem. Lett., 2008, 18, 5796–5799 CrossRef CAS.
- D. L. Nersesian, L. A. Black, T. R. Miller, T. A. Vortherms, T. A. Esbenshade, A. A. Hancock and M. D. Cowart, Bioorg. Med. Chem. Lett., 2008, 18, 355–359 CrossRef CAS.
- A. D. Medhurst, A. R. Atkins, I. J. Beresford, K. Brackenborough, M. A. Briggs, A. R. Calver, J. Cilia, J. E. Cluderay, B. Crook, J. B. Davis, R. K. Davis, R. P. Davis, L. A. Dawson, A. G. Foley, J. Gartlon, M. I. Gonzalez, T. Heslop, W. D. Hirst, C. Jennings, D. N. Jones, L. P. Lacroix, A. Martyn, S. Ociepka, A. Ray, C. M. Regan, J. C. Roberts, J. Schogger, E. Southam, T. O. Stean, B. K. Trail, N. Upton, G. Wadsworth, J. A. Wald, T. White, J. Witherington, M. L. Woolley, A. Worby and D. M. Wilson, J. Pharmacol. Exp. Ther., 2007, 321, 1032–1045 CrossRef CAS.
- L. A. Black, H. Liu, G. J. Diaz, G. B. Fox, K. E. Browman, J. Wetter, K. C. Marsh, T. R. Miller, T. A. Esbenshade, J. Brioni and M. D. Cowart, Inflammation Res., 2008, 57(s1), 45–46 CrossRef.
- T. T. Wager, presented at the 237th ACS National Meeting, Salt Lake City, March, 2009 Search PubMed.
- C. J. Helal, Z. Kang, J. C. Lucas and B. R. Bohall, Org. Lett., 2004, 6, 1853–1856 CrossRef CAS.
- C. Beard and A. Burger, Chem. Ber., 1962, 95, 2535–2540 CrossRef CAS.
- B. Jackson and T. Scholl (Lonza Ltd.), US Pat., 5087756, 1992 Search PubMed.
- H. H. Wasserman, J. U. Piper and E. V. Dehmlov, J. Org. Chem., 1973, 38, 1451–1455 CrossRef CAS.
- H. Bibas, M. W. Wong and C. Wentrup, Chem.–Eur. J., 1997, 3, 237–248 CrossRef CAS.
- P. Herczegh, I. Kovács, L. Szilágyi, M. Zsély and F. Sztaricskai, Tetrahedron Lett., 1992, 33, 3133–3136 CrossRef CAS.
- The Supplementary Material contains some concise computational studies addressing the effect of the cyclobutyl lock†.
Footnotes |
| † Electronic supplementary information (ESI) available: Gradient programs, compound purities, pharmacological procedures, computational studies, synthetic procedures and NMR spectra. See DOI: 10.1039/c0md00056f |
| ‡ Present address: Addex Pharma, Geneva, Switzerland. |
| § Present address: Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach an der Riß, Germany. |
| This journal is © The Royal Society of Chemistry 2010 |