Structural and magnetic study of the annealing of Fe–Co nanoparticles
Céline
Desvaux
b,
Pierre
Lecante
c,
Marc
Respaud
d and
Bruno
Chaudret
*a
aLaboratoire de Chimie de Coordination, 205 route de Narbonne, 31077, Toulouse Cedex 04, France. E-mail: Chaudret@lcc-toulouse.fr
bIMI-NRC-CNRC, 75, Boulevard de Mortagne, Boucherville, Québec, Canada J4B 6Y4
cCEMES, 29, rue Jeanne Marvig, BP 94347, 31055, Toulouse Cedex 4, France
dLaboratoire de Physique et de Chimie des Nano-Objets, INSA, Département de Génie Physique, 135 avenue de Rangueil, 31077, Toulouse Cedex 4, France
First published on 6th November 2009
Abstract
Post-synthesis thermal treatments are often used to induce the crystallization of amorphous nanoparticles obtained by wet chemical synthesis in order for them to display the physical properties of the bulk. We have studied the structural and magnetic evolution of FeCo magnetic nanoparticles synthesized via an organometallic route during a soft controlled annealing treatment. Despite very mild conditions, the structural study, carried out using WAXS, EXAFS and Mössbauer spectroscopy, evidences the presence of carbon atoms in the as-synthesized structure and their continuous insertion in the structure upon heating, as they form a rather ordered structure including carbides, before being expelled from the particles as the crystallization of the bcc alloyed phase takes place at higher annealing temperatures. The magnetic properties, recorded along the process, accordingly show highly depleted values as the carbon atoms insert in the structure, and reach the Ms bulk value as the bcc phase is restored.
Introduction
The synthesis of nanoparticles by a chemical process in solution has led to many spectacular developments during the past few years. However, one drawback of this process may be the formation of poorly crystalline particles which directly results in a depletion of their physical properties.1–4 Magnetic nanoparticles, for example, may need a soft post-synthesis thermal treatment in order to display the magnetic properties of the bulk material.5,6 This kind of process is often used in the literature and leads, when proper conditions are applied, to nanoparticles displaying bulk magnetic properties without any sintering.7–10 The two ends of this process are well characterized,11,12 and some work has been done on the effect of high temperature annealing of FeCo xerogels and aerogels,13–15 but the structural changes within nanoparticles during a soft thermal treatment have not yet been accurately experimentally investigated.In a previous work we have reported the synthesis of perfectly monodisperse FeCo nanoparticles included into millimeter long super-lattices from the co-decomposition of two organometallic precursors in the presence of a long chain amine (hexadecylamine) and acids (oleic acid, stearic acid) as stabilizing agents.7 Tuning of the material's composition was achieved by varying the organometallic precursors ratio. For the needs of the application it was set to Fe60Co40 (2/1 ratio for Fe/Co precursors). At the end of the synthesis, the 15 nm spherical particles obtained displayed a disordered atomic structure evidenced by a broad ring on the Fourier transform of the Transmission Electron Microscopy image. Moreover, a Parallel Electron Energy Loss Spectroscopy (PEELS) analysis showed that within the particles, metals were not truly alloyed, but displayed an onion-like structure consisting in a cobalt core surrounded by an iron shell, itself surrounded by a cobalt shell. This segregated organization was attributed to the mechanism of formation of the particles and, more precisely, to the relative rates of decomposition of the organometallic precursors. An annealing process was reported in a preliminary form, the result of which was a significant improvement of the magnetic properties. Furthermore, the resulting particles exhibited the expected bcc structure with fine mixing of Fe and Co. At the end of the thermal treatment, the particles were surrounded by a 2 nm carbon coating, protecting them from ambient oxidation (Fig. 1).
 | ||
| Fig. 1 HRTEM micrographs of one particle before (left) and after (right) annealing. | ||
In this paper, we study the structural evolution of these FeCo nanoparticles synthesized using an organometallic approach during a controlled annealing process. A dissolution of carbon into the FeCo lattice is first evidenced followed, as the temperature rises, by the segregation of carbon concomitant with the formation of nanoparticles of FeCo alloy adopting the bcc structure. The particles structure is investigated using Wide Angle X-ray Scattering, EXAFS and Mössbauer spectroscopies, and the impact of the structure of the nanoparticles on the magnetic properties is described.
Results and discussion
In order to re-distribute the atomic species within each FeCo particle to form the true alloy and thereby optimize the magnetic properties, a controlled thermal treatment was carried out on the particles. A preliminary thermogravimetry analysis showed that the free ligands (oleic acid, stearic acid and hexadecylamine) totally decompose around 350 °C under a flow of Ar. The thermal treatment hence included different temperature ramps: a fast one (10 °C min−1) up to 300 °C, then a slower one (5 °C min−1) up to 500 °C. A 30 min plateau was finally imposed at 500 °C. The whole process was carried out under a static inert atmosphere of Ar. In a typical experiment, the particles were placed in a ceramic plate in the glove box and introduced in a quartz tube. Once tightly closed, the tube was transferred into a furnace to undergo the thermal treatment. At the end of the plateau, the particles were cooled down naturally in the tube. They were then collected in the glove box and analyzed. A Differential Scanning Calorimetry (DSC) analysis was carried out on the particles using the same temperature ramps as the ones used for the thermal treatment (Fig. 2).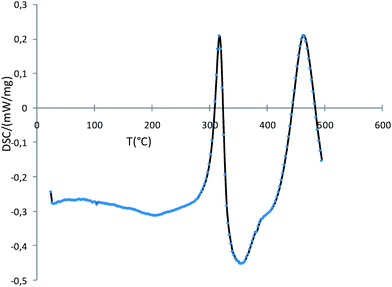 | ||
| Fig. 2 DSC curve showing 2 exothermic peaks corresponding to distinct crystalline phases. | ||
The obtained curves display two well defined exothermic peaks showing that two distinct crystalline phases form during the thermal treatment. The first one appears around 300 °C and collapses when the second one forms at 450 °C. This second peak should correspond to the formation of the bcc phase of the bulk alloy but the first one was unexpected.
From these elements, several intermediate samples were prepared. 150 °C being the synthesis temperature, the lowest annealing temperature was set to 200 °C (Sample 1). Subsequent samples were prepared by quenching the reaction each 50 °C during the thermal treatment up to 500 °C.
In order to structurally investigate these successive phases, X-ray scattering analyses were carried out on each sample, using a diffractometer dedicated to the study of less ordered materials, characterized by a low background and conditions allowing for Fourier transform (Mo wavelength, extended angular range, accurate corrections) (Fig. 3).
 | ||
| Fig. 3 a) Diffractograms of the particles before annealing (b.a.) and annealed at different temperatures, and b) related RDFs. | ||
The study of the diffractogram before annealing and of the related Radial Distribution Function (RDF), reveals a well defined pattern however lacking the features of any compact structure: the coherence length observed on the RDF doesn't exceed 2 nm, much less than the 15 nm value obtained by TEM. Such discrepancy points to a very amorphous organization of the metals. The observation at the different annealing temperatures further reveals several events happening throughout the thermal treatment. The first of these events, occurring at a temperature as low as 200 °C, is a dramatic decrease of all structural features, indicating extensive disorder in the sample. In the 250 °C–300 °C range, several sharp peaks appear and indicate the onset of an intermediate order, in agreement with the first exothermic peak on the DSC. These peaks do not belong to the bcc structure but are in agreement with the diffraction patterns of different carbides (e.g. Fe7C3, Co3C1 see Fig. 4a).
 | ||
| Fig. 4 a) Diffractograms of the particles at 250 °C and after total annealing, compared to data from bulk FeCo and Fe and Co carbides, and b) detail of the RDF: left and right bars point to the M–M distances in bulk FeCo. | ||
This intermediate phase can be observed up to 350 °C. A second collapse takes place around 400 °C. At 450 °C, the bcc pattern appears, in coincidence with the DSC second exothermic peak. At the end of the thermal treatment, the particles are highly crystalline and the sharp peaks perfectly index on the bcc structure of the bulk alloy. The size of the crystalline domains is likely much closer to the values obtained by TEM. It is however not possible to extract valid size measurements from the related RDFs because of the limited spatial resolution of the diffractometer used for measurements. The total damping of RDFs observed above 4.5 nm only indicates that crystallites real size is larger.
These drastic changes of the chemical order in the material can be better observed on the enlargement of the RDF focussed on the peak centered on 0.26 nm, only related to the M–M bonding distances (Fig. 4b). For easier comparison, a scale factor has been applied to make all curves of similar amplitude. From 200 °C until 350 °C, all peaks are very close in shape, and actually very close to the peak before annealing, which indicates a very stable short range order. From 400 °C to 500 °C, we observe the onset of the typical bcc structure characterized by well separated short and long bonding distances (0.2474 nm and 0.2857 nm). The short and long distances observed on the distribution after total annealing are very close to the values expected for the bcc bulk FeCo alloy. On the contrary, for temperatures lower than 400 °C the M–M distances are much less dispersed and a single symmetric pointing to 0.260 nm is observed.
Since WAXS studies can only provide information on the average structure, EXAFS investigations were carried out to more accurately define the relative arrangement of iron, cobalt and hypothetical carbon atoms. Measurements could be performed on the non-annealed samples at both Fe and Co K edges on the X1 beamline of Hasylab (Hamburg, Germany) at room temperature in transmission mode together with reference Fe and Co foils (Fig. 5).
 | ||
| Fig. 5 EXAFS functions of the particles before annealing at Fe and Co K absorption edge (upper and lower, respectively; right: chi function, left: related Fourier Transform) – solid line: sample, dotted line: fit on first peak, dashed line: reference foil (divided by 4 or 5, respectively, for comparison). | ||
At both edges, the Fourier Transforms are very similar in shape and amplitude. Compared to the reference foils, they are characterized by a strong damping, consistent with high static disorder, and also a shoulder on the left of the main peak pointing to a short bonding distance. A least-square refinement procedure was attempted on the first peak. A model including only metallic distances did not lead to an appropriate fit, but a good agreement was obtained using a very simple model including only two shells (Fe or Co, and C). Because of the strong correlations between the amplitude parameters, only one term of disorder was refined for both shells. Similarly, the same E0 term was used for the edge energy adjustment for both shells. Close R factors were obtained (0.002 and 0.003 for Fe and Co respectively). Because of the very strong correlations, a reliable value couldn't be obtained for metal coordination, however the numbers of C atoms in the coordination sphere of both metals are significant and very close (N Fe–C/N Fe–Fe = 0.69, N Co–C/N Co–Co = 0.67). The bonding distances are also close for both edges, if not identical (dFe–C = 0.196 nm, dFe–Fe = 0.254 nm, dCo–C = 0.191 nm, dCo–Co = 0.256 nm). All these elements point to a nearly identical, very simple, environment for Fe and Co atoms, with the lack of the longer distance characteristic of the bcc structure. This arrangement is highly disordered and includes a significant amount of C atoms. Such a description is in agreement with the amorphous structure observed by WAXS and is also confirmed by Mössbauer spectroscopy which was performed using a 57Co source in a Rh matrix calibrating against bulk α-Fe.
The general shape of the spectrum collected at 80 K (Fig. 6a) is indeed similar to amorphous Fe with some C inserted in the material (94.3%, δ = 0.33 mm s−1, ΔQ = 0 mm s−1). Two minor contributions are related to some Fe oxide impurities (3%, δ = 0.35 mm s−1, ΔQ = 0 mm s−1, Hhyp = 485.5 kG) and paramagnetic ions (2.7%, δ = 0.85 mm s−1, ΔQ = 1.7 mm s−1). When performed on the fully annealed sample the expected spectrum for a metallic disordered bcc FeCo material is obtained (Fig. 6b). The hyperfine field distribution and isomer shift match the one measured by M. F. Casula et al.13
 | ||
| Fig. 6 Mossbauer spectrum, fit and hyperfine field of a) FeCo particles before annealing; b) FeCo particles after annealing. | ||
Clearly, the ordering process is more complex than the simple migration of the metallic species. Instead, this structural study shows that the disordered segregated particles obtained at the end of the chemical synthesis undergo two main processes during the thermal treatment: a first crystallization process around 250 °C yielding a stable structure made of, or containing, metal carbides which is then replaced by the bcc bulk phase from which carbon is expelled as the annealing temperature increases.
For each annealing temperature, the magnetic properties of the samples were recorded on a SQuID magnetometer. The evolution of the saturation magnetization (Ms) and of the coercive field (Hc) were studied and compared to the structural data. The results of the magnetic study are in agreement with the conclusions of the structural study (Fig. 7).
 | ||
| Fig. 7 Evolution of the magnetic properties at 2 K during the annealing process: a) evolution of the saturation magnetization with the annealing temperature; b) evolution of the saturation magnetization with the annealing time. | ||
The particles were all found to be ferromagnetic at room temperature, regardless of the annealing temperature. Their magnetization at saturation, Ms, varies however a lot during the thermal treatment. At the end of the synthesis the observed magnetization Ms is quite low compared to that expected for the bulk material. This can be explained by the atomic disorder and the segregated structure of the particles as well as by the presence of C atoms in the structure leading to a decrease of the electronic density around the magnetic species and hence to a low Ms. This is in agreement with WAXS, EXAFS and Mössbauer spectroscopy. For annealing temperatures between 200 °C and 350 °C Ms drops even more down to 110 A m2 kgFeCo−1. This is consistent with the insertion of more carbon atoms into the structure leading to an increase of the M–C/M–M coordination ratio or to the formation of a new phase displaying a very low magnetization. At 350 °C, Ms is minimal as the intermediate phase including carbides is formed. After 400 °C, the process of crystallization into the bcc alloyed phase takes place and most of the carbon atoms are expelled off the particle which results in a sudden rise of Ms which reaches 90% of the bulk magnetization (MsbulkFe60Co40 = 235 A m2 kgFeCo−1).16 When this treatment is carried on for very long periods of time, the saturation magnetization leans towards 100% of the bulk value, showing that only very small structural changes then occur in the particles that also lean to the perfect bcc alloyed structure as the remaining carbon atoms are gradually removed. We studied the evolution of the value of the coercive field during the thermal treatment as a marker of the anisotropy of the system. At 2 K, the coercive field of the particles is found in each case to be very low (<50 mT), as expected for this soft alloy composition and the small size of the particles. Before the thermal treatment, a very low coercive field of 9 mT is recorded. A maximum value of 45.2 mT is obtained for an annealing temperature of 250 °C when the intermediate phase containing the carbides is observed, hence suggesting, in accordance with the WAXS study, the occurrence of a well define phase. The coercive field then decreases continuously and reaches Hc = 18 mT at the end of the thermal treatment, with the formation of the soft bcc FeCo alloy. The evolution of Hc with the annealing temperature follows well the successive phase transformations occurring inside the sample. At the end, the value of Hc for the ordered FeCo alloy remains higher than the expected one considering the bulk magnetocrystalline anisotropy, which could be related to the complex contribution of the dipolar magnetic contribution.
All the characterizations point to the formation of an intermediate stable structure involving carbides, at least in part responsible for the depletion of the magnetic properties of the particles. Insertion of carbon into metal nanoparticles and formation of carbides has precedents in the literature. Indeed, Suslick et al. have shown that sonication of carbonyl complexes leads to the formation of nanoparticles containing carbides in the presence of hydrocarbons.17 Nikitenko et al. also used sonication followed by annealing to synthesize Fe3C coated Fe particles and demonstrated that as the annealing temperature increases, the saturation magnetization increases too, which they attributed to the lowering of the amount of Fe3C in the structure.18 The formation of carbides in metallic structures has also been taken advantage of for the synthesis of steel19 and is believed to be one of the formation mechanisms of carbon nanotubes.20,21 Even if the growth mechanism of the CNTs is not fully understood, various mechanisms have been suggested based on the VLS (Vapor–Liquid–Solid) theory. In this theory, described by Kuznetsov in ref. 21 and references herein, carbon from a source such as hydrocarbons or carbon monoxide is dissolved into catalyst particles (iron) in the liquid (molten) state and precipitates as nanotubes when the metal carbide particle is oversaturated with carbon atoms. In this case, several studies have proven the presence of intermediate Fe–C within the particles and the expulsion of the carbon atoms via precipitation leading to the growth of the CNTs.
In our case, the initial carbon source has to be either CO as in the case of Suslick but in milder conditions or the stabilizing long chain ligands present during the synthesis and surrounding each particle or both. If CO is involved, this process occurs through CO dissociation to form carbides and oxides which could be reduced by the atmosphere of dihydrogen in the synthesis process to form water. This process is likely to explain the presence of the carbon in the initially formed nanoparticles, before annealing. However, if it is necessary to explain the structural and magnetic evolution of the particles with temperature by an increase in their carbon content, CO cannot be the only carbon source. It is then necessary to invoke the dissolution of carbon arising from the ligands. Although this may look surprising, this process is not impossible since Fe, Co and the FeCo alloys are all Fischer–Tropsch catalysts in these conditions which implies the formation but also the dissociation of alkyl chains which in the present case could be provided by the amine and acid ligands. The unusually low temperatures (150 °C) at which these phenomena occur could be explained by the formation of a crystalline phase which could be the driving force of the process. The insertion of carbon could then be thermodynamically favored by the formation of a stable phase. When the annealing temperature is high enough to allow alloy formation and crystallization in the bcc phase, alloy formation becomes the driving force towards a more stable configuration and carbon is expelled from the particles.
Conclusion
In conclusion, we have reported here the first structural and magnetic study of the thermal treatment of FeCo nanoparticles synthesized via a solution chemical route. This study shows that the initially obtained and poorly crystalline particles recrystallize upon heating into the bcc structure of the bulk alloy, which results in the optimization of their magnetic properties. The structural study at different steps of the thermal treatment shows that another phenomenon occurs, namely the insertion and desorption of carbon atoms in and from the particles. The insertion of carbon atoms occurs at very low temperatures in solution and possibly goes on during the annealing process and is probably driven by the formation of a crystalline phase including metal carbide species within the structure. This phase then disappears during the crystallization process of the particles into the bulk alloy structure upon heating, process during which carbon is expelled from the metallic core to form a carbon shell surrounding each annealed particle. The formation of carbides in mild conditions is unexpected and strongly suggests the occurrence of similar processes in other systems where synthesis conditions involve relatively high temperatures. Finally, the result of this process is the coating of FeCo nanoparticles displaying bulk magnetization and a graphene type carbon layer rendering them air-stable and therefore usable for various applications.Experimental section
Nanoparticle synthesis
The synthesis is reported elsewhere.7 Briefly, 2 mmol of Fe(CO)5 and 1 mmol of [Co(η3-C8H13)(η4-C8H12)] were dissolved in toluene in the presence of stabilizing agents (typically a mixture of oleic acid and hexadecylamine) and placed at 150 °C under 3 bars of H2 for 48 h. At the end of the reaction, the supernatant is evacuated and the nanoparticles are dried under vacuum and collected in the glove box.Annealing procedure
Furnace: Nabertherm RS 80/300/11. The whole process was carried out under a static inert atmosphere of Ar. In a typical experiment, the particles were placed in a ceramic plate in the glove box and introduced in a quartz tube. Once tightly closed, the tube was transferred into a furnace to undergo the thermal treatment. At the end of the plateau, the particles were cooled down naturally in the tube. They were then collected in the glove box.WAXS measurements
Data collection was performed on small amounts of the fine powder sealed in 1 mm diameter Lindemann glass capillaries. Measurements of the X-ray intensity scattered by the samples irradiated with graphite-monochromatized molybdenum Kα (λ = 0.071069 nm) radiation were performed using a dedicated two-axis diffractometer. Time for data collection was typically 20 h for a set of 457 measurements collected at room temperature in the range 0° < θ < 65° for equidistant s values (s = 4π(sin θ/λ)). The raw intensity was corrected for polarization and self-absorption corrections. Data were then reduced and Fourier transformed to allow for radial distribution function (RDF) analysis.EXAFS measurements
Fe and Co absorption spectra at K edges were measured on beamline X at Hasylab in Hamburg, Germany (http://www-hasylab.desy.de/index.htm). The samples were prepared as 5 mm large pellets sealed between Kapton foils to protect them from air oxidation. The measurements were done in transmission mode at room temperature using a double silicon monochromator set for diffraction from (111) planes. Treatment of the data was carried out via the Athena and Artemis programs (http://cars9.uchicago.edu/%7Eravel/software/).Mössbauer measurements
The samples are prepared in a glove box by compressing the powder into solid pellets and the spectra are recorded on a spectrometer composed of a Wissel transductor MR360 and a Wissel functions generator DFG1000. The source used is 25 mCi 57Co in a Rh matrix. The data are calibrated against bulk α-Fe.Acknowledgements
Portions of this research were carried out at the light source facilities DORIS III at HASYLAB/DESY. DESY is a member of the Helmholtz Association (HGF). We would like to thank E. Welter for assistance in using beamline X1.The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under grant agreement n° 226716.
References
- S. Zhou, K. Potzger, A. Mucklich, F. Eichhorn, M. Helm, W. Skorupa and J. Fassbender, Nucl. Instrum. Methods Phys. Res., Sect. B, 2008, 266, 589 CrossRef CAS
.
- H. W. Kim, S. H. Shim, J. W. Lee, C. Lee and S. C. Jeoung, Opt. Mater., 2008, 30, 1221 CrossRef CAS
.
- J. Carrey, M. L. Kahn, S. Sanchez, B. Chaudret and M. Respaud, Eur. Phys. J.: Appl. Phys., 2007, 40, 71 CrossRef CAS
.
- M. Wen, H. Qi, W. Zhao, J. Chen, L. Li and Q. Wu, Colloids Surf., A, 2008, 312, 73 CrossRef CAS
.
- S. Momose, H. Kodama, W. Yamagishi and T. Uzumaki, Jpn. J. Appl. Phys., 2007, 46, L1105 CrossRef CAS
.
- S. Yan, J. Yin and E. Zhou, J. Alloys Compd., 2008, 450, 417 CrossRef CAS
.
- C. Desvaux, C. Amiens, P. Fejes, P. Renaud, M. Respaud, P. Lecante, E. Snoeck and B. Chaudret, Nat. Mater., 2005, 4, 750 CrossRef CAS
.
- G. S. Chaubey, C. Barcena, N. Poudyal, C. Rong, J. Gao, S. Sun and J. P. Liu, J. Am. Chem. Soc., 2007, 129, 7214 CrossRef CAS
.
- M. Chen, J. P. Liu and S. Sun, J. Am. Chem. Soc., 2004, 126, 8394 CrossRef CAS
.
- C. Liu, X. Wu, T. Klemmer, N. Shukla, D. Weller, A. G. Roy, M. Tanase and D. Laughlin, Chem. Mater., 2005, 17, 620 CrossRef CAS
.
- C. Liu, X. Wu, T. Klemmer, N. Shukla, X. Yang, D. Weller, A. G. Roy, M. Tanase and D. Laughlin, J. Phys. Chem. B, 2004, 108, 6121 CrossRef CAS
.
- S. Sun, C. B. Murray, D. Weller, L. Folks and A. Moser, Science, 2000, 287, 1989 CrossRef CAS
.
- M. F. Casula, G. Concas, F. Congiu, A. Corrias, A. Falqui and G. Spano, J. Phys. Chem. B, 2005, 109, 23888 CrossRef CAS
.
- A. Corrias, G. Navarra, M. F. Casula, S. Marras and G. Mountjoy, J. Phys. Chem. B, 2005, 109, 13964 CrossRef CAS
.
- M. F. Casula, A. Corrias, A. Falqui, V. Serin, D. Gatteschi, C. Sangregorio, C. de Julián Fernández and G. Battaglin, Chem. Mater., 2003, 15, 2201 CrossRef CAS
.
-
R. C. O'Handley, Modern Magnetic Materials Principles and Applications, Wiley Interscience ed., J. Wiley & Sons, INC, New York, 2000 Search PubMed
.
- K. S. Suslick, M. M. Fang, T. Hyeon and M. M. Mdleleni, Sonochemistry and Sonoluminescence, 1999, 291 Search PubMed
.
- S. I. Nikitenko, Y. Koltypin, O. Palchik, I. Felner, X. N. Xu and A. Gedanken, Angew. Chem., 2001, 113, 4579 CrossRef
.
- B. Decaudin, C. Djega-Mariadassou and G. Cizeron, J. Alloys Compd., 1995, 226, 208 CrossRef CAS
.
- H. Kim and W. Sigmund, Carbon, 2005, 43, 1743 CrossRef CAS
.
- V. L. Kuznetsov, Nanoengineered nanofibrous materials, 2004, 169, 19 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2010 |
