DOI:
10.1039/C0MD00024H
(Concise Article)
Med. Chem. Commun., 2010,
1, 50-53
Received
8th March 2010
, Accepted 7th May 2010
First published on
20th May 2010
Introduction
While appropriate oxygen supply from blood vessels maintains cellular activities, a dysregulation of oxygen homeostasis causes generation of pathological cells that are responsible for several diseases. In particular, hypoxic cells as generated by a supplied oxygen deficiency1 are well-characterized to be related to malignant solid tumors,2 inflammatory diseases3 and cardiac ischemia.4 Since hypoxic cells are identified as one of the most important features of diseases, there is increasing demand for the development of hypoxia-specific molecular probes to achieve the pathophysiological analysis of diseases.
Fluorescent molecular probes have recently attracted much attention to be widely used in the fields of biology, physiology and pharmacology, because of their wide applicability and high sensitivity for bioimaging.5 In the recent study, we proposed a new type of hypoxia-sensing fluorescence probe consisting of an COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundindolequinone unit and a COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcoumarin fluorophore (IQ-Cou),6,7 in which the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundindolequinone unit has a quenching function of fluorescence6 as well as hypoxia-sensitive reduction reactivity.8 Consequently, while fluorescence emission of coumarin in the IQ-Cou conjugation was effectively suppressed, enzymatic treatment under hypoxic conditions resulted in an intense fluorescence in a hypoxia-selective manner as a result of a one-electron reductive bond dissociation of IQ-Cou to release coumarin fluorophore. Thus, IQ-Cou shows several unique properties that are favorable for hypoxia imaging. However, further application of IQ-Cou to cellular imaging has been limited due to its lower solubility in water9 and shorter wavelengths for the absorption and fluorescence emission of the coumarin unit.
These previous results stimulated us to improve a molecular system so as to be applicable to visualization of hypoxic cells. We conjugated a water-soluble fluorophore of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundrhodol10 with the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundindolequinone unit to form a new hypoxia imaging probe (IQ-R). The COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundrhodol has a good solubility in water, long excitation and emission wavelengths (around 550 nm), intense fluorescence emission with the quantum yield (ΦF of 0.20)11 and a favorable on-off switching property of fluorescence emission regulated by the hypoxia-sensitive reactivity of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundindolequinone. According to the density functional calculations of IQ-R at the B3LYP/6-31G(d) level,12 the orbital of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundindolequinone and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundrhodol units was orthogonal. The energy level of the lowest unoccupied molecular orbital (LUMO = −3.03 eV) localized at the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundindolequinone unit was lower than the LUMO + 1 (= −2.16 eV) localized at the rhodol unit. This suggests that intramolecular electron transfer from the rhodol unit in the excited state to the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundindolequinone unit in the ground state is thermodynamically feasible.13 It is therefore predicted that the excited fluorescent state of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundrhodol in the IQ-R conjugate undergoes effective intramolecular quenching by the neighboring COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundindolequinone as in the case of IQ-Cou, while the intense fluorescence would be restored upon enzymatic one-electron reduction under hypoxic conditions to release a nonconjugated free COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundrhodol. We report herein enzymatic activation, fluorescence properties and application to cellular imaging of the IQ-R probe.
Result and discussion
Synthesis and one-electron reduction characteristics of IQ-R
IQ-R was synthesized by coupling COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3-chloromethyl-5-methoxy-1,2-dimethylindole-4,7-dione8d with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundrhodol (ESI, Scheme S1†). We initially confirmed better water solubility up to 120 μM of IQ-R in comparison with a conventional IQ-Cou. We characterized the fluorescence properties of IQ-R in aqueous solution during enzymatic one-electron reduction with NADPH:cytochrome P450 reductase (Fig. 1), which catalyzes the one-electron reduction of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundindolequinone derivatives under hypoxic conditions.6 Before incubation with reductase, IQ-R showed extremely weak fluorescence, indicating that the fluorescence of the rhodol fluorophore is quenched intramolecularly by the neighboring COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundindolequinone unit: the fluorescence quantum yield (ΦF) of IQ-R was 0.0098. A similar weak fluorescence was observed for the sample incubated with reductase under aerobic conditions. In contrast, it is striking that hypoxic incubation of IQ-R resulted in an intense fluorescence emission at around 550 nm: the fluorescence emission was about 11 times stronger than that of the original IQ-R, while the absorption spectra of the sample before and after incubation were quite similar (see ESI, Fig. S1†). As predicted, enzymatic activation to enhance the fluorescence emission occurs substantially under hypoxic conditions.
HPLC analysis of enzymatic reduction of IQ-R
In a separate experiment, we monitored the course of the enzymatic reduction of IQ-R by reversed-phase HPLC. Fig. 2 shows a representative reaction profile of IQ-R under treatment with NADPH:cytochrome P450 reductase. The appearance of a single new peak in Fig. 2C was attributed to the formation of a corresponding fluorophore COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundrhodol upon hypoxic treatment, as confirmed by the overlapped injection of authentic sample in the HPLC analysis. In contrast, the enzymatic decomposition of IQ-R was dramatically suppressed under aerobic conditions, leading to the formation of a small amount of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundrhodol (Fig. 2B). The steady-state kinetic parameters, Vmax and Km, for the release of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundrhodol were derived from a Lineweaver–Burk plot (Table 1).14 These results indicate that the change in rhodol fluorophore concentration on treatment with reductase is responsible for the change in fluorescence intensity.
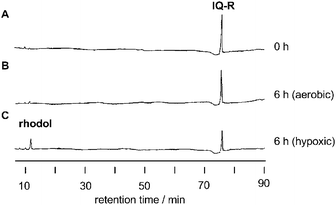 |
| | Fig. 2 HPLC profiles of IQ-R (100 μM) upon treatment with NADPH:cytochrome P450 reductase (10 μg mL−1) in the presence of β-NADPH (0.2 mM) at 37 °C for 0 h (A) and 6 h under aerobic (B) or hypoxic (C) conditions. The elution peaks were monitored at 315 nm wavelength. | |
Table 1 The steady-state kinetic parameters for enzymatic one-electron reduction of IQ-R under hypoxic or aerobic conditions.a
| |
Hypoxic |
Aerobic |
|
IQ-R was incubated with NADPH:cytochrome P450 reductase in the presence of β-NADPH. Vmax and Km were calculated from a Lineweaver–Burk plot.
|
|
V
max
(pmol min−1) |
710 |
180 |
|
K
m
(μM) |
10 |
3.6 |
Hypoxic cancer cell imaging using IQ-R
In light of the behavior in the enzymatic activation, we applied IQ-R to a cellular imaging of a human lung adenocarcinoma cell line, A549, that expresses NADPH:cytochrome P450 reductase in high amount.15 A549 cells were cultured in the presence of IQ-R (50 μM) for 24 h at 37 °C under hypoxic or aerobic conditions.16 As shown in Fig. 3A, blight fluorescence was observed from the cytoplasm of cells incubated under hypoxic conditions. In contrast, there was substantially no fluorescence emission in the case of the similar-cultured cells under aerobic conditions (Fig. 3B). Considering the evidence that treatment of IQ-R with lysate of A549 cells resulted in an intense fluorescence emission in a hypoxia-dependent manner (Fig. 3C), it is reasonable to conclude that IQ-R penetrates into living cells wherein it is activated to release COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundrhodol by intracellular reductase in a hypoxic environment to show an intense fluorescence.
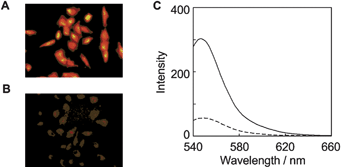 |
| | Fig. 3 (A,B) Fluorescent microscopic observation of A549 cells as incubated with 50 μM IQ-R for 24 h at 37 °C under hypoxic (A: 0.02% oxygen) or aerobic conditions (B: 20% oxygen) and then washed with PBS. (C) The fluorescent spectra of IQ-R (5 μM) upon treatment for 6 h with the cell lysate derived from A549 cells under hypoxic (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ) or aerobic conditions ( ) or aerobic conditions (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ). The fluorescence spectra were measured with excitation at 525 nm. ). The fluorescence spectra were measured with excitation at 525 nm. | |
Conclusion
In summary, we characterized the biological one-electron reduction and fluorescence properties of IQ-R. The fluorescence of IQ-R was significantly suppressed by the intramolecular quenching function of the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundindolequinone unit. On the other hand, hypoxic treatment with isolated or intracellular reductase induced the efficient decomposition of IQ-R and release of free fluorophore COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundrhodol, resulting in the intense fluorescence emission. Thus, imaging of hypoxic cells by means of enzymatic reduction characteristics of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundindolequinone derivatives was successfully accomplished for the first time. IQ-R would be a promising candidate as a hypoxia-specific fluorescence probe. Application of IQ-R to in vivo optical imaging of tumor hypoxia is now in progress.
Experimental section
General procedures
All starting materials and reagents were purchased from Tokyo Kasei Kogyo (Tokyo, Japan), Wako (Tokyo, Japan) and Aldrich Chemical (Milwaukee, WI). All other solvents, purchased from Wako, were GR grade or dry grade and used without further purification. NADPH:cytochrome P450 reductase and β-NADPH were obtained from Oxford Bio-medical research and Oriental Yeast Co. (Japan), respectively. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3-Chloromethyl-5-methoxy-1,2-dimethyl-1H-indole-4,7-dione8d and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundrhodol10a were synthesized as described previously. The 1H NMR spectra were recorded using a JOEL JNM-AL400 (400MHz) spectrometer in CDCl3. Coupling constants are given in hertz. 13C NMR spectra were measured with JOEL JMN-AL-400 (100 MHz) spectrophotometer in CDCl3. The FAB-MS spectra were recorded on a JOEL JMS-SX102A spectrometer, using COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundnitrobenzyl alcohol as matrix. The organic reactions were carried out in oven-dried glassware under an argon atmosphere with magnetic stirring. All absorption spectra of IQ-R were recorded using a JASCO V-530 UV/Vis spectrophotometer with a 1 cm quartz cell. In a similar manner, fluorescence spectra in vitro were recorded on a SHIMADZU RF-5300PC spectrofluorophotometer with a 1 cm quartz cell. The slit widths were set to 5 nm for both excitation and emission. High-performance liquid chromatography (HPLC) was carried out with a Hitachi HPLC system (L-7455 Diode array detector, L-7300 column oven, L-7100 pump, D-7000 interface). Sample solutions were injected onto a reversed-phase column (Inertsil ODS-3, GL Science Inc.). The following program was used: of 70% B followed by 70–0% B (linear gradient over 70 min.; solution A: 100% COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundacetonitrile, solution B: 0.1 M COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundtriethyl ammonium acetate (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundTEAA) buffer pH 7.
Synthesis of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-(6-dimethylamino-3-oxo-3H-xanthen-9-yl)-benzoic acid 5-methoxy-1,2-dimethyl-4,7-dioxo-4,7-dihydro-1H-indol-3-ylmethyl ester (IQ-R)
COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundThionyl chloride (2 mL) was added to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3-hydroxymethyl-5-methoxy-1,2-dimethylindole-4,7-dione (20.9 mg, 0.888 mmol, 1 equiv.) and stirred for 30 min at ambient temperature. After the reaction, the solvent was removed under reduced pressure to give crude COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3-chloromethyl-5-methoxy-1,2-dimethylindole-4,7-dione (22.5 mg). To the solution of crude product of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3-chloromethyl-5-methoxy-1,2-dimethylindole-4,7-dione (22.5 mg) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMF (5 mL), COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundrhodol (32.0 mg, 0.890 mmol) and K2CO3 (200 mg) were added, and then stirred for 5 h at 80 °C. After being cooled to room temperature, the reaction mixture was extracted with CHCl3 two times, and the combined organic layer was washed with brine, dried over MgSO4, filtered and concentrated by evaporation of the solvent. The crude product was purified by silica gel column chromatography (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundChloroform/EtOAc = 1/1 to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundChloroform/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMethanol = 10/1) to afford COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-(6-Dimethylamino-3-oxo-3H-xanthen-9-yl)-benzoic acid 5-methoxy-1,2-dimethyl-4,7-dioxo-4,7-dihydro-1H-indol-3-ylmethyl ester (IQ-R) as a red solid (24.9 mg, 0.432 mmol, 48.5%): m.p. > 172 °C (decomposed), 1H NMR (400MHz, CDCl3) 1.71 (s, 3H), 3.00 (s, 6H), 3.69 (s, 3H), 3.80 (s, 3H), 4.88 (d, 2H, J = 11.7 Hz), 5.17 (d, 2H, J = 11.7 Hz), 5.48 (s, 1H), 6.10–6.40 (4H), 6.65–6.70 (2H), 7.05–7.10 (1H), 7.55–7.60 (2H), 8.20–8.25 (1H).;13C NMR(100MHz, CDCl3) 8.7, 32.4, 40.3, 56.3, 57.0, 96.4, 104.3, 106.6, 110.9, 111.9, 113.5, 114.8, 120.9, 126.3, 128.9, 129.6, 130.0, 130.0, 130.1, 130.5, 131.5, 132.4, 133.9, 137.9, 154.6, 155.3, 158.5, 159.2, 165.2, 176,6, 178.4, 182.0, 182.0.; FABMS m/z 577 [(M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH) +]; HRMS calced for C34H29O7N2+ [(M + COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH) +] 577.1969, found 577.1989.
Bioreduction by NADPH-P450 reductase
To establish hypoxia, a solution of NADPH:cytochrome P450 reductase (final concentration: 20 μg mL−1) and β-NADPH (final concentration: 2 mM) in 5 mM phosphate buffer (pH 7.4) was purged with argon for 10 min at 37 °C. To the resulting solution was added IQ-R (final concentration: 10 μM) and incubated at 37 °C for 30 min. Then, the fluorescence spectra were measured with excitation at 485 nm. A control aerobic sample solution was incubated and analyzed in a similar manner.
For the HPLC analysis, a solution of IQ-R (final concentration: 100 μM) in the presence of NADPH:cytochrome P450 reductase (final concentration: 10 μg mL−1) and β-NADPH (final concentration: 0.2 mM) in 5 mM phosphate buffer (pH 7.4) and incubated at 37 °C for 6 h under hypoxic or aerobic conditions. After the reaction, the samples were analyzed.
Measurement of fluorescence quantum yield
The fluorescence quantum yield (ΦF) was determined by using COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundrhodol, with known ΦF value of 0.20 in water, as in ref. 10a. The quantum yield was calculated according to eqn (1), in which ΦF(S) and ΦF(R) are the fluorescence quantum yields of the sample and the reference, respectively, the terms A(S) and A(R) are the area under the fluorescence spectra, (Abs)(S) and (Abs)(R) are the optical densities of the sample and reference solutions at the excitation wavelength, and n(S) and n(R) are the refractive indices of the solvents used for the sample and the reference.| | | ΦF(S)/ΦF(R) = A(S)/A(R) × (Abs)(R)/(Abs)(S) × n(S)2/n(R)2 | (1) |
Bioreductive activation of IQ-R by A549 cell lysate
A549 cells were cultured in 10 dishes (90% confluent in ϕ100 mm dishes) and washed twice with ice-cold PBS(−). The cell lysate was then harvested with 5 mL of ice-cold CelLytic M Cell Lysis Reagent (SIGMA-ALDRICH, Inc. St. Louis, MO), kept at ambient temperature for 15 min, and centrifuged at 14,000 rpm for 5 min to remove the cell debris. The resultant supernatant was pre-incubated in a well-oxygenized incubator (95% air and 5% CO2 at 37 °C) for aerobic treatment or in Bactron II anaerobic environmental chamber (Sheldon Manufacturing, Cornelius, OR; 94% N2, 5% CO2 and 1% H2 at 37 °C) for hypoxic treatment for 24 h. IQ-R (the final IQ-R concentrations 50 μM) was added to 600 μL of the aerobic or the hypoxic cell lysates and incubated under the same oxygen conditions as the pre-incubation for additional 6 h. The samples were quickly frozen until they were subjected to the spectral analysis. After a dilution (10 times by water) of the samples, fluorescence spectra were measured with excitation at 525 nm.
Cellular imaging of A549 cells
A549 cells (1 × 106 cells/ϕ60 mm dish) were pre-cultured under aerobic or hypoxic conditions for 18 h and then treated with the aqueous solution of IQ-R (final concentration 50 μM) for 24 h under the same oxygen conditions as the pre-culture. After washing the cells twice with phosphate buffer, fluorescence images were observed with a fluorescent microscope (IX-71 Olympus) equipped with HQ type mirror unit (excitation filter: BP535-555HQ, emission filter: BA570-625HQ, and dichromatic mirror: DM565HQ) ideal for the wavelength chracteristics of a red fluorescent protein, DsRed2. Digital images were captured with a digital camera, DP-72 (Olympus).
Acknowledgements
This study is a part of joint researches, which are focusing on development of basis of technology for establishing COE of nanomedicine, carried out through Kyoto City Collaboration of Regional Entities for Advancement of Technology Excellence (CREATE) assigned by Japan Science and Technology Agency (JST), and is partly supported by the Program for Promotion of Fundamental Studies in Health Science of the National Institute of Biomedical Innovation (NIBIO), Japan.
References
-
(a) A. L. Harris, Nat. Rev. Cancer, 2002, 2, 38–47 CrossRef CAS;
(b) P. Vaupel, F. Kallinowski and P. Okunieff, Cancer Res., 1989, 49, 6449–6465 CAS.
-
(a) S. Kizaka-Kondo, M. Inoue, H. Harada and M. Hiraoka, Cancer Sci., 2003, 94, 1021–1028 CrossRef.
- C. Mardoch, M. Muthana and C. E. Lewis, J. Immunol., 2005, 175, 6257–6263.
-
(a) G. L. Semenza, Annu. Rev. Med., 2003, 54, 17–28 CrossRef;
(b) G. L. Semenza, Trends Mol. Med., 2001, 7, 345–350 CrossRef;
(c) G. L. Semenza, Pediatr. Res., 2001, 49, 614–617 CrossRef CAS.
-
(a) D. W. Domaille, E. L. Que and C. J. Chang, Nat. Chem. Biol., 2008, 4, 168–174 CrossRef CAS;
(b) Y. Umezawa, Annu. Rev. Anal. Chem., 2008, 1, 397–421 Search PubMed;
(c) E. E. Graves, R. Weissleder and V. Ntziachristos, Curr. Mol. Med., 2004, 4, 419–430 Search PubMed.
- K. Tanabe, N. Hirata, H. Harada, M. Hiraoka and S. Nishimoto, ChemBioChem, 2008, 9, 426–432 CrossRef CAS.
- For recent reports on the molecular probe for hypoxia, see:
(a) K. Tanabe, H. Harada, M. Narazaki, K. Tanaka, K. Inafuku, H. Komatsu, T. Ito, H. Yamada, Y. Chujo, T. Matsuda, M. Hiraoka and S. Nishimoto, J. Am. Chem. Soc., 2009, 131, 15982–15983 CrossRef CAS;
(b) E. Nakata, Y. Yukimachi, H. Kariyazono, S. Im, C. Abe, Y. Uto, H. Maezawa, T. Hashimoto, Y. Okamoto and H. Hori, Bioorg. Med. Chem., 2009, 17, 6952–6958 CrossRef CAS;
(c) W. P. Zhu, M. Dai, Y. F. Xu and X. H. Qian, Bioorg. Med. Chem., 2008, 16, 3255–3260 CrossRef CAS;
(d) Y. Liu, Y. Fu, X. H. Qian, Y. Xiao, J. W. Liu, L. Y. Shen, J. H. Li and Y. X. Zhang, Bioorg. Med. Chem. Lett., 2006, 16, 1562–1566 CrossRef CAS;
(e) Y. Liu, Y. F. Xu, X. H. Qian, J. W. Liu, L. Y. Shen, J. H. Li and Y. X. Zhang, Bioorg. Med. Chem., 2006, 14, 2935–2941 CrossRef CAS;
(f) R. J. Hodgkiss, R. W. Middleton, J. Parrick, H. K. Rami, P. Wardman and G. D. Wilson, J. Med. Chem., 1992, 35, 1920–1926 CrossRef CAS;
(g) R. J. Hodgkiss, G. W. Jones, A. Long, R. W. Middleton, J. Parrick, M. R. L. Stratford, P. Wardman and G. D. Wilson, J. Med. Chem., 1991, 34, 2268–2274 CrossRef CAS.
-
(a) S. A. Everett, E. Swann, M. A. Naylor, M. R. L. Stratford, K. B. Patel, A. Tian, R. G. Newman, B. Vojnovic, C. J. Moody and P. Wardman, Biochem. Pharmacol., 2002, 63, 1629–1639 CrossRef CAS;
(b) M. Hernick, C. Flader and R. F. Borch, J. Med. Chem., 2002, 45, 3540–3548 CrossRef CAS;
(c) E. Swann, P. Barraja, A.M. Oberlander, W. T. Gardipee, A. R. Hundnott, H. D. Beall and C. J. Moody, J. Med. Chem., 2001, 44, 3311–3319 CrossRef CAS;
(d) M. A. Naylor, E. Swann, S. A. Everett, M. Jaffar, J. Nolan, N. Robertson, S. D. Lockyer, K. B. Patel, M. F. Dennis, M. R. L. Stratford, P. Wardman, G. E. Adams, C. J. Moody and I. J. Stratford, J. Med. Chem., 1998, 41, 2720–2731 CrossRef CAS.
- Organic solvent such as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundacetonitrile was required to dissolve IQ-Cou.
-
(a) L. G. Lee, G. M. Berry and C. H. Chen, Cytometry, 1989, 10, 151–164 CAS;
(b) J. E. Whitaker, R. P. Haugland, D. Ryan, P. C. Hewitt, R. P. Haugland and F. G. Prendergast, Anal. Biochem., 1992, 207, 267–279 CrossRef CAS.
- COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundRhodol showed brighter fluorescence emission than coumarin fluorophore. The quantum yield of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundrhodol was 0.20, while that of coumarin-3-carboxilic acid, which was released from IQ-Cou, was 0.042.
- We used SPARTAN program for density functional calculations.
- A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515–1566 CrossRef.
-
W. J. Moore, Physical Chemistry, Prentice-Hall, Inc., New Jersey, 1972 Search PubMed.
- L. J. Yu, J. Matis, D. A. Scudiero, K. M. Hite, A. Monk, E. A. Sausville and D. J. Waxman, Drug Metab. Dispos., 2001, 29, 304–312 CAS.
- Fluorescence enhancement in hypoxic cells was also observed even in administration of 5 μM IQ-R (see ESI, Fig. S2†).
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ); incubated for 30 min under aerobic conditions (
); incubated for 30 min under aerobic conditions (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ); and before incubation (⋯). The fluorescence spectra were measured with excitation at 485 nm.
); and before incubation (⋯). The fluorescence spectra were measured with excitation at 485 nm.

![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ) or aerobic conditions (
) or aerobic conditions (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ). The fluorescence spectra were measured with excitation at 525 nm.
). The fluorescence spectra were measured with excitation at 525 nm.