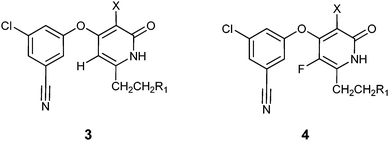
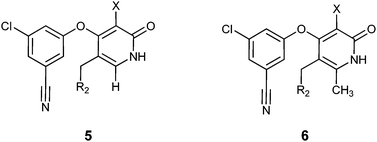
Synthesis and biological activity of new pyridone diaryl ether non-nucleoside inhibitors of HIV-1 reverse transcriptase†
Joshua J.
Kennedy-Smith
*a,
Nidhi
Arora
a,
J. Roland
Billedeau
a,
Jennifer
Fretland
e,
Julie Q.
Hang
d,
Gabrielle M.
Heilek
c,
Seth F.
Harris
b,
Donald
Hirschfeld
a,
Hassan
Javanbakht
c,
Yu
Li
d,
Weiling
Liang
a,
Ralf
Roetz
a,
Mark
Smith
a,
Guoping
Su
c,
Judy M.
Suh
a,
Armando G.
Villaseñor
b,
Jeffrey
Wu
a,
Dennis
Yasuda
a,
Klaus
Klumpp
d and
Zachary K.
Sweeney
*a
aDepartment of Medicinal Chemistry, Roche Palo Alto, 3431 Hillview Avenue, Palo Alto, CA 94304. E-mail: joshua.kennedy_smith@roche.com; sweeney.zachary@gene.com
bDepartment of Discovery Technologies, Roche Palo Alto, 3431 Hillview Avenue, Palo Alto, CA 94304
cDepartment of Viral Disease Biology, Roche Palo Alto, 3431 Hillview Avenue, Palo Alto, CA 94304
dDepartment of Viral Disease Biochemistry, Roche Palo Alto, 3431 Hillview Avenue, Palo Alto, CA 94304
eDepartment of Non-Clinical Safety, Roche Palo Alto, 3431 Hillview Avenue, Palo Alto, CA 94304
First published on 28th April 2010
Abstract
New pyridone non-nucleoside reverse transcriptase inhibitors (NNRTIs) were prepared and several flexible routes to this class of inhibitor were identified. These NNRTIs were active inhibitors of the replication of wild-type and NNRTI-resistant HIV. Structure-based drug design was used to optimize the activity of the compounds against NNRTI-resistant mutants. The co-crystal structure of inhibitor 2b in the NNRTI binding pocket of HIV reverse transcriptase (HIVRT) is also described.
Non-nucleoside reverse transcriptase inhibitors (NNRTIs) are important components of the preferred combination antiretroviral therapy for the treatment of HIV infection.1,2 The most commonly used NNRTIs, efavirenz and nevirapine, were approved for use more than 10 years ago,3 and the efficacy of several other drugs in this class are being studied in clinical trials.4 Current efforts are focused on the identification of NNRTIs with convenient dosing regimens, increased genetic barriers to resistance development, and improved safety profiles.5
We have recently described the discovery of a series of diaryl ether NNRTIs.6 These compounds are potent inhibitors of the wild-type virus and commonly observed NNRTI-resistant mutant viruses in vitro, and have promising pharmacokinetic profiles in several animal species. A fluorinated central aromatic ring is a common feature of these inhibitors (Figure 1, A), as these compounds were generally more potent inhibitors of polymerase activity than their non-fluorinated analogs. Structural analysis revealed that the fluorine atom engaged in weak hydrophobic interactions. We also speculated that the fluorine might affect the strength of a C–H hydrogen bond between the hydrogen atom meta to the fluorine (bold in Figure 1) and the Lys101 carbonyl oxygen of the viral reverse transcriptase.7 Most NNRTIs feature direct or water-mediated hydrogen bonding interactions with the carbonyl oxygen of Lys101.8
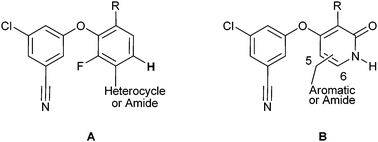 | ||
| Fig. 1 Diaryl ether (A) and pyridone aryl ether (B) NNRTIs. | ||
The diaryl ether NNRTIs A also contain a heterocyclic or amide functionality that engages in hydrogen-bonding interactions with the backbone of Lys103. This unit stabilizes an expanded NNRTI binding pocket and strongly influences the potency of the inhibitors against NNRTI-resistant viruses.6
These considerations led to the design of a series of pyridone diaryl ether inhibitors that feature aliphatic or aromatic groups attached to C(5) or C(6) of the pyridone ring (Figure 1, B). Such pyridone inhibitors would be expected to interact with the Lys101 carbonyl group of HIVRT through a strong traditional hydrogen bonding interaction. Appropriate substitution of the pyridones should allow for stabilization of the expanded NNRTI binding pocket, potential interactions with the backbone amide of Lys103, and optimization of the physical properties of the inhibitors.
Pyridone NNRTIs have been known for over 20 years and the field has been reviewed.9 Furthermore, substituted 4-arylthio- and 4-aryloxypyridones have recently been reported in publications by the Tibotec/CNRS group.10,11 However, the structural diversity of the pyridone NNRTI class is limited by the availability of synthetic precursors for the diversity-oriented preparation of substituted pyridines. As a consequence, most pyridone NNRTIs contain a number of metabolically unstable functional groups (e.g. thioethers, alkylaromatics). In addition, there has been relatively little published data regarding the structure–activity relationships for this class of inhibitors. This report describes novel synthetic routes and the biological activity of diversely substituted pyridone NNRTIs that have excellent antiviral properties.
In order to confirm that the pyridone ring would effectively replace the central phenyl ring of our diaryl ether NNRTIs, inhibitors 1, 2a and 2b were prepared (Fig. 2). Both pyridone compounds 2 were found to strongly inhibit the polymerase activity of wild type HIV reverse transcriptase. Compounds 2 also prevented HIV-induced cell death in vitro.12 Finally, the pyridone inhibitors were determined to have promising activity in assays assessing the ability of compounds to inhibit the polymerase activity of the NNRTI-resistant Lys103Asn and Tyr181Cys mutant enzymes. The comparable efficacy of compounds 1 and 2 encouraged us to further explore the effects of substitution of the pyridone inhibitors. This optimization effort was assisted by a co-crystal structure of compound 2b and wild-type HIVRT, which confirmed the expected binding mode of our pyridone inhibitors (Fig. 3).13,14
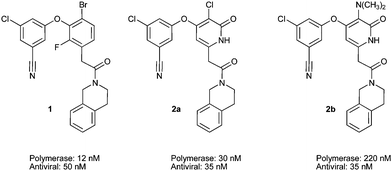 | ||
| Fig. 2 Structures of pyridone and phenyl amide inhibitors 1 and 2 and associated IC50s for inhibition of the wild type polymerase and wild-type HIVRT. | ||
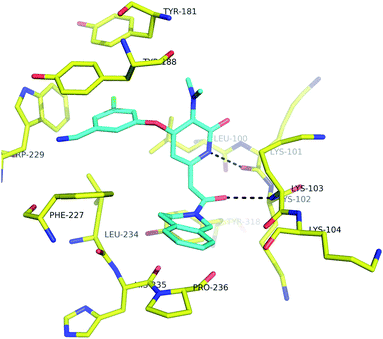 | ||
| Fig. 3 Co-crystal structure of 2b in the NNRTI binding pocket (3FFI). | ||
In an effort to replace the amide linkage of 2 while maintaining the open NNRTI binding pocket, we prepared a series of pyridone NNRTIs that contained aromatic groups attached to the C(6) carbon of the pyridone (Table 1). This effort was enabled by the synthesis of the new, versatile aryl bromide intermediate 8. All of the compounds prepared in this exercise strongly inhibited the polymerase activity of the wild-type and NNRTI-resistant enzymes. Two important conclusions were drawn from this initial work. First, in contrast to our previous observations, fluorination of the central ring did not significantly influence the potency of the inhibitors. Second, while a wide variety of aromatic structures were accommodated in this flexible region of the NNRTI binding pocket, there appeared to be no significant advantage for hydrogen bond donor–acceptor interactions between the pyridone inhibitors and the K103 amide backbone such as those found in initial compounds 2.
| Compd | X | R1 | WT | K103Nb | Y181Cc |
|---|---|---|---|---|---|
| a IC50 in nM. See ref. 10 for assay details. b Experiment using the Lys103Asn mutant enzyme. c Experiment using the Tyr181Cys mutant enzyme. | |||||
| 3a | Cl | Ph | 8 | — | — |
| 3b | Cl | 2-Benzoxazole | 6 | 16 | 4 |
| 3c | Cl | 2-Pyridyl | 21 | 78 | 33 |
| 3d | Cl | 3-Pyridyl | 22 | 16 | 22 |
| 3e | Cl | 4-Pyridyl | 19 | 14 | 21 |
| 3f | Cl | 3-(Cl-phenyl) | 12 | 6 | 7 |
| 4a | Cl | Ph | 7 | — | — |
| 4b | Br | 3-(Cl-phenyl) | 17 | 8 | 8 |
| 4c | Br | 4-Pyridyl | 2 | 1 | 1 |
Analysis of the modeled structure of compound 4c in the expanded NNRTI binding pocket,6,15 suggested that inhibitors with an alkyl substituent at C(5) of the pyridone ring might be able to effectively contact the conserved L234 and F227 residues. Synthesis of functionalized haloaromatics 16 and 18 allowed for the preparation of a series of compounds with aromatic groups attached to C(5) of the pyridone ring. Testing in the biological assays revealed that benzyl-substituted compounds 5a and 6a could function as potent inhibitors of polymerase activity, and that the methyl substituent at C(6) modestly increased potency against NNRTI-resistant mutants (Table 2). Extension of the linkage between the aromatic group and the pyridone ring provided a number of potent NNRTIs, including pyridine-containing compounds that had acceptable thermodynamic aqueous solubility.16 A model of compound 6c in the NNRTI binding pocket is shown in Fig. 4.17,18
| Compd | X | R2 | WT | K103Nb | Y181Cc |
|---|---|---|---|---|---|
| a IC50 in nM. See ref. 10 for assay details. b Experiment using the Lys103Asn mutant enzyme. c Experiment using the Tyr181Cys mutant enzyme. | |||||
| 5a | Br | Ph | 34 | 71 | 41 |
| 5b | Br | CH2O(3-pyridyl) | 11 | 16 | 8 |
| 5c | Br | CH2O(4-pyridyl) | 10 | 14 | 8 |
| 5d | Br | OCH2(4-pyridyl) | 21 | 13 | 11 |
| 6a | Br | Ph | 10 | 7 | 8 |
| 6b | Cl | CH2OPh | 6 | 5 | 6 |
| 6c | Cl | (CH2)2(3-pyridyl) | 4 | 2 | 3 |
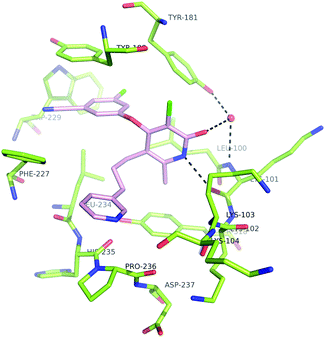 | ||
| Fig. 4 Modeled structure of inhibitor 6c in the NNRTI binding pocket. | ||
Pyridone NNRTIs that strongly inhibited polymerase activity were advanced into cellular assays that measure the ability of the NNRTIs to inhibit HIV-replication in MT4 cells (Table 3). Efavirenz, which was used as a standard in this assay, had significantly reduced potency in experiments conducted either in the presence of additional human serum or with the NNRTI-resistant K103N virus. Compound 4c had activity comparable to efavirenz in assays with the wild-type virus and was a much stronger inhibitor of the K103N mutant. Inhibitor 6c was approximately 3-fold more active than efavirenz in the serum-shifted assay and strongly inhibited both NNRTI-resistant viruses.
| Cmpd | WT | WT+s | K103N | Y181C |
|---|---|---|---|---|
| a IC50 in nM. See ref. 10 for assay details. WT+s refers to experiments run in the presence of 40% human serum. K103N and Y181C refer to experiments using the Lys103Asn mutant virus or the Tyr181Cys mutant virus, respectively. | ||||
| efv | 2 | 18 | 65 | 3 |
| 4c | 5 | 23 | 5 | 11 |
| 5d | 7 | 10 | 13 | 19 |
| 6a | 6 | 32 | 16 | 29 |
| 6b | 4 | 13 | 24 | 11 |
| 6c | 2 | 5 | 2 | 6 |
The synthesis of 2a began with compound 719 (Scheme 1). Reduction of the nitro group, selective bromination, and a Sandmeyer reaction provided the flexible intermediate 8. Installation of the acetic acid functionality in 9 was then accomplished by Negishi coupling of the bromide, followed by selective deprotection of the tert-butyl ester with TFA. Standard EDC coupling and removal of the methyl ether with TMSI provided the target amide 2a.
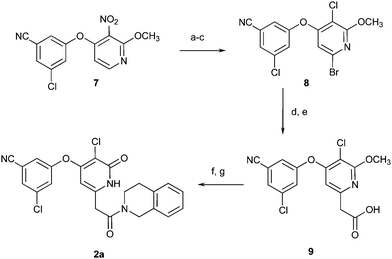 | ||
| Scheme 1 Reagents and conditions: (a) Fe, NH4Cl, H2O, EtOH, 100 °C, 97%. (b) NBS, DMF, 0 °C, 97%. (c) tBuONO, LiCl, CuCl2, MeCN, 60 °C, 64%. (d) 2-tBu-2-oxoethylzinc chloride, Pd(PtBu3)2, dioxane, 25 °C, 75%. (e) TFA, DCM, 25 °C, 99%. (f) Tetrahydroisoquinoline, EDCI, HOBT, DMAP, DIPEA, DMF, 99%. (g) TMSCl, NaI, MeCN, 25 °C, 46%. | ||
Compounds 3 were also prepared using intermediate 8 (Scheme 2). For example, inhibitors 3a and 3g were produced by Negishi coupling of the commercially available phenethylzinc bromide reagents followed by deprotection with TMSI. The heteroaryl compounds 3c–3e could be prepared by Sonogashira coupling, alkyne hydrogenation, and deprotection.
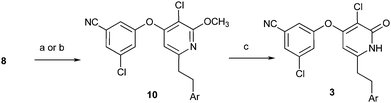 | ||
| Scheme 2 Reagents and conditions: (a) ArCH2CH2ZnCl, Pd(PPtBu3)2, dioxane, 25 °C. (b) ArCCH, CuI, Cl2Pd(PPh3)2, TEA, 25 °C, then H2, Pd/C, 25 °C. (c) TMSCl, NaI, MeCN, 25 °C. | ||
The synthesis of compounds 4 commenced from aryl ether 1120 (Scheme 3). Regioselective hydrazine addition followed by oxidative halogenation21 provided brominated intermediate 12. Installation of the phenethyl and heteroaryl groups as above, conversion of the fluoropyridine to a pyridone, and halogenation provided the desired pyridone inhibitors.
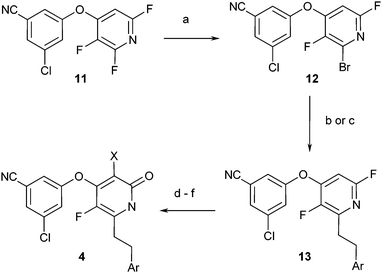 | ||
| Scheme 3 Reagents and conditions: (a) H2NNH2, THF, 25 °C, then Br2, CHCl3, 60 °C, 38%. (b) ArCH2CH2ZnCl, Pd(PtBu3)2, dioxane, 25 °C. (c) ArCCH, CuI, Cl2Pd(PPh3)2, TEA, 25 °C, then H2, Pd/C, 25 °C. (d) BnOH, NaH, THF, 25 °C. (e) TFA, 50 °C. (f) NXS, MeCN, 25–60 °C. | ||
In order access compounds 5, known pyridone 1422 was converted in two steps to benzyl ether 15. Reduction to the aniline and conversion to the iodide then provided key intermediate 16. This intermediate could be selectively functionalized to provide a range of C(5) substituted pyridone NNRTIs (Scheme 4).
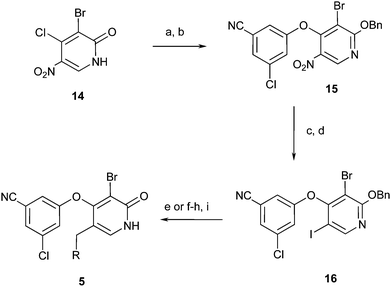 | ||
| Scheme 4 Reagents and conditions: (a) Ag2CO3, BnBr, PhH, 60 °C, 44%. (b) 3-Chloro-5-cyanophenol, K2CO3, DMF, 50 °C, 55% (two steps). (c) Fe, NH4Cl, H2O, EtOH, 100 °C, 97%. (d) tBuONO, CH2I2, 60 °C, 48%. (e) BnZnBr, Pd(PtBu3)2, dioxane, 25 °C, 75%. (f) Allyltributyltin, Pd(PPh3)4, DMF, 100 °C. (g) OsO4, NMO, NaIO4, H2O, THF, 25 °C, then NaBH4, MeOH, 25 °C. (h) ArOH, DIAD, PPh3, THF, 0–25 °C. (i) TFA, DCM, 25 °C. | ||
The culmination of our chemistry efforts led to the synthesis of compounds 6 (Scheme 5). Key intermediate 18 was prepared from known compound 1723 in 5 steps. Either a Stille/Mitsunobu or Grubbs/Suzuki24 sequence followed by deprotection allowed for the preparation of 6b–6c.
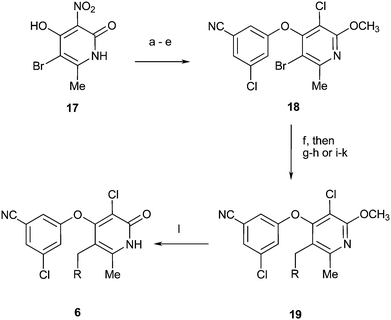 | ||
| Scheme 5 Reagents and conditions: (a) POCl3, benzyltriethylammonium chloride, MeCN, 40 °C. (b) Ag2CO3, MeI, PhH, 60 °C. (c) 3-Chloro-5-cyanophenol, K2CO3, DMF, 50 °C, 55% (two steps). (d) Fe, NH4Cl, H2O, EtOH, 100 °C, 97%. (e) tBuONO, LiCl, CuCl2, MeCN, 60 °C, 64%. (f) Allyltributyltin, Pd(PPh3)4, PhMe, 100 °C. (g) OsO4, NMO, NaIO4, H2O, THF, 25 °C, then NaBH4, MeOH, 25 °C. (h) ArOH, DIAD, PPh3, THF, 0 °C to 25 °C. (i) Propenylpinacolatoborane, Grubbs II, DCM, 50 °C. (j) ArI, Pd(PPh3)4, K2CO3, H2O, DME, 90 °C. (k) H2, PtO2, THF, 25 °C. (l) TMSCl, NaI, MeCN, 25 °C. | ||
In this communication we have described novel synthetic routes to polysubstituted pyridone NNRTIs. These syntheses provide for facile exploration of the C(3), C(5) and C(6) substituents of the pyridone ring, and permit the introduction of a wider variety of aryl ethers than previously reported approaches. The pyridone NNRTIs described herein are active inhibitors of HIV-induced cell death in experiments employing both wild-type and common NNRTI-resistant viruses, and X-ray crystallography and molecular modeling permitted the determination of the binding modes of the inhibitors in the NNRTI binding pocket. We hope that these compounds and their synthetic precursors will enable the identification of new medicines for the treatment of HIV infection.
Notes and references
- C. Flexner, Nat. Rev. Drug Discovery, 2007, 6, 959 CrossRef CAS.
- F. Maggiolo, M. Airoldi, H. D. Kleinloog, A. Callegaro, V. Ravasio, C. Arici, E. Bombana and F. Suter, HIV Clin. Trials, 2007, 8, 282 CrossRef.
- S. M. Vrouenraets, F. W. Wit, J. van Tongeren and J. M. Lange, Expert Opin. Pharmacother., 2007, 8, 851 Search PubMed.
- D. Ripamonti and F. Maggiolo, Curr. Opin. Invest. Drugs, 2008, 9, 899 Search PubMed.
- Z. K. Sweeney and K. Klumpp, Curr. Opin. Drug Discovery Develop., 2008, 11, 458 Search PubMed.
- (a) Z. K. Sweeney, S. F. Harris, N. Arora, H. Javanbakht, Y. Li, J. Fretland, J. P. Davidson, J. R. Billedeau, S. K. Gleason, D. Hirschfeld, J. J. Kennedy-Smith, T. Mirzadegan, R. Roetz, M. Smith, S. Sperry, J. Suh, J. Wu, S. Tsing, A. G. Villasenor, A. Paul, G. Su, G. Heilek, J. Q. Hang, A. S. Zhou, J. A. Jernelius, F.-J. Zhang and K. Klumpp, J. Med. Chem., 2008, 51, 7449 CrossRef CAS; (b) Z. K. Sweeney, S. Acharya, A. Briggs, J. P. Dunn, T. R. Elworthy, J. Fretland, A. M. Giannetti, G. Heilek, Y. Li, A. C. Kaiser, M. Martin, Y. D. Saito, M. Smith, J. M. Suh, S. Swallow, J. Wu, J. Q. Hang, A. S. Zhou and K. Klumpp, Bioorg. Med. Chem. Lett., 2008, 18, 4348 CrossRef CAS; (c) Z. K. Sweeney, J. Dunn, Y. Li, G. Heilek, P. Dunten, T. R. Elworthy, X. Han, S. F. Harris, D. R. Hirschfeld, J. H. Hogg, W. Huber, A. C. Kaiser, D. J. Kertesz, W. Kim, T. Mirzadegan, M. G. Roepel, Y. D. Saito, T. M. P. C. Silva, S. Swallow, J. L. Tracy, A. Villasenor, H. Vora, A. S. Zhou and K. Klumpp, Bioorg. Med. Chem. Lett., 2008, 18, 4352 CrossRef CAS; (d) Z. K. Sweeney, J. J. Kennedy-Smith, J. Wu, N. Arora, J. R. Billedeau, J. P. Davidson, J. Fretland, J. Q. Hang, G. M. Heilek, S. Harris, D. Hirschfeld, P. Inbar, H. Javanbakht, J. A. Jernelius, Q. Jin, Y. Li, W. Liang, R. Roetz, K. Sarma, M. Smith, D. Stefanidis, G. Su, J. M. Suh, A. G. Villasenor, M. Welch, F.-J. Zhang and K. Klumpp, ChemMedChem, 2009, 4, 88 CrossRef CAS.
- Ab initio calculations on a model system confirmed that fluorination increased the electropositive character of the relevant aromatic hydrogen atom.
- A. Beyer, L. Lawtrakul, P. Pungpo and P. Wolschann, Curr. Comput.-Aided Drug Des., 2007, 3, 87 Search PubMed.
- J. L. Medina-Franco, K. Martinez-Mayorga, C. Juarez-Gordiano and R. Castillo, ChemMedChem, 2007, 2, 1141 CrossRef CAS.
- (a) J. Guillemont, A. Benjahad, S. Oumouch, L. Decrane, P. Palandjian, D. Vernier, L. Queguiner, K. Andries, M.-P. de Bethune, K. Hertogs, D. S. Grierson and C. H. Nguyen, J. Med. Chem., 2009, 52, 7473 CrossRef CAS; (b) D. Himmel, K. Das, A. D. Clark, S. H. Hughes, A. Benjahad, S. Oumouch, J. Guillemont, S. Coupa, A. Poncelet, I. Csoka, C. Meyer, K. Andries, C. H. Nguyen, D. S. Grierson and E. Arnold, J. Med. Chem., 2005, 48, 7582 CrossRef CAS.
- A very recent patent application from Merck published after our work was complete also describes 4-aryloxypyridones: T. J. Tucker, R. Tynebor, J. T. Sisko, N. Anthony, R. Gomez and S. M. Jolly, WO2009067166.
- For a detailed description of the in vitro assays see: Z. K. Sweeney and M. Welch, WO2008119662.
- Protein purification and crystallization was performed as described in ref. 6a. Crystallographic data for the cocrystal structure of 2b and HIVRT has been deposited in the PDB (3FFI), http://www.rcsb.org.
- Graphics were prepared using Pymol. See: W. L. DeLano, The PyMOL Molecular Graphics System, 2002, DeLano Scientific, Palo Alto, CA, http://www.pymol.org Search PubMed.
- For the model of 4c in the NNRTI binding pocket see the ESI.† J. Ren, C. Nichols, L. E. Bird, T. Fujiwara, H. Sugimoto, D. I. Stuart and D. K. Stammers, J. Biol. Chem., 2000, 275, 14316 Search PubMed.
- The solubility of 5b at pH 6.5 was determined to be 10 µg mL−1 in a medium-throughput thermodynamic solubility assay.
- The open NNRTI binding pocket of diaryl ether pyridazinone inhibitors (ref. 6c) was selected for modeling inhibitors presented in this paper. An essential water molecule (captured in the pyridazinone structure) is required to dock the pyridone correctly so that the pyridone CO makes a water-mediated hydrogen bond with K101 backbone NH, while the NH group of the pyridone makes a hydrogen bond with the backbone carbonyl of K101.
- Lead optimization was guided using models prepared using the molecular mechanics program MOLOC. Ligand molecules were optimized in the protein environment using the MAB forcefield and 500 steps of conjugate gradient optimization. No constraints were placed on the ligand atoms during minimization. See: (a) P. R. Gerber and K. Müller, J. Comput.-Aided Mol. Des., 1995, 9, 251 CrossRef CAS; (b) P. R. Gerber, J. Comput.-Aided Mol. Des., 1998, 37, 12 , http://www.moloc.ch.
- Compound 7 was prepared from commercially available 4-chloro-3-nitropyridone by methylation with silver carbonate/methyl iodide, followed by nucleophilic substitution with 3-chloro-5-cyanophenol in the presence of potassium carbonate.
- Compound 11 was prepared by addition of 3-chloro-5-cyanophenol to 2,3,4,6-tertrafluoropyridine in the presence of potassium carbonate. See: Q. Li, D. T. Chu, A. Claiborne, C. S. Cooper, C. M. Lee, K. Raye, K. B. Berst, P. Donner, W. Wang, L. Hasvold, A. Fung, Z. Ma, M. Tufano, R. Flamm, L. L. Shen, J. Baranowski, A. Nilius, J. Alder, J. Meulbroek, K. Marsh, D. Crowell, Y. Hui, L. Seif, L. M. Melcher and J. Plattner, J. Med. Chem., 1996, 39, 3070 Search PubMed.
- M. Schlosser, C. Bobbio and T. Rausis, J. Org. Chem., 2005, 70, 2494 CrossRef CAS.
- D. Lee and R. A. Stavenger, WO 2005034866.
- C. S. Wang, J. Heterocycl. Chem., 1970, 7, 389 CrossRef CAS.
- C. Morrill and R. H. Grubbs, J. Org. Chem., 2003, 68, 6031 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data for the co-crystal structure of 2b and HIV-RT. See DOI: 10.1039/c0md00009d |
| This journal is © The Royal Society of Chemistry 2010 |