Macroporous oxide structures with short-range order and bright structural coloration: a replication from parrot feather barbs†
Lei
Shi
a,
Haiwei
Yin
a,
Renyuan
Zhang
b,
Xiaohan
Liu
ac,
Jian
Zi
*ac and
Dongyuan
Zhao
*bc
aDepartment of Physics and Surface Physics Laboratory, Fudan University, Shanghai 200433, P. R. China. E-mail: jzi@fudan.edu.cn
bDepartment of Chemistry and Molecular Catalysis and Innovative Materials Laboratory, Fudan University, Shanghai 200433, P. R. China. E-mail: dyzhao@fudan.edu.cn
cAdvanced Materials Laboratory, Fudan University, Shanghai 200433, P. R. China
First published on 6th October 2009
Abstract
Three-dimensional (3-D) macroporous structures with a short-range order of pore arrangements are of both scientific significance and consequent technological impact. Inspired by parrot feather barbs that display a bright blue structural color, artificial 3-D macroporous SiO2 and TiO2 structures were successfully fabricated by using the barbs as templates. Structural and optical characterization show that the fabricated structures are 3-D bi-continuous macroporous structures with short-range order and display bright structural colors.
Three-dimensional (3-D) macroporous structures1–3 represent a special class of composite materials with important potential applications in catalysis, optics, and clean energy.4–7 Many of their physical and chemical properties strongly depend on the size and spatial arrangements of pores.8,9 Macropore structures with submicron sizes can produce interesting optical effects in the visible range. For example, a periodic arrangement of equal pores manifests a new kind of optical media known as photonic crystals,10 which could offer unprecedented opportunities in the manipulations of light. From the optical aspect, macroporous photonic-crystal structures have been extensively studied and their optical properties have been well understood by photonic band structures.11 Like in solids,12 amorphous photonic structures with only short-range order have many unusual optical transport properties, leading to exotic optical phenomena such as light localization13 and random lasing.14 However, they have been rarely studied and understood because of the serious challenges in fabrication and theoretical treatment due to the lack of long-range ordering, especially in the visible regime. On the other hand, macroporous structures with pore sizes comparable to the visible wavelengths can produce structural colors,15–17 found in bird feathers.18 These biological macroporous structures may inspire both our design and fabrication of optical devices.
Self-assembly methods offer an efficient and inexpensive way to fabricate 3-D macropore structures in large-domains.2,10,11,19 However, they often result in periodic structures rather than ones with only short-range order. The phase separation method can be used to prepare such 3-D macrostructures with block copolymers20,21 or gold compositions.22,23 The pore sizes of the 3-D macrostructures for block copolymers are always too large to produce interesting optical effects in the visible region. The pore sizes of the nanoporous golds can vary from several nanometres to hundreds of nanometres.7 These structures are, however, metallic. So far, the fabrication of 3-D macroporous dielectric structures with submicron pore sizes and with only short-range order is still challenging.
The Peach-faced Lovebird (Agapornis Roseicollis) is a small member of the parrot family. It has lively green plumage on the back, yellow-greenish plumage on the chest, and bright blue feathers at the tail. Unlike many other birds, their feather colors come from barbs rather than barbules. The blue feather barbs were observed by optical microscopy and scanning electron microscopy (SEM), shown in Fig. 1. The outermost of the cross-section of the feather barbs (Fig. 1a) is a keratin cortex layer of several micrometres in thickness. The medulla consists of an 3-D macroporous structure that is a bi-continuous random network of keratin backbones. In the central part, there exists spherical air voids of less than ten micrometres. The perceived non-iridescent blue color is a structural color produced by the 3-D macropore structure24 rather than by pigments.25 As seen from Fig. 1d, there is a ring-like pattern in the 2-D Fourier transform of the SEM cross-section image for the 3-D macroporous structure. This suggests that the 3-D macroporous structure is an amorphous photonic structure with short-range order rather than a random structure.18
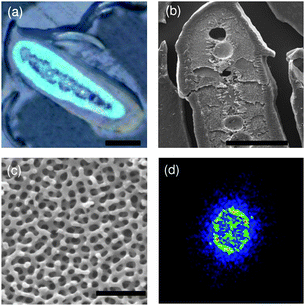 | ||
| Fig. 1 (a) Optical and (b) SEM cross-section images of a blue feather barb. (c) Close-up SEM cross-section image of the blue region. (d) 2-D Fourier transform. Scale bars: (a) and (b) 20 µm, and (c) 1 µm. | ||
In the present work, a sol–gel method was adopted to fabricate 3-D macroporous SiO2 and TiO2 structures by using the blue feather barbs as templates.26 These artificial 3-D macroporous structures have a well-defined short-range order of pore arrangements. Optical measurements revealed that the fabricated macroporous structures display interesting wavelength selective scattering properties, such as bright and non-iridescent structural colors.
3-D macroporous SiO2 and TiO2 structures were prepared by a simple sol–gel process combined with nanocasting, using tetraethylorthosilicate (TEOS) or a blend of isopropyl titanate and titanium tetrachloride as a precursor. For a typical preparation, the blue feather barbs were embedded into epoxy resin and then cut into tissue sections with a microtome. Each section was about 10 µm in thickness. 0.354 g of TEOS, 0.75 g of 0.2 M HCl and 18.6 mL of ethanol were mixed and stirred for 3 h to obtain an SiO2 sol solution. Afterwards the solution was dropped onto the barb sections. After the ethanol solvent was completely evaporated for more than 20 min, the sections were put into a oven and dried at 80 °C for 15 min. The above steps were repeated three or four times until the blue color of the sections disappeared. Although, the nanocasting process of the silica replica is dependent on the concentration of TEOS and drying process, to obtain a faithful replication of the macrostructure, we adopted a low concentration of hydrolyzed TEOS and multi-casting process. After being dried overnight at 80 °C, the sections were put into a muffle furnace and heated up to 550 °C with a rate of 1 °C/min and held at 550 °C for 300 min in air in order to remove the blue feather barb hard templates completely. For the TiO2 sol solution, 0.076 g of titanium tetrachloride, 0.209 mL of isopropyl titanate and 150 mL of ethanol were mixed and stirred for 3 h. The other processes were similar to the process of the macroporous SiO2 case.
Fabricated macroporous SiO2 samples were observed with both optical microscopy and SEM, shown in Fig. 2. SEM images show that the morphology and main structural features of the feather templates are retained, suggesting a faithful replication. The distribution of macropore sizes is narrow with an average value of about 100 nm. The SiO2 backbones are about 200 nm in length and ∼70 nm in width. In feather barbs, the volume fraction of solid is smaller than that of air. Intuitively, it seems that this should be reversed in the inverse replica. However, it is not the case by inspecting the SEM images, due to the shrinkage of precursor.19,27 The 2-D Fourier transform of the SEM cross-section image (Fig. 2d) shows that the inverted macroporous silica structure has a relatively uniform ring-like pattern, implying that the replicated structure is a disordered photonic structure with only short-range order.18 Interestingly, the inverted 3-D macrostructure is also a bi-continuous random network of SiO2 backbones, resembling its template structure. This is a feature of bi-continuous random networks.
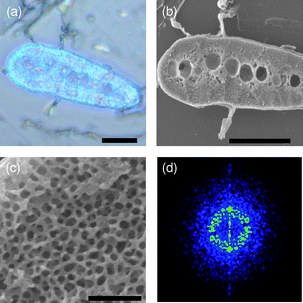 | ||
| Fig. 2 (a) Optical and (b) SEM cross-section images of the fabricated macroporous SiO2 sample. (c) Close-up SEM cross-section image of the blue region. (d) 2-D Fourier transform. Scale bars: (a) and (b) 20 µm, and (c) 1 µm. | ||
Obviously, the fabricated macroporous silica samples prepared by using the blue feather barbs as hard-templates display a bright blue color (Fig. 2a). This blue color is non-iridescent, which can be easily seen with the viewing angle changed to oblique (see ESI, Fig. S1†). Moreover, the blue color is a structural color15,16 produced by the macropore structure itself rather than by pigmentation since there are no pigments presented in the system. This can be verified by the following two facts. After infiltration with ethanol, the blue color disappears (Fig. S2, ESI†). This is due to the fact that the refractive index contrast becomes very small after the infiltration of ethanol into the pores, leading to the transparency of the structure. One can also observe the transmitted optical image which obtains a complementary color with respect to the reflected one (Fig. S3, ESI†). This is a distinct feature of structure colors. We can thus conclude unambiguously that the perceived blue color originates from the inverted structure alone.
TiO2 has been considered as one of the promising materials for photonic applications.19 Its large refractive index may facilitate strong scattering of light in disordered or amorphous photonic structures. We also fabricated 3-D macroporous TiO2 structures by using the blue feather barbs as templates and an acid–base pair precursor (mixture of isopropyl titanate and TiCl4) as the titanium source.28
Fig. 3 shows the optical and SEM cross-section images of the inverted 3-D macroporous TiO2 structure. It can be seen from Fig. 3a that the obtained macroporous TiO2 structure also displays a bright blue color. The color complementarity of the reflected and transmitted optical images indicates that the blue color is caused by the macroporous structure (Fig. S4, ESI†). Similar to the inverted silica structures, the inverted TiO2 sample has a macropore structure with relatively uniform pore sizes replicated from the feather barb templates. The interdistance of the pores is about 200 nm. The average macropore size of TiO2 structures is about 65 nm. Our results show that the filling fraction of TiO2 is much larger than that of the macroporous SiO2 due to the higher density, leading to smaller pore sizes for the macroporous TiO2. This can be understood by the high hydrolysis rate28 of TiO2 in the final stage of ethanol evaporation which is difficult to control, leading to a relatively low structural regularity for the replicated 3-D macroporous TiO2 structure. This is the reason why we chose a blend of the isopropyl titanate and TiCl4 pair, which can improve the macropore structural regularity.
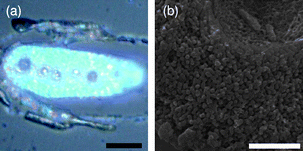 | ||
| Fig. 3 (a) Optical and (b) SEM cross-section images of the fabricated macroporous TiO2 sample. Scale bar: (a) 20 µm and (b) 2 µm. | ||
The optical properties of the fabricated macroporous SiO2 and TiO2 samples were measured by a home-made microscopic spectral measurement system, shown in Fig. 4. Compared with that of the feather template, the reflection spectrum for the replicated 3-D macroporous SiO2 structure shows a blue shift in wavelength, which can be understood by the smaller reflective index of SiO2 with respect to that of keratin and the smaller filling fraction of the backbones in the inverted macrostructure. The inverted macroporous TiO2 structure, on the other side, undergoes a red shift in peak wavelength, due to the high refractive index and filling fraction of TiO2.29
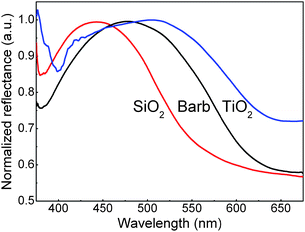 | ||
| Fig. 4 Measured reflectance at normal incidence for the feather barb template, 3-D macroporous SiO2, and TiO2 samples. | ||
The physical mechanism to produce the non-iridescent blue color relies on the coherent scattering of light by the macroporous structure.18 The macroporous structures in both the feather barbs and the fabricated samples possess only short-range order. As a result, strong scattering due to short-range order are expected, producing a ring-like pattern in the 2-D Fourier transform of the SEM cross-section images. This ring in the reciprocal space acts as a quasi-Brillouin zone, leading to strong coherent scattering. This eventually causes a selective reflection for wavelengths that can satisfy coherent scattering.18 The non-iridescence can be understood by the fact that light is scattered evenly in all directions since the macroporous structures in both the feather barbs and the fabricated samples have only short-range order.
Electron dispersive X-ray spectroscopy (EDS) was used to determine the chemical composition of the feather barbs and the fabricated oxide macrostructures (Fig. 5). In the 3-D macroporous SiO2 samples, O and Si peaks dominate, clearly indicating a silica composition. Similarly, the EDS spectrum shows that the 3-D macroporous TiO2 samples are composed mainly of Ti and O. The C peak in both macroporous SiO2 and TiO2 samples almost disappears, indicating that the feature template keratin was successfully removed by calcining at 550 °C. We also carried out X-ray diffraction (XRD) measurements to check whether the macroporous TiO2 fabricated is crystalline or not. The XRD results show clearly that the TiO2 samples after calcined at 550 °C are crystalline with an anatase phase. It is known that the annealing temperature influences the crystallization of TiO2 considerably.30 We conducted annealing experiments at 550 and 850 °C. At 850 °C, the rutile phase is observed (Fig. S5, ESI†). Due to the absence of the hard templates at this high temperature, grain coarsening occurs, leading to poor structural quality (Fig. S6, ESI†).
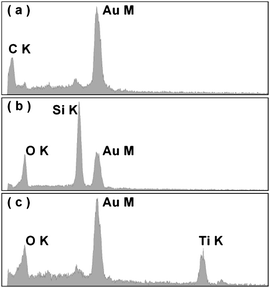 | ||
| Fig. 5 EDS spectra of (a) the feather barb, (b) the fabricated 3-D macroporous SiO2 sample, and (c) TiO2 samples. The Au peak is due to the Au coverage on samples for the SEM observation. | ||
Conclusions
3-D macroporous SiO2 and TiO2 structures were fabricated by using parrot feather barbs as hard-templates. The replicated macroporous structures are bi-continuous random networks of silica or titania backbones with short-range order, manifesting a special kind of amorphous photonic structures. The pore sizes of the replicated SiO2 and TiO2 materials are relatively uniform. The fabricated disordered macroporous structures of both silica and titania display bright blue colors stemming from coherent ligh scattering due to short-range order. Non-iridescence is due to the structural feature of the fabricated macroporous structures that can scatter light evenly in all directions. The biomimetic macroporous structures could have potential applications in photonics and other fields. Moreover, these macrostructures with short-range order may also serve as promising candidates for showing interesting optical transport properties such as light localization.Acknowledgements
This work was supported by the 973 Program (grant no. 2007CB613200 and 2006CB921700). The research was further supported by the NSFC and the Shanghai Science and Technology Commission.References
- E. C. Peters, F. Svec and J. M. J. Fréchet, Adv. Mater., 1999, 11, 1169 CrossRef CAS
.
- A. Stein and R. C. Schroden, Curr. Opin. Solid State Mater. Sci., 2001, 5, 553 CrossRef CAS
.
- K. Nakanishi and N. Tanaka, Acc. Chem. Res., 2007, 40, 863 CrossRef CAS
.
- C. M. Ho, J. C. Yu, X. C. Wang, S. Lai and Y. F. Qiu, J. Mater. Chem., 2005, 15, 2193 RSC
.
- A. Ressine, S. Ekström, G. Marko-Varga and T. Laurell, Anal. Chem., 2003, 75, 6968 CrossRef CAS
.
- F. Ramiro-Manzano, P. Atienzar, I. Rodriguez, F. Meseguer, H. Garcia and A. Corma, Chem. Commun., 2007, 242 RSC
.
- J. Biener, G. W. Nyce, A. M. Hodge, M. M. Biener, A. V. Hamza and S. A. Maier, Adv. Mater., 2008, 20, 1211 CrossRef CAS
.
- S. Polarz and B. Smarsly, J. Nanosci. Nanotechnol., 2002, 2, 581 CrossRef CAS
.
- P. D. Yang, T. Deng, D. Y. Zhao, P. Y. Feng, D. Pine, B. F. Chmelka, G. M. Whitesides and G. D. Stucky, Science, 1998, 282, 2244 CrossRef CAS
.
- O. D. Velev and A. M. Lenhoff, Curr. Opin. Colloid Interface Sci., 2000, 5, 56 CrossRef CAS
.
- A. Blanco, E. Chomski, S. Grabtchak, M. Ibisate, S. John, S. W. Leonard, C. López, F. Meseguer, H. Miguez, J. P. Mondia, G. A. Ozin, O. Toader and H. M. van Driel, Nature, 2000, 405, 437 CrossRef CAS
.
- D. A. Drabold, Eur. Phys. J. B, 2009, 68, 1 CrossRef
.
- C. Conti and A. Fratalocchi, Nat. Phys., 2008, 4, 794 CrossRef CAS
.
- S. Gottardo, R. Sapienza, P. D. García, A. Blanco, D. Wiersma and C. López, Nat. Photonics, 2008, 2, 429 Search PubMed
.
- P. Vukusic and J. R. Sambles, Nature, 2003, 424, 852 CrossRef CAS
.
- S. Kinoshita and S. Yoshioka, ChemPhysChem, 2005, 6, 1442 CrossRef CAS
.
- R. C. Schroden, M. Al-Daous, C. F. Blanford and A. Stein, Chem. Mater., 2002, 14, 3305 CrossRef CAS
.
- R. O. Prum, R. H. Torres, S. Williamson and J. Dyck, Nature, 1998, 396, 28 CrossRef CAS
.
- J. E. G. J. Wijnhoven and W. L. Vos, Science, 1998, 281, 802 CrossRef CAS
.
- J. L. Blin, A. Léonard, Z. Y. Yuan, L. Gigot, A. Vantomme, A. K. Cheetham and B. L. Su, Angew. Chem., Int. Ed., 2003, 42, 2872 CrossRef CAS
.
- M. Breulmann, S. A. Davis, S. Mann, H. P. Hentze and M. Antonietti, Adv. Mater., 2000, 12, 502 CrossRef CAS
.
- J. Erlebacher, M. J. Aziz, A. Karma, N. Dimitrov and K. Sieradzki, Nature, 2001, 410, 450 CrossRef CAS
.
- L. P. Lefebvre, J. Banhart and D. C. Dunand, Adv. Eng. Mater., 2008, 10, 775 CrossRef CAS
.
-
D. L. Fox, Animal Biochromes and Structural Colors, Univ. California Press, Berkeley 1976 Search PubMed
.
- In some other bird feathers their colors result from pigments rather than structures, e.g.,
(a) R. Stradi, E. Pini and G. Celentano, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2001, 128, 529 CrossRef CAS
; (b) M. Veronelli, G. Zerbi and R. Stradi, J. Raman Spectrosc., 1995, 26, 683 CAS
; (c) K. J. McGraw and M. C. Nogare, Biol. Lett., 2005, 1, 38 Search PubMed
.
- E. W. White, J. N. Weber, D. M. Roy, E. L. Owen, R. T. Chiroff and R. A. White, J. Biomed. Mater. Res., 1975, 9, 23 CrossRef CAS
.
- O. D. Velev, T. A. Jede, R. F. Lobo and A. M. Lenhoff, Nature, 1997, 389, 447 CrossRef CAS
.
- B. Z. Tian, X. Y. Liu, B. Tu, C. Z. Yu, J. Fan, L. M. Wang, S. H. Xie, G. D. Stucky and D. Y. Zhao, Nat. Mater., 2003, 2, 159 CrossRef CAS
.
- The refractive indices of SiO2, keratin, and TiO2 are about 1.45, 1.54, and 2.5, respectively.
-
(a) W. Y. Dong, Y. J. Sun, C. W. Lee, W. M. Hua, X. C. Lu, Y. F. Shi, S. C. Zhang, J. M. Chen and D. Y. Zhao, J. Am. Chem. Soc., 2007, 129, 13894 CrossRef CAS
; (b) H. F. Yang, Q. H. Shi, X. Y. Liu, S. H. Xie, D. C. Jiang, F. Q. Zhang, C. Z. Yu, B. Tu and D. Y. Zhao, Chem. Commun., 2002, 2842 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Optical and SEM cross-sections and XRD pattern. See DOI: 10.1039/b915625a |
| This journal is © The Royal Society of Chemistry 2010 |
