DOI:
10.1039/C0MD00015A
(Concise Article)
Med. Chem. Commun., 2010,
1, 145-148
Received
18th February 2010
, Accepted 31st March 2010
First published on
5th May 2010
Introduction
Since the discovery of penicillin, β-lactam antibiotics have been the most important family of antibacterial agents. Cephalosporins, a class of β-lactam antibiotics, are known to exert their biological activity by reacting with bacterial enzymes to open the β-lactam ring. This process is accompanied by liberation of the 3′-substituent, when the substituent can function as a leaving group (Scheme 1).1 Examples are known where cytotoxic components, including COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundmitomycin C,2 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddoxorubicin,3 as well as antimicrobials, such as quinolones,4 have been incorporated at the 3′ position. The cephalosporin-COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundquinolone conjugates have been shown to exhibit a broad spectrum of antibacterial activity derived from both cephalosporin-like and COMPOUND LINKS
Read more about this on ChemSpider
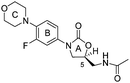
Download mol file of compoundquinolone-like components. These dual-action cephems can also be used to overcome the destructive action of β-lactamases (βL) (Enz = βL, Scheme 1), a main cause of bacterial resistance to β-lactam antibiotics.5 While, as indicated, many cephalosporin prodrug conjugates have been described, linezolid (Fig. 1), a relatively new FDA approved drug for treatment of MRSA and VRE, or any of the related COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone antibiotics, have not been evaluated in an analogous fashion. Linezolid is the first COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone drug approved for the treatment of Gram-positive bacterial infections.6 Cephalosporin-COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone conjugates are anticipated to possess activity against both Gram-negative and Gram-positive bacteria. Herein we report the syntheses and biological evaluation of two cephalosporin-COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone congeners and demonstrate the controlled release of the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone in the presence of a β-lactamase GES-II.
Results and discussion
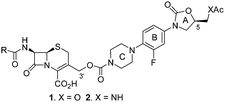
Based on the ample precedent mentioned earlier for the use of the βL-induced release process as depicted in Scheme 1, we considered using a carbamate linkage between the 3′ position of a representative cephalosporin and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpiperazine-based linezolid analogs as depicted in Fig. 2. This strategy required the preparation and eventual conjugation of the appropriately functionalized cephalosporin and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone components.
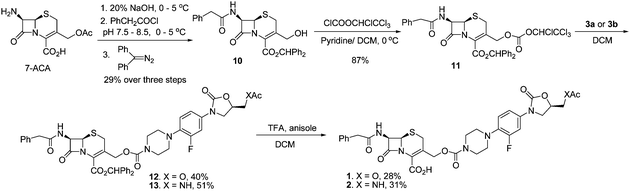
The syntheses of the component linezolid analogs 3a and 3b are shown in Scheme 2. The syntheses utilized considerable literature precedent7 and began with a SNAr reaction of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3,4-difluoronitrobenzene and mono Boc-protected piperazine 4 to afford para-substituted COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundnitrobenzene derivative 5 in 49% yield. Palladium catalyzed hydrogenation of compound 5 followed by reaction with CbzCl gave protected COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundaniline COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6. Then, as reported,7a compound COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound6 was sequentially treated with nBuLi and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundR-glycidyl butyrate at −78 °C to form the key COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone 7 in 71% yield with defined stereochemistry at the C-5 position of ring A. Reaction of 7 with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundacetic anhydride in the presence of DMAP and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpyridine afforded the corresponding C-5 acetate analog, 8, in 95% yield. TFA-induced removal of the Boc group afforded the desired linezolid analog 3a in 92% isolated yield. Alternatively, stepwise reaction of 7 with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundmethanesulfonyl chloride, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundammonia and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundacetyl chloride produced the acetamidomethyl-containing analog, 9, in 61% overall yield for three steps. Analog 3b was then obtained in good yield by treatment of 9 with TFA to remove the Boc group.
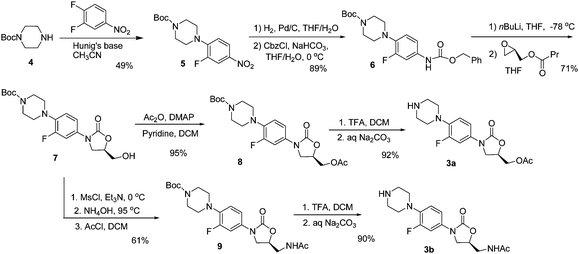 |
| | Scheme 2 Syntheses of linezolid analogs 3a and 3b | |
With oxazolidinones 3a and 3b in hand, the syntheses of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcarbamate-linked cephalosporin-oxazolidione compounds 1 and 2 were carried out as shown in Scheme 3. The syntheses began with a controlled hydrolysis to remove the acetyl group from the 3′-hydroxymethyl substituent of commercially available 7-amino cephalosporanic acid (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7-ACA). Reaction with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundphenylacetyl chloride followed by treatment with freshly prepared COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddiphenyldiazomethane8 provided COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcephem COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound10 in 29% overall yield for three steps.9 Subsequent reaction with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundtetrachloroethyl chloroformate gave intermediate COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcarbonate COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound11 in 81% yield. Reaction of the activated carbonate COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound11 with 3a in the presence of pyridine and DMAP was attempted. Desired coupling product 12 was generated; however, as often during reactions of cephalosporins, partial isomerization of the cephem nucleus occurred,10 to give a nonseparable mixture of the Δ3 and Δ2 double bond isomers (12 and 12b, Fig. 3). Cephalosporin Δ2 isomers 12b are not effective antibiotics10c,11 and also are not substrates for the planned β-lactamase induced prodrug process depicted earlier in Scheme 1. Further studies of the coupling reaction revealed that without any basic additives, reaction between COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound11 and 3a still took place and provided the desired protected conjugate 12 in 40% yield. No Δ2–Δ3 isomerization was observed. Subsequent removal of the benzhydryl protecting group from 12 gave the desired conjugate 1 in 28% yield after crystallization. With this approach validated, cephalosporin-COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone conjugate 2 was also synthesized in a similar manner, involving the formation of conjugate 13 with a C-5 acetamidomethyl substitute and subsequent removal of benzhydryl group of 13.
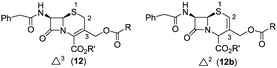 |
| | Fig. 3 General structures for Δ3–Δ2 double bond cephem isomers. | |
To determine whether cephalosporin-COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone conjugates 1 and 2 could be specifically activated in the presence of β-lactamase as planned, a LC/MS assay was performed (Fig. 4).12 Conjugates 1 and 2 were separately incubated with catalytic amounts of β-lactamase GES-II (0.4 mol%) in phosphate buffered saline (50 mM, pH 7.0) at room temperature. We were pleased to find that in the presence of β-lactamase, conjugates 1 and 2 were completely cleaved to the hydrolyzed cephalosporin and the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone components 3a and 3b, respectively, after 4 h. Control experiments were also conducted by treatment of 1 and 2 under the same conditions in the absence of β-lactamase. As expected, conjugates 1 and 2 were stable even after 24 h. These data demonstrate that compounds 1 and 2 are good substrates for βL GES-II and are stable in a neutral aqueous environment until specifically activated by the β-lactamase.
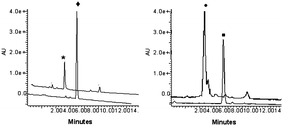 |
| | Fig. 4 HPLC trace of compounds 1, 2 and products 3a, 3b released by β-lactamase activation. Left to right: (*) 3a (with βL, 4 h), (◆) 1 (no βL, 24 h), (●) 3b (with βL, 4 h), (■) 2 (no βL, 24 h). | |
Cephalosporin-COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone conjugates 1 and 2, as well as linezolid analogs 3a and 3b, and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundciprofloxacin as a control, were tested for their antibacterial activities against various strains of Gram-positive and Gram-negative bacteria as well as Mycobacterium vaccae, using an agar diffusion assay (Table 1). Cephalosporin derivative 14 was synthesized from COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound7-ACA (Scheme 4) and included in the assay as an additional control. In general, conjugates 1 and 2 were found to retain similar biological profiles compared to cephem 14 as well as individual oxazolidinones 3a and 3b. More interestingly, for most organisms tested, conjugates 1 and 2 displayed extended activity relative to linezolid itself. For example, they exhibited good activity against the Gram-negative strain Pseudomonas aeruginosa K799/61, while linezolid was relatively inactive. Conjugates 1 and 2, as well as components 3a and 3b, were also evaluated against MRSA and VRE, and good activity was observed. Again, conjugate and components matched in activity. All compounds, including conjugates 1 and 2, as well as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone 3a and 3b, also exhibited antimycobacterial activity and could potentially be useful for treatment of M. tuberculosis as they induced large inhibition zones against M. vaccae, a common model for M. tuberculosis.13 Interestingly, linezolid components 3a and 3b were roughly equipotent in vitro with linezolid against several Gram-positive organisms, including Bacillus subtilis, Staphylococcus aureus, Enterococcus faecalis and Micrococcus luteus. These biological data suggested that the cephalosporin-COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone conjugates might possess additive biological activity corresponding to that of both the cephem and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone components.
Table 1 Antibacterial activity of conjugates 1 and 2 in the agar diffusion assay
| compdsa |
Growth inhibition zones in mm (9 mm well diameter) |
| Gram-positive bacteria |
Gram-negative bacteria |
M. vaccae IMET |
|
B. subtilis ATCC |
S. aureus
|
E. faecalis
|
M. luteus ATCC |
P. aeruginosa
|
E. coli
|
| 6633b |
SG 511b |
Efs1c |
Efs4c |
134/93 MRSAc |
1528cVRE |
10240b |
K799/WTc |
K799/61c |
SG 458b |
10670b |
Exactly 50 μL of a 2.0 or 1.0 mM solution in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO : MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) of each compound was filled in 9 mm wells in agar media (Standard I Nutrient Agar, Serva or Mueller Hinton II Agar, Becton, Dickinson and Company). Inhibition zones read after incubation at 37 °C for 24 h. Cipro (COMPOUND LINKS 9) of each compound was filled in 9 mm wells in agar media (Standard I Nutrient Agar, Serva or Mueller Hinton II Agar, Becton, Dickinson and Company). Inhibition zones read after incubation at 37 °C for 24 h. Cipro (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundciprofloxacin) was dissolved in H2O to give a 5 μg/mL solution.
1.0 mM solution of linezolid, 1–2, 3a–3b, and 14 was used.
2.0 mM solution of linezolid, 1–2, 3a–3b, and 14 was used.
p, partially clear inhibition zone/colonies in the inhibition zone.
P, unclear inhibition zone/many colonies in the inhibition zone.
h, faint indication of inhibition zone.
|
| linezolid |
33/37pd |
35 |
34/39p |
35/39p |
42 |
34 |
38/45p-Pe |
0 |
10P |
23/28p |
52 |
|
14
|
46/52p |
39 |
43 |
35 |
13 |
23 |
38/44p-P |
11hf |
23 |
35 |
0 |
|
3a
|
24/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound26p |
21 |
31 |
19/23P |
29 |
25 |
31p/40P |
15h |
16/26h |
13P |
42 |
|
3b
|
26 |
18 |
31/35p |
14/18P |
25 |
25 |
23/30p-P |
11P/18h |
20/30h |
14P |
35 |
|
1
|
43/47p |
36 |
40 |
31 |
27 |
26 |
42 |
17h |
22/31p |
33 |
44 |
|
2
|
42/45p |
34 |
40 |
30 |
17 |
23 |
38/42p |
15P/20h |
24/33h |
33 |
30 |
| cipro |
31 |
18 |
0 |
0 |
0 |
15 |
0 |
29 |
35 |
34 |
23 |
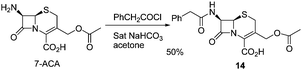 |
| | Scheme 4 Synthesis of cephem 14 | |
Conclusions
We have described straightforward syntheses of cephalosporin-COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone conjugates. The conjugates retained antibacterial activity comparable to individual cephalosporin and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone components, and showed stability in aqueous media until specifically activated by a β-lactamase. Future work will focus on in vitro enzyme assays to examine the release of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazolidinone in the presence of β-lactamase producing bacteria. Additional structural analogs are also under consideration.
Acknowledgements
This work was supported by grant from the National Institutes of Health (GM 075855). We thank Professor Shahriar Mobashery for providing β-lactamase GES-II. We gratefully acknowledge Uta Wohlfeld for performing antibacterial assays, and Dr Viktor Krchnak for help with LC/MS. We also thank the Lizzadro Magnetic Resonance Research Center at Notre Dame for NMR facility and Nonka Sevova for mass spectroscopic analyses. TAW acknowledges the University of Notre Dame Chemistry-Biochemistry-Biology Interface (CBBI) Program and NIH Training Grant T32GM075762 for a fellowship.
Notes and references
-
(a) J. M. T. Hamilton-Miller, G. G. F. Newton and E. P. Abraham, Biochem. J., 1970, 116, 371 CAS;
(b) C. H. O'Callaghan, S. M. Kirby, A. Morris, R. E. Waller and R. E. Duncombe, J. Bacteriol., 1972, 110, 988 CAS;
(c) W. S. Faraci and R. F. Pratt, J. Am. Chem. Soc., 1984, 106, 1489 CrossRef CAS;
(d) A. D. Russell and R. H. Fountain, J. Bacteriol., 1971, 106, 65 CAS;
(e) D. B. Boyd and W. H. Lunn, J. Med. Chem., 1979, 22, 778 CrossRef CAS;
(f) D. B. Boyd, J. Org. Chem., 1985, 50, 886 CrossRef CAS;
(g) M. L. Page and P. Proctor, J. Am. Chem. Soc., 1984, 106, 3820 CrossRef;
(h) E. J. Grabowski, A. W. Douglas and G. B. Smith, J. Am. Chem. Soc., 1985, 107, 267 CrossRef CAS.
- V. M. Vrudhula, H. P. Svensson and P. D. Senter, J. Med. Chem., 1997, 40, 2788 CrossRef CAS.
-
(a) V. M. Vrudhula, H. P. Svensson and P. D. Senter, J. Med. Chem., 1995, 38, 1380 CrossRef CAS;
(b) L. N. Jungheim, T. A. Shepherd and J. K. Kling, Heterocycles, 1993, 35, 339 CrossRef CAS.
-
(a) H. A. Albrecht, G. Beskid, J. G. Christenson, N. H. Georgopapadakou, D. D. Keith, F. M. Konzelmann, D. L. Pruess, P. L. Rossman and C. C. Wei, J. Med. Chem., 1994, 37, 400 CrossRef CAS and references therein;
(b) G. Y. Zhao, M. J. Miller, S. Franzblau, B. J. Wan and U. Möllmann, Bioorg. Med. Chem. Lett., 2006, 16, 5534 CrossRef CAS.
- G. H. Hakimelahi, K.-S. Shia, C. H. Xue, S. Hakimelahi, A. A. M. Movahedi, A. A. Saboury, A. K. Nezhad, M. N. S. Rad, V. Osyetrov, K.-P. Wang, J.-H. Liao and F.-T. Luo, Bioorg. Med. Chem., 2002, 10, 3489 CrossRef CAS and references therein.
-
(a) M. R. Barbachyn and C. W. Ford, Angew. Chem., Int. Ed., 2003, 42, 2010 CrossRef CAS;
(b) S. J. Brickner, D. K. Hutchinson, M. R. Barbachyn, P. R. Manninen, D. A. Ulanowicz, S. A. Garmon, K. C. Grega, S. K. Hendges, D. S. Toops, C. W. Ford and G. E. Zurenko, J. Med. Chem., 1996, 39, 673 CrossRef CAS.
-
(a) J. A. Tucker, D. A. Allwine, K. C. Grega, M. R. Barbachyn, J. L. Klock, J. L. Adamski, S. J. Brickner, D. K. Hutchinson, C. W. Ford, G. E. Zurenko, R. A. Conradi, P. S. Burton and R. M. Jensen, J. Med. Chem., 1998, 41, 3727 CrossRef CAS;
(b) M. R. Barbachyn, D. S. Toops, D. A. Ulanowicz, K. C. Grega, S. J. Brickner, C. W. Ford, G. E. Zurenko, J. C. Hamel, R. D. Schaadt, D. Stapert, B. H. Yagi, J. M. Buysse, W. F. Demyan, J. O. Kilburn and S. E. Glickman, Bioorg. Med. Chem. Lett., 1996, 6, 1003 CrossRef;
(c) D. M. Gleave and S. J. Brickner, J. Org. Chem., 1996, 61, 6470 CrossRef CAS;
(d) D. M. Gleave, S. J. Brickner, P. R. Manninen, D. A. Allwine, K. D. Lovasz, D. C. Rohrer, J. A. Tucker, G. E. Zurenko and C. W. Ford, Bioorg. Med. Chem. Lett., 1998, 8, 1231 CrossRef CAS.
- S. Kumar and R. W. Murray, J. Am. Chem. Soc., 1984, 106, 1040 CrossRef CAS.
- M. Gonzalez, Z. Rodriguez, B. Tolon, J. C. Rodriguez, H. Velez, B. Valdes, M. A. Lopez and A. Fini, Farmaco, 2003, 58, 409 CrossRef CAS.
-
(a) R. Prasada, V. V. Korrapati, R. Dandala, V. K. Handa, K. Subramanyeswara, G. Anand, I. V. Rao, A. Rani and A. Naidu, J. Heterocycl. Chem., 2007, 44, 1513 CrossRef;
(b) M. Botta, M. C. De Rosa, R. Di Fabio, C. Mozzetti, A. Santini and F. Corelli, Electron. J. Theor. Chem., 1996, 1, 52 Search PubMed;
(c) E. Pop, M. E. Brewster, N. Bodor and J. J. Kaminski, In. J. Quantum Chem., 1989, 16, 291 Search PubMed.
- R. R. Chauvette and E. H. Flynn, J. Med. Chem., 1966, 9, 741 CrossRef CAS.
- The LC/MS analyses were carried out on a Waters ZQ instrument consisting of chromatography module Alliance HT, photodiode array detector 2996, and mass spectrometer Micromass ZQ, using a 3 × 50 mm Pro C18 YMC reverse phase column (Waters, Milford, MA, http://www.waters.com/). Mobile phases: 10 mM COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundammonium acetate in HPLC grade water (A) and HPLC grade COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundacetonitrile (B). A gradient was formed from 5% to 80% of B in 10 min at 0.7 mL/min. The MS electrospray source operated at capillary voltage 3.5 kV and a desolvation temperature 300 °C.
-
(a) L. J. Xu, Y. Y. Wang, X. D. Zheng, X. D. Gui, L. F. Tao and H. M. Wei, Cellular and Molecular Immunology, 2009, 6, 67 Search PubMed;
(b) J. Stanford, C. Stanford and J. Grange, Front. Biosci., 2004, 9, 1701 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental procedures, characterization data and copies of 1H NMR and 13C NMR spectra, protocols of antibacterial assays. See DOI: 10.1039/C0MD00015A/ |
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here. 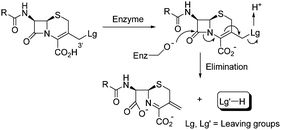



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) of each compound was filled in 9 mm wells in agar media (Standard I Nutrient Agar, Serva or Mueller Hinton II Agar, Becton, Dickinson and Company). Inhibition zones read after incubation at 37 °C for 24 h. Cipro (ciprofloxacin) was dissolved in H2O to give a 5 μg/mL solution.
b 1.0 mM solution of linezolid, 1–2, 3a–3b, and 14 was used.
c 2.0 mM solution of linezolid, 1–2, 3a–3b, and 14 was used.
d p, partially clear inhibition zone/colonies in the inhibition zone.
e P, unclear inhibition zone/many colonies in the inhibition zone.
f h, faint indication of inhibition zone.
9) of each compound was filled in 9 mm wells in agar media (Standard I Nutrient Agar, Serva or Mueller Hinton II Agar, Becton, Dickinson and Company). Inhibition zones read after incubation at 37 °C for 24 h. Cipro (ciprofloxacin) was dissolved in H2O to give a 5 μg/mL solution.
b 1.0 mM solution of linezolid, 1–2, 3a–3b, and 14 was used.
c 2.0 mM solution of linezolid, 1–2, 3a–3b, and 14 was used.
d p, partially clear inhibition zone/colonies in the inhibition zone.
e P, unclear inhibition zone/many colonies in the inhibition zone.
f h, faint indication of inhibition zone.

