An automated microdroplet passive pumping platform for high-speed and packeted microfluidic flow applications†
Pedro J.
Resto
a,
Brian J.
Mogen
b,
Erwin
Berthier
b and
Justin C.
Williams
*b
aMaterials Science Program, University of Wisconsin, Madison, WI 53706, USA. E-mail: presto@wisc.edu
bDepartment of Biomedical Engineering, University of Wisconsin, Madison, WI 53706, USA. E-mail: jwilliams@cae.wisc.edu
First published on 30th October 2009
Abstract
Surface tension driven passive pumping is a microfluidic technology that uses the surface tension present in small droplets to generate flow. To enhance the potential of this type of passive pumping, a new ‘micro passive pumping’ technique has been developed that allows for high throughput fluidic delivery by combining passive pumping with a small droplet-based fluidic ejection system. Flow rates of up to four milliliters per minute (mL/min) were achieved that are solely limited by the channel geometry and droplet size. Fluid exchange rates can be performed within tens of milliseconds (ms) by delivering fluids from multiple nozzles. The technique can be extended to a multitude of platforms, as channels are not pressurized and therefore do not require bonding to a substrate. This technique provides a novel flow control for high-speed and packeted flow applications without requiring external tubing connections or substrate bonding.
Introduction
Surface tension driven passive pumping is an emerging method for delivering fluid in microfluidic devices that relies on surface tension as the driving force for flow.1 Theoretically, the smaller the drop size, the higher the internal pressure.2 We investigate whether small droplet (0.05–0.5 mm radius) surface tension pumping can be used to achieve high microfluidic flow rates and fast intra-channel fluidic exchange times. This type of passive pumping eliminates the need for expensive external equipment or complex internal structures to move or control flow. No tubing is connected to the device so the system can remain independent and dead volumes in delivery systems are reduced. However, passive pumping is limited by the capabilities of the user to deliver fluid to the inlet of a microfluidic device. Typically one would manually pipette fluid drops to the inlet, however recent advances in robotic technology have allowed for precise high-throughput passive pumping for cell culture applications.3We propose extending the functionality of passive pumping by using an automated fluid delivery system using a set of voltage-controlled micro-nozzles to deliver small volume fluid droplets to the inlet of a microfluidic device in a high frequency manner. This provides an accurate and precise method for high-speed droplet delivery. By using passive pumping in conjunction with the delivery system, we are able to demonstrate how this technique achieves high flow rates and low exchange times.
Theory
Passive pumping uses the surface energy of a small drop of liquid to pump fluid through a microchannel.1 The Laplace law explains how the spherical profile of a drop results in a change in pressure between its interior and exterior. This change in pressure, ΔP = 2γ/Rd, between the inside and outside of a drop is inversely proportional to the drop radius, Rd, where γ is the surface tension of the fluid. As a result a smaller drop possesses a higher internal pressure than a larger drop; thus, when two drops are connected via a microfluidic channel, fluid flows from the small drop to the large drop.1 For a given inlet diameter, Di, a drop that creates a 90° contact angle with the inlet will result in the maximum inlet pressure2 (see Fig. 3 for inlet drop profile), and subsequently maximize the velocity and flow rate in the channel. The pressure drop in the channel can be written as a function of the flow rate, Q, and the fluidic resistance, as shown in eqn (1):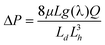 | (1) |
Using eqn (1), under the assumption of the 90° contact angle criteria (i.e. Rd = ½ Di), Figs. 1(a) and 1(b) show the average channel velocity for different channel widths and heights (at a constant length). Fig. 1(a) shows the case where the inlet width changes proportionally to the channel width. Fig. 1(b) shows the case where the inlet width is constant at 1 mm diameter. In Fig. 1(a), fluid flow through the channel is maximized when the channel has a square cross-sectional area. The consequent decrease in velocity with increasing channel width is caused by a decrease in inlet pressure due to a larger drop at the inlet (i.e. drop diameter = channel width). In Fig. 1(b), as the width and/or height get larger, so does velocity, without the progressive drop in velocity seen in Fig. 1(a). This is because the inlet drop remains constant while the channel offers less fluidic resistance. Therefore, from a theoretical standpoint, to achieve high velocities one must balance a high inlet pressure with a low channel resistance.
 | ||
| Fig. 1 Intrachannel fluid velocities when (a) the inlet diameter is equal to the channel width and (b) the inlet diameter is constant at 1 mm. | ||
Materials and methods
A straight, single-channel, microfluidic device was created via conventional soft-lithography techniques using poly(dimethylsiloxane) (PDMS) cast over a silicon master.4 The channel dimensions used in this study are such that the inlet diameter, Di, is nearly equal to the channel width. The outlet diameter is approximately twice that of the inlet, providing for a large pressure difference.One of the main advantages of passive pumping is that the PDMS device may be reversibly attached to a glass slide and not permanently bonded. A reversible attachment allows the device to be re-used multiple times. The method can also be used with permanently bonded devices, although it is not required.3 Upon placing the PDMS on the glass slide, the device is filled with liquid and air bubbles are removed, as bubbles interfere with flow patterns.
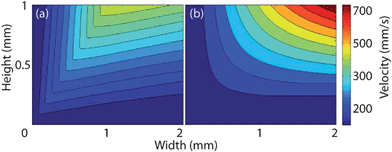
A small droplet delivery system was developed using The Lee Company's microdispending electro-fluidic VHS kit (The Lee Company, Westbrook, CT). By using a LabView interface and a microcontroller, a programmable sequence of volumetrically precise fluidic drops can be ejected to the inlet. Multiple valves may be aimed at the inlet and used to form a variety of alternating flow patterns (Fig. 2(a) and 2(b)). The volume dispensed by a nozzle in one pulse is a linear function of its open time and its backpressure; see Fig. 2 (inset).
 | ||
| Fig. 2 (a) Schematic of two nozzles aimed at inlet. Insert: Delivered volume as a function of open time at different pressures. (b) Top view picture of two nozzles aimed at inlet and a drop placed at the outlet. Device dimensions are 2.2 mm, 7.5 mm, 250 µm (width, length, height). | ||
 | ||
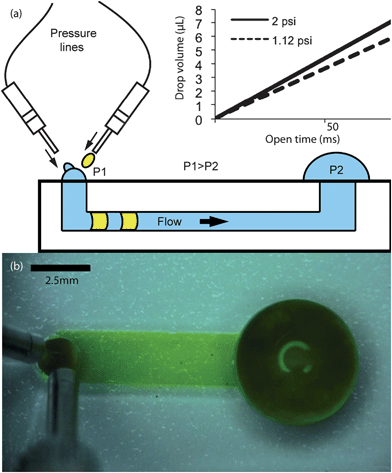
| Fig. 3 Upper left: Average fluid velocity inside a channel as a function of time as caused by the placement of a single drop, manually or otherwise, at the channel inlet. Bottom left: schematic of drop collapse. (a)–(h) Drop collapse time steps corresponding to the schematic. | ||
Magnetic micromanipulators (Bioscience Tools, San Diego, CA) were used to hold and fix the nozzles in position relative to the inlet. Hydrostatic pressure was used to provide a pressure head to drive the nozzles, although any means of pressurization can be used (i.e. compressed gas). All imaging was done with a high-speed, high-definition video camera (Sony Corp. New York, NY, HDR-SR11).
Results and discussion
As soon as a drop is placed at the inlet it begins to collapse. The fluid velocity inside the channel is not constant (Fig. 3) due to the changing droplet radius during collapse. Theoretically, to achieve the fastest flow, one would like to keep the droplet contact angle as close to 90° as possible. Practically, one must allow the inlet drop to collapse a volume of at least the volume of the next incoming refill drop. If the incoming refill drop is greater than the volume that has collapsed, the inlet drop (at the time the refill arrives and the two drops coalesce) will have a contact angle greater than 90°. Once the inlet drop has a greater contact angle than 90°, it will have a reduced pressure, will collapse slower and subsequent refill drops will cause the inlet to continue to increase in volume and eventually overflow.Practically it is convenient to deliver drops such that the contact angle fluctuates between approximately 30° and 60° to avoid the problems inherent with trying to maintain a drop at 90°. Because this delivery method is technically pulsatile in nature, it will produce a pseudo-continuous flow inside the channel. By controlling the pulse frequency and the open time the user can control the total flow rate out of a nozzle, thus controlling the volumetric flow rate through the microfluidic device.
Fig. 3 presents the behavior of a 1.5 µL (0.9 mm radius) drop collapsing. The peak of the velocity curve in Fig. 3 corresponds to a drop contact angle of 90° as calculated by theory.2 The schematic and image in Fig. 3 (a)–(h) show drop collapse as observed in the experimental device.
It should be noted that in the later time steps in Fig. 3 a slight rebound of the inlet drop occurs, suggesting the existence of some inertial relationship between momentum caused by flow inside the channel and surface tension of the fluid. At equilibrium, these forces must balance after drop collapse occurs. The same phenomenon can be observed within the channel by using a suspension of red 10 µm polystyrene microspheres (Invitrogen, Carlsbad, CA). The resurgence of the inlet drop shows up as a very small backflow of the fluid in the channel (on Fig. 4(a)–(d)). Observe that the fluorescent beads initially flow towards the outlet, and then suffer a small backflow towards the inlet.
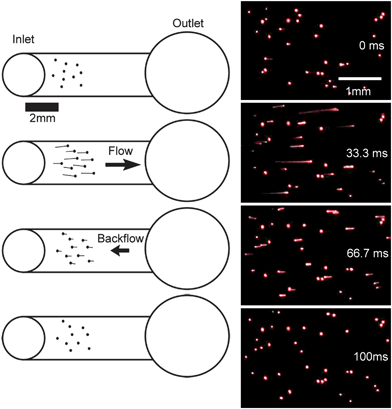 | ||
| Fig. 4 Red fluorescent polystyrene microspheres in channel. While some beads are adhered to the PDMS, most flow towards the outlet and then undergo a small backflow towards the inlet. The tail produced by the fluorescent beads shows the direction of flow. | ||
It is likely that the added momentum inherent in the creation of microdrops causes the inlet drop to overshoot its natural equilibrium point causing a rebound of the surface and a resulting backflow in the channel. Although a similar phenomenon has been previously explored, it can be seen in Fig. 3 and 4 that the time scale in this rebound lies in the tens of milliseconds, orders of magnitude less than in previous studies.5 This suggests a very dynamic interaction likely caused by the high velocity of an incoming inlet drop. Backflow may be unavoidable as the creation of small droplets requires high velocity ejection of fluids into the inlet. Further studies are needed to investigate this phenomenon.
With the general experimental parameters used in this study, flow rates of four milliliters per minute can easily be achieved. Compare this to a flow rate of 30 microliters per minute when manually placing a 1.5 µL drop at the inlet every 3 seconds. Higher flow rates can be achieved with more traditional pumping methods, but these require permanent bonding of the device to a surface. Further experimentation will explore the limits of high speed flow as a function of device geometry and delivery frequency. The drop size shown in this study illustrates a fair comparison to the smallest drop size that can be pipetted manually, however the system is capable of even smaller drops.
Fluidic exchange
For many biological applications, the fluidic exchange of the intra-channel media is important, particularly for drug studies and development. Fluid exchange may take place as a complete substitution of the channel medium, or as a change in concentration from one fluid to another. Using multiple nozzles we are able to introduce different fluids into the device in rapid succession (Fig. 5). It was observed that these packets follow laminar behavior as expected. However, the exact profile of the packets is dependent on nozzle control and position; notice the fluid flow is compressed to the opposite side of the channel in Fig. 5(c)–(f). This velocity profile is likely due to refill drops being applied at an angle to the inlet rather than directly from the top. In general this phenomenon is highly dependent on nozzle placement and continued experimentation will explore the flow profile behavior as a function of nozzle positioning. | ||
| Fig. 5 Fluidic exchange inside a channel taken by slow motion recording with a high speed camera. | ||
As would be expected with laminar flow, it was observed that fluid moves quickly through the center of the device leaving a residual volume near the walls. The efficiency of fluidic exchange in a microfluidic device is a function of the inlet drop volume to channel volume ratio.6 The larger the channel cross-sectional area to inlet port size ratio, the more residual space will be at the walls and the longer it will take to complete total fluidic exchange.
Under the experimental channel dimensions used in this study, a volume of approximately 8 µL is needed to evacuate the entire channel, including the inlet and outlet ports and the volume of the liquid spherical cap present at the inlet. Within these parameters, it takes approximately 60 ms for an 8 µL drop to collapse and reach the outlet, as can be seen in Fig. 5. It has been shown that 5 channel volumes will effectively flush 95% of the standing volume inside a channel using passive pumping.6 We propose that when using high frequency passive pumping efficient washing can be achieved in a fraction of the time required for manual wash due to the high frequency of inlet drop placement in an automated system. The highest drop frequency we achieved in this study while maintaining pseudo-continuous flow was eight milliseconds between alternating drops.
Conclusions
A novel technique has been created by using passive pumping with controlled micro droplet delivery. We have shown that this new ‘micro passive pumping’ technique is capable of high flow rates and fast channel exchange times, both of which are largely limited by device dimensions. Experimentally, flow rates of up to 4 milliliters per minute were achieved without any external tubing or bonding. Alternating drops were delivered as fast as 8 milliseconds, with the experimental device geometries used in this study. In the course of experimentation it was observed that the incoming droplets carry momentum that assists in the collapse of the inlet drop and reduces the time scale of channel backflow. The momentum–surface tension interaction is important because it results in instantaneous fluid flow stop within the channel. An instantaneous stop may help in the compartmentalization of fluids within the channel, which may be advantageous to biological experiments using passive pumping. By experimentally exploring the limits of ‘micro passive pumping’ flow as a function of device geometry and refill dynamics, we aim to extend the functionality of this new technique to suit many biological applications.Notes and references
- G. M. Walker and D. J. Beebe, A passive pumping method for microfluidic devices, Lab Chip, 2002, 2, 131–134 RSC.
- E. Berthier and D. J. Beebe, Flow rate analysis of a surface tension driven passive micropump, Lab Chip, 2007, 7, 1475–1478 RSC.
- I. Meyvantsson, J. W. Warrick, S. Hayes, A. Skoin and D. J. Beebe, Automated cell culture in high density tubeless microfluidic device arrays, Lab Chip, 2008, 8, 717–724 RSC.
- J. C. McDonald and G. M. Whitesides, Poly(dimethylsiloxane) as a Material for Fabricating Microfluidic Devices, Acc. Chem. Res., 2002, 35, 491–499 CrossRef CAS.
- J. Ju, J. Y. Park, K. C. Kim, H. Kim, E. Berthier, D. J. Beebe and S. H. Lee, Backward flow in a surface tension driven micropump, J. Micromech. Microeng., 2008, 18.
- J. Warrick, I. Meyvantsson, J. Ju and D. J. Beebe, High-throughput microfluidics: improved sample treatment and washing over standard wells, Lab Chip, 2007, 7, 316–321 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Two movie files. See DOI: 10.1039/b917147a |
| This journal is © The Royal Society of Chemistry 2010 |
