Hierarchical porous carbon from cell assemblies of rice husk for in vivo applications†
Seiichiro
Tabata
*,
Horonori
Iida
,
Takeshi
Horie
and
Shinichiro
Yamada
Advanced Materials Laboratories, Sony Corporation, 4-16-1 Okata, Atsugi-shi, Kanagawa 243-0021, Japan. E-mail: Seiichiro.Tabata@jp.sony.com; Fax: + 81-46-226-2437; Tel: + 81-46-226-3481
First published on 5th May 2010
Abstract
Hierarchical porous carbon material, comprising micro-, meso- and macropores, was developed by a environmentally friendly process using unique structural cell assemblies of rice husk. They showed effects on renal failure rats with low-toxicity in vivo.
Recently, porous carbon (PC) materials with high surface area and connected pore channels have become a fascinating area of research for various applications.1–5 Hierarchical porous carbon (HPC) materials are also gaining significant attention due to their simultaneous availability of macro- (<50 nm) meso- (2–50 nm) and/or micro- (<2 nm) pores.6–9 The macropore can act as an easy transport system of reagents to the meso/micropore.10,11 The meso- and micropores together with the macropore provide high surface area and enhancements of host–guest interactions, selectivity, catalytic activity and lifetime.12–15
Fabrication of HPC generally requires chemical synthesis of a template such as nano-structural silica at high temperature and artificial preparation of its composite (carbon precursor-template) before carbonization and/or removal of the template. As the hierarchical structure is found in nature and can be accelerated by solar energy, air, water and minerals in soil, its use as a precursor of HPC is an environmentally friendly method for the reduction of energy consumption. Here, we reported a novel fabrication process of HPC using cell assemblies of rice husks, which consist of 20% silica, as the template and cellulose and lignin as the carbon precursors. This process involves removal of the silica between the cells followed by steam activation to fabricate macro-, meso- and microporous structures (Fig. 1).
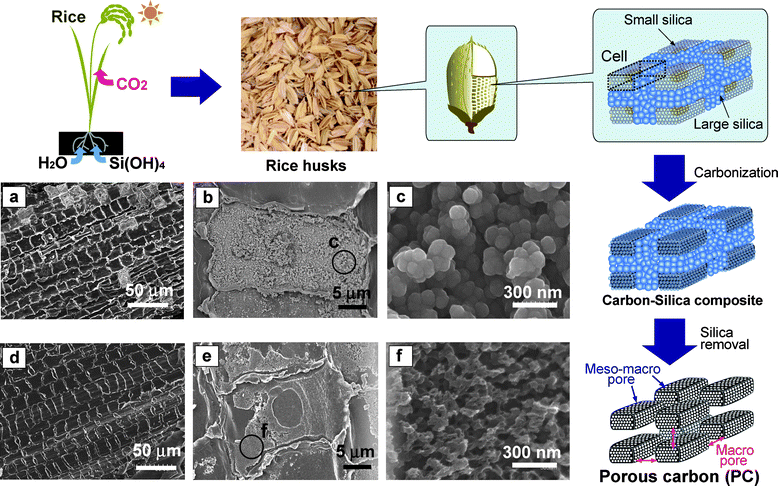 | ||
| Fig. 1 Preparation scheme for porous carbon (PC) from rice husk and SEM images of silica-carbon composites (a–c) and PC (d–f). | ||
To date, applications of PC materials prepared by template methods using a silica-etching process have been confined to electrode materials,16–19 separation purposes,5,20,21 and in vitro studies.22 To the best of our knowledge, little is known about the oral safety and in vivo properties of PC prepared by these template methods. In this study, we described a novel fabrication process and report for the first time the oral toxicity test of PC that was prepared using the silica template method on normal rats. Then, we determined the effects of our HPC on renal failure rats in vivo.
Rice (Oryza sativa) grows by taking in silicic acid as well as CO2, water and various minerals.23 Rice can deposit amorphous silica on their cell walls, forming silica-cuticle and silica-cellulose double layers on the surface of leaves, stems and husks.24,25 About 20% of rice is typically comprised of husks, most of which are presently disposed as waste.23,24 In rice husks, aggregations of small and large silica particles are formed on the intercellular layers and surface of cell walls. In this study, rice husks were carbonized under nitrogen in a furnace for 1 h at 800 °C to produce a carbon–silica composite, and then analyzed by scanning electron micrography (SEM) (Fig. 1a–c). As shown by elemental mapping (ESI Fig. S1†), the white areas charged by electrons on SEM were confirmed to be silica. Under magnification (Fig. 1b), small silica particles can be seen closely packed on the surface of the cell, to give its closely packed porous structure. Further enlargement (Fig. 1c) clearly revealed that the size of the silica was below 100 nm. Brunauer-Emmett-Teller (BET) analysis by nitrogen adsorption measurements indicated that the specific surface area and pore volume of the composite were only 40 m2g−1 and 0.1 cm3g−1, respectively, before immersion in hydrofluoric acid (HF) solution at room temperature. After this silica removal process, the specific surface area and pore volume of PC increased to 612 m2g−1 and 0.6 cm3g−1, respectively. X-ray analysis and Fourier transform infrared spectroscopy (ESI Fig. S2 and S3†) showed that HF treatment resulted in the complete dissolution of silica, while SEM (Fig. 1d) showed PC with large, relatively ordered pores (∼1 μm). Moreover, after silica removal, connected small pores of 50–100 nm were found on the surface, where small silica particles had accumulated (Fig. 1e, f). Thus, the natural primary microstructure of PC enables us to fabricate unique pore structures by simple carbonization and silica removal.
Next, PC was steamed at 250 mL min−1 for 3.0 h at 900 °C under nitrogen to obtain HPC with increased BET-specific surface area and pore volume of 1340 m2g−1 and 1.2 cm3g−1, respectively. Increasing the activation time increased the BET surface area and pore volume (ESI Fig. S5†).
Pore distribution of carbons in each process was estimated by measuring nitrogen adsorption and mercury porosimetry. Macropores (≈1 μm) were generated by the removal of silica in the intercellular layer (Fig. 2a). Interestingly, BJH and MP analyses showed that HPC inherently comprised both mesopores (≈4 nm) and micropores (≈0.7 nm) on its surface (Fig. 2b, c); that is, ragged periodicity of pores every 4 nm was observed on the HPC surface (Fig. 2d). Thus, steam activation of PC lead to the deepening of the mesopores and micropores; this method differed from previous steam activation methods which generated just micropores (Fig. 3).26 We also determined that our HPC had an exceedingly superior adsorption capability for organic molecules in the liquid phase (ESI Fig. S6†). Using an aqueous solution containing acid green 25 dye, dyed molecules were found to adsorb completely to HPC. Interestingly, the amount of adsorption for HPC was approximately seven times as large as that for activated carbon at higher concentrations. Compared to the single-step adsorption observed for PC after HF treatment, HPC showed a two-step adsorption. By estimating the acid green 25 adsorption strengths for the initial adsorption step using linear plots of Langmuir isotherms (ESI Fig. S7†), we found the binding constant for AC, PC and HPC to be approximately 49,000, 64,000 and 160,000 M−1, respectively. Taken together, the strong interaction with acid green 25 and its carbon surface, as well as the unique meso- and macropore structure of HPC induced multi-layer adsorption at high concentrations.20
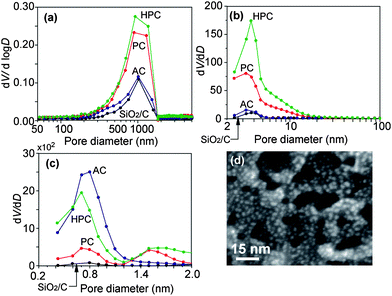 | ||
| Fig. 2 Pore distribution estimated by the mercury porosimetry (a), BJH method (b) and MP method (c). Silica-carbon composites were estimated before HF treatment (black) and after treatment with AC (blue), PC (red) and HPC (green). The SEM image (d) shows the periodically ragged surface of HPC. | ||
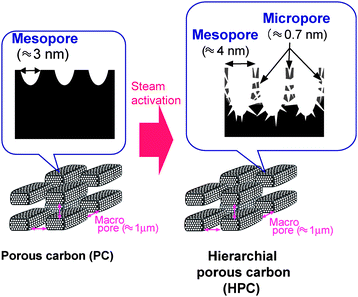 | ||
| Fig. 3 Fabrication scheme of deep mesopore on the surface of HPC by steam activation of PC with shallow mesopores. | ||
Examination of the oral safety and toxicity of new materials is required for use in health care. In this study, results of the oral safety and toxicity test on HPC showed no significant difference in weight change in normal rats administered either feed containing 5 wt% HPC powder (ca. 5 μm), activated carbon (AC) or no additive for 2 weeks (Fig. 4a). The stasis of the feed, AC and HPC in the gastrointestinal tract of these rats was confirmed by laparotomy after administration (Fig. 4b–d). Although more detailed examinations may be needed, HPC shows ultimately low toxicity. As low toxicity and superior adsorption of HPC are very attractive characteristics for medical adsorbents or drug delivery carriers,27–30 we also examined effectiveness of HPC as an adsorbent of uremic toxins in vivo in pneumonia renal failure model rats. High pressure liquid chromatography analysis of the plasma concentration of uremic toxin in these rats after 3 days of 5% HPC feed administration showed various peaks for uremic toxins that were remarkably decreased (ESI Fig. S8†). Moreover, administration of 5% HPC significantly decreased uric acid and creatinine plasma concentrations compared with that fed the control feed (no additives) (Fig. 5b, c). In particular, HPC more effectively decreased uric acid than AC. Although further studies are needed to optimize the HPC formulation for application as a medical adsorbent, we demonstrated for the first time in vivo exceedingly high safety and superior adsorption properties of HPC prepared by our template method.
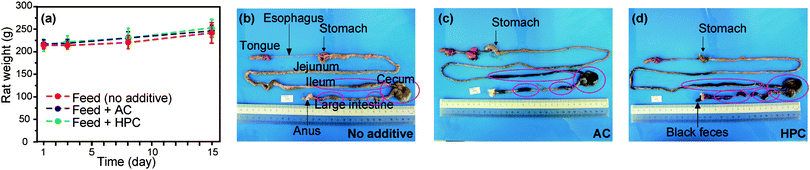 | ||
| Fig. 4 Change in body mass of rats administered feed mixtures containing 5% HPC and 5% AC, compared with feed with no additive (a). Photographs of the gastrointestinal tract by laparotomy after 2 week administration of non-additive (b), 5% AC (c), and 5% HPC (d) feed groups. Circled sections indicate the location of digested material. | ||
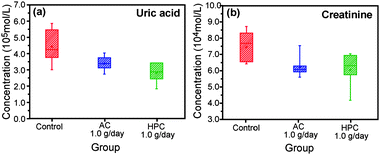 | ||
| Fig. 5 Effect of HPC on pneumonia renal failure in vivo. Plasma concentrations of uric acid and creatinine were obtained 48 h after administration of feed mixtures containing 5% HPC and 5% AC or no additive (control) to rats. | ||
In conclusion, our novel fabrication process using cell assemblies of rice husk could develop a hierarchical macro-meso-microporous carbon structure. Our HPC with its low-toxicity and superior in vivo properties can be applied to many significant applications not only in the electronic and industrial fields but also in the medical field for use as adsorbents, drug delivery and bio-interface materials, and scaffolds for cell incubation.
Notes and references
- A. A. Zakhidov, R. H. Baughman, Z. Iqbal, C. Cui, I. Khayrullin, S. O. Dantas, J. Marti and V. G. Ralchenko, Science, 1998, 282, 897 CrossRef CAS.
- S. Jun, S. H. Joo, R. Ryoo, M. Kruk, M. Jaroniec, Z. Liu, T. Ohsuna and O. Terasaki, J. Am. Chem. Soc., 2000, 122, 10712 CrossRef CAS.
- W. Fan, M. A. Snyder, S. Kumar, P.-S. Lee, W. C. Yoo, A. V. McCormick, R. L. Penn, A. Stein and M. Tsapatsis, Nat. Mater., 2008, 7, 984 CrossRef CAS.
- C. Liang, Z. Li and S. Dai, Angew. Chem., Int. Ed., 2008, 47, 3696 CrossRef CAS.
- A. Stein, Z. Wang and M. A. Fierke, Adv. Mater., 2009, 21, 265 CrossRef CAS.
- S.-W. Woo, K. Dokko, K. Sasajima, T. Takei and K. Kanamura, Chem. Commun., 2006, 4099 RSC.
- D.-W. Wang, F. Li, M. Liu, G. Q. Lu and H.-M. Cheng, Angew. Chem., Int. Ed., 2008, 47, 373 CrossRef CAS.
- M. Jaroniec, J. Choma, J. Gorka and A. Zawislak, Chem. Mater., 2008, 20, 1069 CrossRef CAS.
- Z. Wang, F. Li, N. S. Ergang and A. Stein, Chem. Mater., 2006, 18, 5543 CrossRef CAS.
- J. C. Groen, W. Zhu, S. Brouwer, S. J. Huynink, F. Kaptteijn, J. A. Moulijn and J. Perez-Ramirez, J. Am. Chem. Soc., 2007, 129, 355 CrossRef CAS.
- A. Stein, F. Li and N. R. Denny, Chem. Mater., 2008, 20, 649 CrossRef CAS.
- M. Choi, H. S. Cho, R. Srivastava, C. Venkatesan, D.-H. Choi and R. Ryoo, Nat. Mater., 2006, 5, 718 CrossRef CAS.
- R. Srivastava, M. Choi and R. Ryoo, Chem. Commun., 2006, 4489 RSC.
- E. S. Toberer and R. Seshadri, Adv. Mater., 2005, 17, 2244 CrossRef CAS.
- F. Iskandar, A. B. D. Nandiyanto, K. M. Yun, C. J. Hogan, K. Okuyama and P. Biswas, Adv. Mater., 2007, 19, 1408 CrossRef CAS.
- J.-S. Yu, S. Kang, S. B. Yoon and G. Chai, J. Am. Chem. Soc., 2002, 124, 9382 CrossRef CAS.
- S. Tabata, Y. Isshiki and M. Watanabe, J. Electrochem. Soc., 2008, 155, K42 CrossRef CAS.
- I. Moriguchi, F. Nakahara, H. Yamada and T. Kudo, Electrochem. Solid-State Lett., 2004, 7, A221 CrossRef CAS.
- V. L. Budarin, J. H. Clark, R. Luque and D. J. Macquarrie, Chem. Commun., 2007, 634 RSC.
- K. Ariga, A. Vinu, M. Miyahara, J. P. Hill and T. Mori, J. Am. Chem. Soc., 2007, 129, 11022 CrossRef CAS.
- K. Ariga, A. Vinu, Q. Ji, O. Ohmori, J. P. Hill, S. Acharya, J. Koike and S. Shiratori, Angew. Chem., Int. Ed., 2008, 47, 7254 CrossRef CAS.
- T.-W. Kim, P.-W. Chung, I. I. Slowing, M. Tsunoda, E. S. Yeung and V.-S.-Y. Lin, Nano Lett., 2008, 8, 3724 CrossRef CAS.
- J. F. Ma and E. Takahashi, Soil, Fertilizer, and Plant Silicon Research in Japan, 1st edn, Elsevier, Oxford, Amsterdam, 2002. pp 5–180 Search PubMed.
- H. A. Currie and C. C. Perry, Annals of Botany, 2007, 1 Search PubMed.
- J. F. Ma, K. Tamai, N. Yamaji, N. Mitani, S. Konishi, M. Katsuhara, M. Ishiquro, Y. Murata and M. Yano, Nature, 2006, 440, 688 CrossRef CAS.
- H. Marsh and F. Rodriguez-Reinoso, Activated Carbon, 1st edn, Elsevier, Oxford, 2006. pp 243–365 Search PubMed.
- H. Yatzidis, Nephron, 1964, 1, 310 CrossRef.
- R. E. Sparks, N. S. Mason, P. M. Meier, M. H. Litt and O. Lindan, Trans. Am. Soc. Artif. Int. Organs, 1971, 17, 229 Search PubMed.
- T. Miyazaki, I. Aoyama, M. Ise, H. Seo and T. Niwa, Nephrol., Dial., Transplant., 2000, 15, 1773 CrossRef CAS.
- M. Melillo, V. M. Gun'ko, S. R. Tennison, L. I. Mikhalovska, G. J. Phillips, J. G. Davies, A. W. Lloyd, O. P. Kozynchenko, D. J. Malik, M. Streat and S. V. Mikhalovsky, Langmuir, 2004, 20, 2837 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The experimental procedure and materials characterization data. See DOI: 10.1039/c0md00011f |
| This journal is © The Royal Society of Chemistry 2010 |
