Facile synthesis and strong antiproliferative activity of disubstituted diphenylmethylidenyl-[3]ferrocenophanes on breast and prostate cancer cell lines†
Meral
Görmen
,
Pascal
Pigeon
,
Siden
Top
*,
Anne
Vessières
,
Marie-Aude
Plamont
,
Elizabeth A.
Hillard
and
Gérard
Jaouen
Chimie ParisTech (Ecole Nationale Supérieure de Chimie de Paris), Laboratoire Charles Friedel, UMR CNRS 7223, 11 rue Pierre et Marie Curie, 75231 Paris Cedex 05, France. E-mail: siden-top@chimie-paristech.fr; Fax: +33 1 43 26 00 61; Tel: +33 1 44 27 66 99
First published on 6th May 2010
Abstract
A series of new 1-[di-(4-R-phenyl)-methylidenyl)]-[3]ferrocenophanes, where R = OH, NH2, NHAc, and the phenyl substitution is mixed or identical, are highly antiproliferative against MDA-MB-231 and PC-3 cancer cells, with IC50 values ranging from 0.05–5.6 μM on MDA-MB-231 and 0.02–12.5 μM on PC-3.
As part of our study on the antiproliferative effects of compounds based on the ferrocenyl-diphenyl-butadiene skeleton, we have recently shown that [3]ferrocenophane is an attractive pharmacophore in the development of cytotoxic agents for hormone-refractory breast cancer cells.1,2 The ferrocenophane motif is found in a variety of compounds which have been used in catalysis, polymerization, sensors and electronics applications.3–6 For the series of molecules based on the 1-[(4-R-phenyl)-phenyl-methylidenyl]-[3]ferrocenophane skeleton, where R = OH (1), NH2 (2) and NHAc (3), the incorporation of the rigid [3]ferrocenophane moiety resulted in up to a four-fold increase in activity compared to the corresponding ferrocene series, with IC50 values of 0.2–0.5 μM on ER-breast cancer cells, (Chart 1).2 The cytotoxicity of such molecules seemed to be further enhanced by substitution on both of the phenyl rings; for example, the 4,4′-bis-hydroxy compound 4 gave an IC50 value of 0.09 μM on MDA-MB-231, compared to 0.47 μM for the monosubstituted 4-hydroxy analogue 1 (Chart 1).1 These two observations together suggest that a combination of NH2, NHAc, and OH moieties on each of the phenyl rings should give access to the most active molecules yet. Therefore, we report here the synthesis and characterization of the unsubstituted compound 5 and compounds 6–9, possessing mixed or identical bis-substitution, and their in vitro antiproliferative effects against hormone-independent breast (MDA-MB-231) and prostate (PC-3) cancer cell lines.
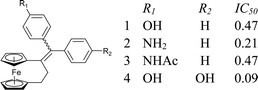 | ||
| Chart 1 Previously reported compounds and their IC50 values (μM) against MDA-MB-231 hormone-independent breast cancer cells. | ||
Compounds 5, 6 and 7 were obtained by a McMurry cross coupling reaction between [3]ferrocenophane-1-one7 and the appropriate benzophenone (Scheme 1a).2 As a precursor to 6, 4-nitro-4′-hydroxybenzophenone was synthesized in 46% overall yield via a Friedel Crafts acylation of anisole with p-nitrobenzoyl chloride followed by a deprotection of the OMe group with HBr in acetic acid.8,9 The nitro-substituted compound was used because of its commercial availability and because the in situ reduction of NO2 to NH2 under McMurry conditions is well known.10 McMurry himself recognized this type of reduction, and noted that it could be avoided by conducting the reaction at low temperature.11 Concerning the low yield of 6, it has been reported that this type of coupling is decelerated by the presence of an amino group.12 Compound 8 was subsequently obtained in 63% yield from the selective acetylation of 6 in a pyridine–THF solution (Scheme 1b).
![Synthesis of new 1-[di-(4-R-phenyl)-methylidenyl]-[3]ferrocenophanes.](/image/article/2010/MD/c0md00026d/c0md00026d-s1.gif) | ||
| Scheme 1 Synthesis of new 1-[di-(4-R-phenyl)-methylidenyl]-[3]ferrocenophanes. | ||
As a precursor for 7, 4,4′-bisacetylaminobenzophenone was synthesized in 88% yield by acetylation of commercially available 4,4′-bisaminobenzophenone with acetyl chloride.13 Compound 9 was obtained in 78% yield by deacetylation of 7 in the presence of HCl in EtOH (Scheme 1c). Direct McMurry cross coupling with 4,4′-diaminobenzophenone was not successful, yielding only the ferrocenyl homo-coupled product and unreacted benzophenone.
Crystals of 5 and Z-8 suitable for X-ray structure analysis were obtained from hexanes and acetonitrile solutions, respectively. Compound 5 crystallized in an orthorhombic space group, while 8 crystallized in a monoclinic space group with one molecule of Z-8 and one molecule of acetonitrile per asymmetric unit (Fig. 1).141H NMR analysis of the crystals showed the presence of one isomer; the chemical shifts corresponded to the minor isomer in the mixture obtained after HPLC purification.
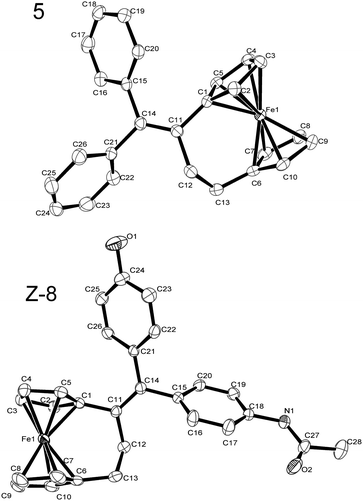 | ||
| Fig. 1 ORTEP diagrams of 5 and Z-8. Thermal ellipsoids shown at 50% probability. Hydrogen atoms and the acetonitrile molecule of solvation from Z-8·CH3CN have been omitted for clarity. | ||
The structure of the [3]ferrocenophane moiety in 5 and 8 is very similar to that reported by Erker and coworkers, with the corresponding boat-like conformation of the bridge.15 The bridge is slightly too short to connect the two Cp rings without distortion in the ferrocene group. The eclipsed η5-C5H4 rings are, therefore, not coplanar, but rather tilted towards the bridge, such that the angle made by the centroids of the two η5-C5H4 rings and the ferrocene is 173°, and the angle between the best planes of the η5-C5H4 ring is approximately 10°. Due to this distortion, the Fe–C distances in the η5-C5H4 rings range from about 2.03 to 2.08 Å. The bridge itself is also slightly strained; the respective angles deviate slightly from tetrahedral and trigonal geometries. See the supplementary information for selected bond distances and angles.†
All of the new compounds were tested for their anti-proliferative effects against hormone-independent MDA-MB-231 breast and PC-3 prostate cancer cells (Table 1).
As predicted, the new compounds, possessing OH/NH2, OH/NHAc and NH2/NH2 aromatic bis-substitution are all highly active, with IC50 values in the nanomolar region, while the monosubstituted compounds (Chart 1) are approximately an order of magnitude less potent. The toxicity profile on PC-3 prostate cancer cells is remarkably similar to that found on MDA-MB-231 breast cancer cells. It is notable that 6, 8, and 9 are much more effective against MDA-MB-231 and PC-3 cells than even the most active ferrocenyl derivatives of tamoxifen (IC50 = 0.5 μM, MDA-MB-231),16 estradiol (13.4 μM, MDA-MB-231),17 nilutamide (5.4 μM, PC-3),18 and testosterone (4.7 μM, PC-3).19 Surprisingly, 5 is also quite active, even lacking aromatic substitution, further demonstrating the antiproliferative properties of the [3]ferrocenophane moiety.
However, a rather startling result was obtained; compound 7, possessing two amide groups shows an anomalously mild toxicity on both cell lines. With respect to the other disubstituted compounds, the behavior of this compound contradicts all of our previous observations. Potential insolubility of 7 has been ruled out; its solubility in DMSO/water is similar to that of monosubstituted compound 3 and much better than that of unsubstituted compound 5. It should be mentioned, however, that esterases, amidases,20–23 and arylamine N-acetyltransferases,24–26 theoretically allow the conversion between the two forms, and it is thus difficult to identify the active species in the cell. This point warrants further study.
In conclusion, we have found that the cytotoxic activity of ferrocenyl compounds based on the diphenylethylene structure can be markedly improved by the exchange of ferrocene for ferrocenophane and the para-substitution of both phenyl rings with protic groups. In particular, such compounds possessing OH/OH, OH/NH2, OH/NHAc and NH2/NH2 substitution are active in nanomolar concentrations, placing them among the most effective organometallic cancer drug candidates heretofore discovered. We have postulated that, for the ferrocene series, quinone methides27,28 or imine methides10 could be active metabolites of these types of compounds, and this hypothesis remains consistent with the data reported here.
Acknowledgements
We thank P. Herson for X-ray structural characterization, as well as the Agence Nationale de la Recherche (ANR-06-BLAN-0384-01, “FerVect”) for financial support. M. G. acknowledges fellowship from the Ministère des Affaires Etrangères de la France.Notes and references
- D. Plazuk, A. Vessieres, E. A. Hillard, O. Buriez, E. Labbe, P. Pigeon, M. A. Plamont, C. Amatore, J. Zakrzewski and G. Jaouen, J. Med. Chem., 2009, 52, 4964–4967 CrossRef CAS.
- M. Gormen, P. Pigeon, E. A. Hillard, M. A. Plamont, D. Plażuk, S. Top, A. Vessières and G. Jaouen, Tetrahedron Lett., 2010, 51, 118–120 CrossRef CAS.
- A. Csampai, A. Z. Gyorfi, Gy. I. Turos and P. Sohar, J. Organomet. Chem., 2009, 694, 3667–3673 CrossRef CAS.
- M. Ogasawara, S. Watanabe, K. Nakajima and T. Takahashi, J. Am. Chem. Soc., 2010, 132, 2136–2137 CrossRef CAS.
- D. P. Huber, G. Kehr, K. Bergander, R. Froehlich, G. Erker, S. Tanino, Y. Ohki and K. Tatsumi, Organometallics, 2008, 27, 5279–5284 CrossRef CAS.
- J.-S. Park, T. R. Lee, Modern Cyclophane Chemistry, ed. R. Gleiter and H. Hopf, 2004, 131–157 and references therein Search PubMed.
- T. D. Turbitt and W. E. Watts, J. Organomet. Chem., 1972, 46, 109–117 CrossRef CAS.
- J. Shani, A. Gazit, T. Livshitz and S. Biran, J. Med. Chem., 1985, 28, 1504–1511 CrossRef CAS.
- H. Dehmlow, J. D. Aebi, S. Jolidon, Y.-H. Ji, E. M. Von Mark, J. Himber and O. H. Morand, J. Med. Chem., 2003, 46, 3354–3370 CrossRef CAS.
- P. Pigeon, S. Top, O. Zekri, E. A. Hillard, A. Vessieres, M. A. Plamont, O. Buriez, E. Labbe, M. Huche, S. Boutamine, C. Amatore and G. Jaouen, J. Organomet. Chem., 2009, 694, 895–901 CrossRef CAS.
- J. E. McMurry, Chem. Rev., 1989, 89, 1513–1524 CrossRef CAS.
- X.-F. Duan, J. Zeng, J.-W Lü and Z.-B. Zhang, J. Org. Chem., 2006, 71, 9873–9876 CrossRef CAS.
- S. Kirkwood and P. H. Philips, J. Am. Chem. Soc., 1947, 69, 934–936 CrossRef CAS.
- Crystal data for 5: C26H22Fe, M = 390.31, orthorhombic, a = 5.8601(9), b = 31.829(4), c = 40.783(4) Å; V = 7606.9(17) Å3, T = 200 K, space group Fdd2, Z = 16, 16586 reflections measured, 4545 unique [Rint = 0.001], the final wR(F2) was 0.0736; for Z-8·CH3CN: C30H28FeN2O2, M = 504.41, monoclinic, a = 5.8841(7), b = 19.872(2), c = 11.1212(17) Å; β = 95.141(10)°, V = 1295.1(3) Å3, T = 200 K, space group P21, Z = 2, 21998 reflections measured, 3870 unique [Rint = 0.001], the final wR(F2) was 0.1068..
- P. Liptau, S. Knüppel, G. Kehr, O. Kataeva, R. Fröhlich and G. Erker, J. Organomet. Chem., 2001, 637–639, 621–630 CrossRef CAS.
- A. Nguyen, E. A. Hillard, A. Vessières, S. Top, P. Pigeon and G. Jaouen, Chimia, 2007, 61, 716–725 CrossRef CAS.
- A. Vessières, D. Spera, S. Top, B. Misterkiewicz, J. M. Heldt, E. A. Hillard, M. Huché, M.-A. Plamont, E. Napolitano, R. Fiaschi and G. Jaouen, ChemMedChem, 2006, 1, 1275–1281 CrossRef CAS.
- O. Payen, S. Top, A. Vessières, E. Brulé, M.-A. Plamont, M. J. McGlinchey, H. Müller-Bunz and G. Jaouen, J. Med. Chem., 2008, 51, 1791–1799 CrossRef CAS.
- S. Top, C. Thibaudeau, A. Vessières, E. Brulé, F. Le Bideau, J.-M. Joerger, M.-A. Plamont, S. Samreth, A. Edgar, J. Marrot, P. Herson and G. Jaouen, Organometallics, 2009, 28, 1414–1424 CrossRef CAS.
- T. Bisogno, K. Katayama, D. Melck, N. Ueda, L. D. Petrocellis, S. Yamamoto and V. D. Marzo, Eur. J. Biochem., 1998, 254, 634 CrossRef CAS.
- J. Katz, M. Levitz, S. S. Kadner and T. H. Finlay, J. Steroid Biochem. Mol. Biol., 1991, 38, 17 CrossRef CAS.
- J. Katz, T. H. Finlay, S. Banerjee and M. Levitz, J. Steroid Biochem., 1987, 26, 687 CrossRef CAS.
- J. V. Watson, S. H. Chambers, P. Workman and T. S. Horsnell, FEBS Lett., 1977, 81, 179 CrossRef CAS.
- J. Dairou, N. Atmane, F. Rodrigues-Lima and J.-M. Dupret, J. Biol. Chem., 2004, 279, 7708 CAS.
- J. A. Williams and D. H. Phillips, Cancer Res., 2000, 60, 4667 CAS.
- P. J. Adam, J. Berry, J. A. Loader, K. L. Tyson, G. Craggs, P. Smith, J. De Belin, G. Steers, F. Pezzella, K. F. Sachsenmeir, A. C. Stamps, A. Herath, E. Sim, M. J. O'Hare, A. L. Harris and J. A. Terrett, Mol. Cancer Res., 2003, 1, 826–835 CAS.
- E. A. Hillard, A. Vessières, L. Thouin, G. Jaouen and C. Amatore, Angew. Chem., 2005, 118, 291 ( Angew. Chem., Int. Ed. , 2006 , 45 , 285–290 ).
- D. Hamels, P. M. Dansette, E. A. Hillard, S. Top, A. Vessières, P. Herson, G. Jaouen and D. Mansuy, Angew. Chem., Int. Ed., 2009, 48, 9124–9126 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures and characterization data for 5–9. Crystallographic data (excluding structure factors) for 7 have been deposited with the Cambridge Crystallographic Data Centre. CCDC reference numbers 768981–768982. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0md00026d |
| This journal is © The Royal Society of Chemistry 2010 |
