Synthesis and biological evaluation of 7-arylindoline-1-benzenesulfonamides as a novel class of potent anticancer agents†
Jang-Yang
Chang‡
bc,
Mei-Jung
Lai‡
a,
Yi-Ting
Chang
a,
Hsueh-Yun
Lee
a,
Yun-Ching
Cheng
b,
Ching-Chuan
Kuo
b,
Min-Chieh
Su
a,
Chi-Yen
Chang
b and
Jing-Ping
Liou
*a
aCollege of Pharmacy, Taipei Medical University, 250 Wu-Xin Street, Taipei 110, Taiwan, Republic of China. E-mail: jpl@tmu.edu.tw; Tel: +886-2-27361661 ext. 6130
bNational Institute of Cancer Research, National Health Research Institutes, Tainan 704, Taiwan, Republic of China
cDivision of Hematology/Oncology, Department of Internal Medicine, National Cheng Kung University Hospital, Tainan 704, Taiwan, Republic of China
First published on 18th May 2010
Abstract
A series of 7-arylindoline-1-benzenesulfonamides were prepared and evaluated for anticancer activity. 7-(4′-Cyanophenyl)indoline-1-benzenesulfonamide 15 exhibited substantial antiproliferative activity with IC50 values ranging from 17–32 nM against a variety of human cancer cell lines, including MDR resistant line. Compound 15 (IC50 = 1.5 μM) also showed more potent inhibition of tubulin polymerization than 4a (combretastatin A-4, IC50 = 2.0 μM) and displayed strong binding to the colchicine binding site of the tubulin.
Introduction
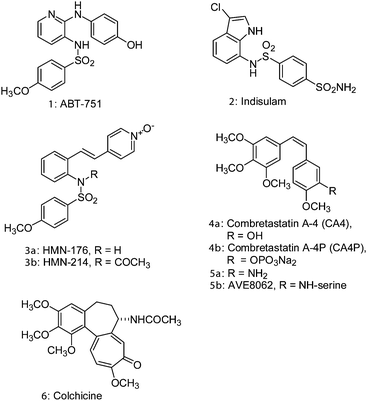
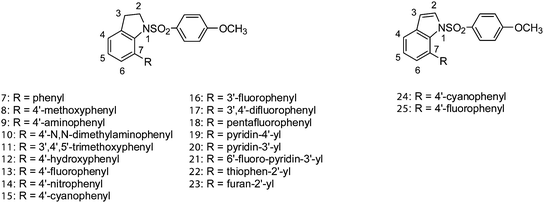
Microtubules are dynamic structures that play a crucial role in cellular division and are recognized as an important target for anticancer therapy.1 A number of naturally occurring compounds, such as paclitaxel, vinblastine, combretastatin A-4 (4a), dolastatin 10, epothilone A and colchicine (6), all exhibit their anticancer properties by interfering with the dynamics of tubulin polymerization and depolymerization, resulting in mitotic arrest. Recent reports2 have revealed that compounds binding to the colchicine domain can act as vascular-disrupting agents (VDA), rapidly depolymerizing the microtubules of vasculatures to block the blood supply to tumors; for example, combretastatin A-4P (4b, ZYBRESTAT) and AVE8062 (5b, Ombrabulin) are currently in clinical trials.Small molecules with sulfonamide functionality, for instance, N-pyridinyl sulfonamide (1, ABT-751),3 chloroindolyl sulfonamide (2, Indisulam),4 and styrylpyridine N-oxide sulfonamide (3b, HMN-214),5 have been reported as potent anticancer agents and are currently undergoing clinical trials for a variety of cancers (Fig. 1).6 Compound 1 showed efficacious inhibition of tubulin polymerization and was found to be a potent antimitotic agent. To our knowledge, there have been no reports on the inhibition of tubulin polymerization with 7-arylindoline-1-benzenesulfonamides. Thus, we report the structure–activity relationship of 7-aryl- and 7-heteroaryl-indoline-1-benzenesulfonamides as a novel class of tubulin depolymerization inhibitors (Fig. 2).
 | ||
| Fig. 1 | ||
 | ||
| Fig. 2 | ||
Results and discussion
Chemistry
Indolines 7–23 and indoles 24–25 were synthesized as shown in Scheme 1, by Suzuki coupling7 of various arylboronic acids and heteroarylboronic acids at the 7-position of indoline-1-benzenesulfonamide (28) and indole-1-benzenesulfonamide (29), respectively. The key intermediate 28, 7-bromo-1-(4′-methoxybenzenesulfonyl)indoline, was synthesized starting from the 7-bromoindole (26). The sodium cyanoborohydride-mediated reduction of 26 resulted in the 7-bromoindoline (27), which reacted with the 4-methoxybenzenesulfonyl chloride in pyridine to give 28. Compound 28 was reacted with the appropriate arylboronic acid and heteroarylboronic acid in the presence of Pd(PPh3)4 and K2CO3 in toluene–EtOH to give the desired 7-aryl(heteroaryl)indoline-1-benzenesulfonamides (7–8 and 10–23). The reduction of the 4′-nitro group in compound 14 with Fe/NH4Cl in isopropanol gave the corresponding amine 9. Indole-sulfonamides 24 and 25 were obtained from an intermediate, 7-bromo-1-(4′-methoxybenzenesulfonyl)indole (29), which was prepared by treatment of 26 with 4-methoxyphenylsulfonyl chloride in the presence of tetrabutylammonium hydrogen sulfate and KOH in CH2Cl2. The Suzuki coupling of 29 with 4-cyanophenylboronic acid and 4-fluorophenylboronic acid gave the desired compounds 24 and 25, respectively.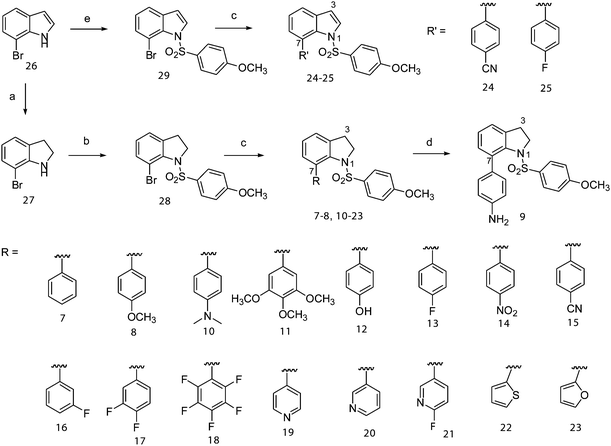 | ||
| Scheme 1 Reagents and conditions: (a) NaCNBH3, CH3COOH, rt; (b) 4-methoxybenzenesulfonyl chloride, pyridine, reflux; (c) various aryl or heteroaryl boronic acids, Pd(PPh3)4, K2CO3, toluene–EtOH, reflux; (d) Fe, NH4Cl, isopropanol; (e) 4-methoxybenzenesulfonyl chloride, Bu4NHSO4, KOH, CH2Cl2, rt. | ||
Biological evaluation
| Compound | Cell type (IC50 nM ± SDa) | ||||
|---|---|---|---|---|---|
| KB | H460 | HT29 | MKN45 | KB-vin10 | |
| a SD: standard deviation. All experiments were independently performed at least three times. | |||||
| 7 | 119.5 ± 85.6 | 212.5 ± 67.2 | 96.5 ± 92.6 | 116.8 ± 125.6 | 107.2 ± 18.0 |
| 8 | 337.0 ± 230.5 | 301.5 ± 170.4 | 277.0 ± 226.9 | 211.3 ± 130.5 | 352.4 ± 25.2 |
| 9 | 249.4 ± 68.7 | 287.5 ± 98.3 | 300.5 ± 106.8 | 161.9 ± 70.0 | 261.5 ± 41.5 |
| 10 | 538.5 ± 188.8 | 556.0 ± 183.8 | 467.0 ± 244.2 | 237.3 ± 130.6 | 553.0 ± 128.4 |
| 11 | 3685.5 ± 869.0 | 6336.0 ± 518.7 | 5100.0 ± 276.2 | 1078.7 ± 869.1 | 4672.3 ± 519.0 |
| 12 | 129.3 ± 40.0 | 113.5 ± 14.8 | 133.0 ± 15.9 | 82.6 ± 36.2 | 123.2 ± 21.0 |
| 13 | 86.0 ± 15.6 | 95.5 ± 20.5 | 85.5 ± 19.1 | 79.0 ± 28.3 | 94.6 ± 9.2 |
| 14 | 52.5 ± 4.9 | 53.1 ± 17.0 | 51.8 ± 8.5 | 34.0 ± 9.6 | 51.3 ± 8.2 |
| 15 | 31.1 ± 5.4 | 29.5 ± 14.8 | 24.2 ± 7.2 | 17.2 ± 8.6 | 29.2 ± 2.1 |
| 16 | 98.5 ± 12.0 | 166.5 ± 23.3 | 119.3 ± 58.6 | 84.7 ± 24.5 | 92.4 ± 21.7 |
| 17 | 45.0 ± 7.1 | 46.0 ± 12.7 | 57.0 ± 28.3 | 55.5 ± 14.8 | 46.0 ± 6.2 |
| 18 | 4040.5 ± 84.1 | 4755.0 ± 770.7 | 3897.5 ± 664.0 | 1777.0 ± 200.8 | 4632.0 ± 256.0 |
| 19 | 96.0 ± 14.1 | 101.0 ± 8.0 | 87.5 ± 40.3 | 69.5 ± 7.6 | 106.0 ± 18.0 |
| 20 | 95.9 ± 36.6 | 89.6 ± 36.2 | 76.3 ± 26.9 | 68.5 ± 47.3 | 84.5 ± 8.0 |
| 21 | 143.0 ± 27.3 | 135.0 ± 56.0 | 108.5 ± 81.5 | 145.3 ± 40.0 | 150.6 ± 19.4 |
| 22 | 214.5 ± 16.3 | 229.0 ± 7.1 | 199.5 ± 6.4 | 165.0 ± 9.9 | 251.3 ± 38.2 |
| 23 | 82.0 ± 18.4 | 94.5 ± 21.9 | 62.5 ± 2.1 | 42.0 ± 1.4 | 79.2 ± 21.3 |
| 24 | 152.9 ± 45.7 | 171.7 ± 35.3 | 164.0 ± 66.1 | 142.7 ± 32.1 | 146.1 ± 32.6 |
| 25 | 203.1 ± 3.0 | 211.9 ± 12.6 | 196.2 ± 10.9 | 170.2 ± 54.5 | 201.7 ± 16.5 |
| colchicine | 10.3 ± 0.9 | 19.8 ± 0.1 | 15.2 ± 0.5 | 11.5 ± 1.5 | 121.2 ± 9.6 |
First, we evaluated whether the substitution of the phenyl group at the 7-position of indoline-1-benzenesulfonamide core would result in antiproliferative activity. 1-(4-Methoxybenzenesulfonyl)-7-phenyl-2,3-dihydro-1H-indole (7) demonstrated potent cell growth inhibitory activity with mean IC50 values of 130 nM (KB, H460, HT29, MKN45, and KB-vin10). Next, investigation of electronic effects on the 7-aryl group of the indoline ring showed that electron-donating groups like 4-methoxyphenyl (8), 4-aminophenyl (9), and 4-(N,N-dimethyl)aminophenyl (10) result in a slight decrease in activity as compared to parent compound 7, exhibiting mean IC50 values of 295, 252 and 470 nM, respectively. It was also noteworthy that the more water soluble 7-(4′-hydroxyphenyl)indoline-1-benzenesulfonamide 12, with the para-hydroxyphenyl group, maintained substantial cytotoxicity, presumably due to the weaker electron-donating ability of the hydroxyl group. As many microtubule inhibitors, such as compounds 4 and 6, have the structure of trimethoxyphenyl group, the addition of trimethoxybenzene moiety may affect activity positively. Compound 11, with a 3′,4′,5′-trimethoxyphenyl group, exhibited dramatically diminished activities with IC50 values ranging from 1078–6336 nM. The steric effect of the substitutions in the 7-arylindoline system seems to influence antiproliferative activity. The important role of the inductive effect on the C7-phenyl moiety of indoline-1-benzenesulfonamides was revealed by the stronger cellular growth inhibition by para(4′)-fluoro-, nitro-, and cyano-substituted phenyl compounds 13, 14 and 15, respectively (mean IC50 values of 88, 49, and 26 nM against the panel of the cell lines), as compared to compounds 7, 8 and 9 with phenyl, 4′-methoxyphenyl and 4′-aminophenyl substitutions. Notably, compound 15, had a mean IC50 value of 26 nM in five cancer cell lines. Compounds 16 and 17 with meta (3′)-fluoro and 3′,4′-difluoro, respectively, were also synthesized and evaluated for antiproliferative activity. SAR information indicated that the fluoro group located at the 3′-position (16) and 4′-position (13) in the 7-arylindoline core resulted in similar activities to each other, but interestingly, the difluoro substitution at the 3′ and 4′-position of phenyl group (17) resulted in improvement of the IC50 values as compared with the mono-fluoro-substituted 13 or 16, with around 0.5–1-fold elevation in potency against five cancer cell lines (KB, H460, HT29, MKN45 and KB-vin10). Notably, compound 17 showed a mean IC50 value of 50 nM in five cancer cell lines, including MDR-positive KB-vin10, which was around 2-fold more potent than the 7-phenylindoline-1-benzenesulfonamides (7). Increase of the number of fluoro groups in pentafluoro-substituted compound 18 resulted in a dramatic decrease in activity to the μM range, thus revealing that steric hindrance on the C7-phenyl regimen of the indoline ring affects the cellular growth inhibitory activity. The improved activity of the 7-aryl-substituted indoline-1-benzenesulfonamides inspired us to investigate the effect of the heteroaryl group at the C-7 position. Compound 19 with a 4′-pyridinyl substitution, compound 20 with a 3′-pyridinyl group, and compound 23 with a 2′-furanyl group at the C-7 position of the indoline ring exhibited stronger antiproliferative activity against human cancer cell lines as compared to compound 7. The 6′-fluoro-3′-pyridinyl (21) and 2′-thiophenyl (22) analogs, however, showed decreased cell growth inhibition as compared to the 7-phenyl compound 7, showing mean IC50 values of 136 and 212 nM, respectively.
In an effort to further understand whether the indoline ring of 7-arylindoline-1-benzenesulfonamides plays an important role for activity, indole sulfonamides 24 and 25 were also prepared. Compounds 24 and 25 showed a decreased growth inhibition by around 2- and 5-fold magnitude in five cancer cell lines, respectively, as compared to the corresponding indolines 15 and 13, thus indicating that the indoline core in this system was preferred over indole (24vs.15 and 25vs.13).
| Compound | Tubulina IC50 ± SD/μM | Colchicine bindingb (% ± SD) | |
|---|---|---|---|
| 1 μM inhibitor | 5 μM inhibitor | ||
| a Inhibition of tubulin polymerization.8 b Inhibition of [3H]colchicine binding.9 Tubulin was at 1 μM; [3H]colchicine was at 5 μM. | |||
| 13 | 2.5 ± 0.1 | 59 ± 3 | 73 ± 2 |
| 14 | 1.7 ± 0.2 | 56 ± 3 | 74 ± 1 |
| 15 | 1.5 ± 0.1 | 81 ± 2 | 92 ± 1 |
| 17 | 2.3 ± 0.4 | 52 ± 2 | 72 ± 3 |
| 19 | 2.2 ± 0.6 | 54 ± 3 | 72 ± 2 |
| 23 | 2.6 ± 0.2 | 43 ± 2 | 68 ± 1 |
| colchicine | 4.1 ± 0.5 | ||
| CA4 | 2.0 ± 0.2 | 86 ± 1 | 96 ± 1 |
Conclusion
We have identified the 7-arylindoline-1-benzenesulfonamides as a novel class of potent inhibitors of tubulin polymerization acting through the colchicine-binding domain of tubulin. The lead compounds 14 and 15 showed significant inhibition of tubulin polymerization with IC50 values of 1.7 and 1.5 μM, respectively, which were superior to CA4. They also demonstrated substantial antiproliferative activity, with IC50 values ranging from 17–54 nM in a variety of human cancer cell lines, including one MDR-positive resistant cell line (KB-vin10). The SAR information of the indoline substitution pattern revealed that the phenyl group located at the 7-position of indoline-1-benzenesulfonamides is beneficial for activity. The electron-withdrawing substituent on the 7-aryl group improved activity (13, 14, 15, 17, 19vs.7). A 2–5-fold decrease in potency was observed on replacing the indoline ring with the indole, thus indicating the important role of the indoline moiety of 7-arylindoline-1-sulfonamides (24vs.15 and 25vs.13). In summary, 7-arylindoline-1-benzenesulfonamides (14 and 15) have potential for further investigation as anticancer agents with strong inhibition of tubulin polymerization and antiproliferative activities.This research was supported by the National Science Council of the Republic of China (grant no. NSC 98-2323-B-038-003, NSC 98-2113-M-038-002-MY2), Department of Health of the Republic of China (grant no. DOH99-TD-C-111-004), and National Health Research Institutes, Taiwan (grant no. CA-097-PP-02).
Notes and references
- M. A. Jordan and L. Wilson, Nat. Rev. Cancer, 2004, 4, 253 CrossRef CAS.
- (a) C. Gridelli, A. Rossi, P. Maione, E. Rossi, V. Castaldo, P. C. Sacco and G. Colantuoni, Oncologist, 2009, 14, 612 CrossRef CAS; (b) G. G. Dark, S. A. Hill, V. E. Prise, G. M. Tozer, G. R. Pettit and D. J. Chaplin, Cancer Research, 1997, 57, 1829 CAS; (c) P. Hinnen and F. A. L. M. Eskens, Br. J. Cancer, 2007, 96, 1159–1165 CrossRef CAS; (d) J. W. Lippert 3rd, Bioorg. Med. Chem., 2007, 15, 605 CrossRef; (e) D. W. Siemann, Vascular-Targeted Therapies in Oncology, John Wiley & Sons, Chichester, UK, 2006 Search PubMed; (f) D. W. Siemann, M. C. Bibby, G. G. Dark, A. P. Dicker, F. A. L. M. Eskens, M. R. Horsman, D. Marme and P. M. LoRusso, Clinical Cancer Res., 2005, 11, 416 Search PubMed; (g) A. M. Gaya and G. J. Rustin, Clinical Oncology, 2005, 17, 277 Search PubMed; (h) G. M. Tozer, C. Kanthou and B. C. Baguley, Nat. Rev. Cancer, 2005, 5, 423 CrossRef CAS; (i) D. M. Patterson and G. J. S. Rustin, Clinical Oncology, 2007, 19, 443 Search PubMed; (j) D. W. Siemann, D. J. Chaplin and P. A. Walicke, Expert Opin. Invest. Drugs, 2009, 18, 189 Search PubMed; (k) G. M. Tozer, S. Akerman, N. A. Cross, P. R. Barber, M. A. Björndahl, O. Greco, S. Harris, S. A. Hill, D. J. Honess, C. R. Ireson, K. L. Pettyjohn, V. E. Prise and C. C. Reyes-Aldasoro, Cancer Res., 2008, 68, 2301 CrossRef CAS.
- (a) H. Yoshino, N. Ueda, J. Niijima, H. Sugumi, Y. Kotake, N. Koyanagi, K. Yoshimatsu, M. Asada, T. Watanabe, T. Nagaau, K. Tsukahara, A. Iijima and K. Kitoh, J. Med. Chem., 1992, 35, 2496 CrossRef; (b) N. Koyanagi, T. Nagasu, F. Fujita, T. Watanabe, K. Tsukahara, Y. Funahashi, M. Fujita, T. Taguchi, H. Yoshino and K. Kitoh, Cancer Res., 1994, 54, 1702 CAS; (c) K. Yoshimatsu, A. Yamaguchi, H. Yoshino, N. Koyanagi and K. Kitoh, Cancer Res., 1997, 57, 3208 CAS.
- T. Owa, H. Yoshino, T. Okauchi, K. Yoshimatsu, Y. Ozawa, N. H. Sugi, T. Nagasu, N. Koyanagi and K. Kitoh, J. Med. Chem., 1999, 42, 3789 CrossRef CAS.
- (a) H. Hideki Tanaka, N. Ohshima, M. Ikenoya, K. Komori, F. Katoh and H. Hidaka, Cancer Res., 2003, 63, 6942; (b) M. Takagi, T. Honmura, S. Watanabe, R. Yamaguchi, M. Nogawa, I. Nishimura, F. Katoh, M. Matsuda and H. Hidaka, Invest. New Drugs, 2003, 21, 387 CrossRef CAS; (c) M. A. DiMaio, A. Mikhailov, C. L. Rieder, D. D. Von Hoff and R. E. Palazzo, Mol. Cancer Ther., 2009, 8, 592 CrossRef CAS.
- (a) A. M. Mauer, E. E. Cohen, P. C. Ma, M. F. Kozloff, L. Schwartzberg, A. I. Coates, J. Qian, A. E. Hagey and G. B. Gordon, J. Thorac. Oncol., 2008, 3, 631 Search PubMed; (b) E. Fox, J. M. Maris, B. C. Widemann, W. Goodspeed, A. Goodwin, M. Kromplewski, M. E. Fouts, D. Medina, S. L. Cohn, A. Krivoshik, A. E. Hagey, P. C. Adamson and F. M. Balis, Clin. Cancer Res., 2008, 14, 1111 CrossRef CAS; (c) A. S. Zandvliet, J. H. M. Schellens, C. Dittrich, J. Wanders, J. H. Beijnen and A. D. R. Huitema, Br. J. Clin. Pharmacol., 2008, 66, 485 CrossRef CAS; (d) W. S. Siegel-Lakhai, A. S. Zandvliet, A. D. R. Huitema, M. M. Tibben, G. Milano, V. Girre, V. Diéras, A. King, E. Richmond, J. Wanders, J. h. Beijnen and J. h. Schellens, M. Brit. J. Cancer, 2008, 98, 1320 Search PubMed; (e) L. L. Garland, C. Taylor, D. L. Pilkington, J. L. Cohen and D. D. Von Hoff, Clin. Cancer Res., 2006, 12, 5182 CrossRef CAS.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS.
- (a) C. Y. Nien, Y. C. Chen, C. C. Kuo, H. P. Hsieh, C. Y. Chang, J. P. Liou and J. Y. Chang, J. Med. Chem., 2010, 53, 2309 CrossRef CAS; (b) J. P. Liou, Z. Y. Wu, C. C. Kuo, C. Y. Chang, P. Y. Lu, C. M. Chen, H. P. Hsieh and J. Y. Chang, J. Med. Chem., 2008, 51, 4351 CrossRef CAS.
- C. C. Kuo, H. P. Hsieh, W. Y. Pan, C. P. Chen, J. P. Liou, S. J. Lee, Y. L. Chang, L. T. Chen and J. Y. Chang, Cancer Res., 2004, 64, 4621 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Spectral data of compounds 7–25 and experimental procedures for synthesis and biological evaluations, and HPLC purity data for compounds 7–25. See DOI: 10.1039/c0md00052c |
| ‡ Contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2010 |
