Gold encapsulation of star-shaped FePt nanoparticles†
Nicolás
Pazos-Pérez
*a,
Benito
Rodríguez-González
a,
Michael
Hilgendorff
b,
Michael
Giersig
bc and
Luis M.
Liz-Marzán
*a
aDepartamento de Química Física, Unidad Asociada CSIC- Universidade de Vigo, 36310, Vigo, Spain. E-mail: nicolaspazos@uvigo.es; lmarzan@uvigo.es; Fax: +34 986 812556; Tel: +34 986 812298
bFreie Universität Berlin, Institut für Experimentalphysik, D-14195 Berlin, Germany
cHelmholtz-Zentrum Berlin für Materialien und Energie GmbH, 14109, Berlin, Germany
First published on 14th August 2009
Abstract
We present a seeded-growth method for the encapsulation of star-like FePt nanoparticles with gold shells in aqueous solution, which allows not only further functionalization (such as silica coating) but also the organization of the synthesized core-shell nanoparticles into mesoscopic one-dimensional nanostructures under the influence of an externally applied magnetic field.
Both magnetic and metallic nanoparticles present very interesting properties, which can be exploited in a number of applications, including information storage,1 electronics2,3 as well as diagnosis and therapy.4 While each type of material has been extensively studied, incorporation of magnetic and (surface plasmon related) optical responses in a single nanoparticle is still at an early development stage, and it has been approached in two different ways, either by forming nanohybrids where the components are separated from each other,5–7 or by forming core-shell structures where one component completely covers the other.8–11 Among the many magnetic and optical materials, the magnetic component is usually made of either an iron oxide or a magnetic alloy, whereas the metal is usually gold, because of its exciting optical properties12 and its biocompatibility, as well as easy biofunctionalization.13,14 Although several reports have been published regarding the coating of iron oxide nanoparticles with gold, the results are still rather controversial and this seems to be related to the chemical and structural differences between the oxide and the noble metal. In contrast, using magnetic alloys (FePt/CoPt/MnPt) appears as a good alternative, since a noble metal (Pt) is already present in the core material, which is likely to facilitate the deposition of a second metal (Au). We have recently reported the use of different magnetic alloy nanoparticles as seeds for the growth of gold in aqueous solution, which however invariably resulted in the formation of hybrid nanoparticles where the components were separated from each other, rather than the ideal core-shell structure.5 In the present work we present a variation of this process where star-like FePt nanoparticles were used as seeds, so as to increase the number of possible nucleation points for the growth of the gold shell and ensure complete encapsulation.
The production of FePt@Au core-shell nanoparticles started with the synthesis of the seeds in high-boiling-point organic solvents,15,16 followed by phase transfer into water using the cationic surfactant cetyltrimethylammonium bromide (CTAB)5,17 and growth by reduction of HAuCl4 with a weak reducing agent (ascorbic acid). Star-shaped FePt alloy nanocrystals were synthesized using a modification of the procedure described in ref. 15, using a combination of oleic acid and oleylamine as capping agents. In a typical synthesis (∼14 nm star-shaped FePt nanocrystals, see size distribution histograms in the ESI†), Pt(acac)2 (0.5 mmol) was mixed with phenyl ether (10 mL) under nitrogen and heated to 100 °C. Fe(CO)5 (1 mmol), oleylamine (20 mmol), and oleic acid (20 mmol) were then added and the mixed solution was heated to 240 °C (heating rate ∼0.5 °C/min) and kept at this temperature for 1 h to ensure complete Fe(CO)5 decomposition, and then heated to reflux (∼260 °C) for 2 h. After cooling to room temperature, the FePt nanoparticles were separated, purified18 and stored in hexane (30 mL).
Transfer of FePt nanoparticles to water was accomplished using CTAB as a phase transfer agent.5,17 Typically, 5 mL of the prepared magnetic nanoparticles was centrifuged (7500 rpm, 20 min) upon addition of 80%vol ethanol (non-solvent). Upon redispersion in chloroform (15 mL), aqueous CTAB (30 mL, 0.1 M) was added, forming a two-phase system. This mixture was then transferred into a typical distillation setup, followed by evaporation of the organic solvent under vigorous mechanical stirring. After several hours at 90 °C, chloroform was completely removed and a clear aqueous nanoparticle solution was obtained. Centrifugation (4000 rpm, 10 min) and redispersion were finally carried out to remove possible remaining aggregates.
Growth of the gold shell was achieved using a seeded-growth approach,5,19 using the aqueous FePt colloid as seed solution. Prior to gold reduction, NaBH4 (2.5 M, 10 mL) was added to the aqueous FePt CTAB solution and the mixture was left under vigorous stirring overnight for complete NaBH4 decomposition, so as to reduce any oxide that may have formed on the FePt surface, followed by cleaning by centrifugation (9000 rpm, 10 min) and redispersion in aqueous CTAB (10 mL, 0.1 M). The growth solution comprising 10 mL of CTAB (0.1 M) and HAuCl4 (5.00 × 10−4 M) was thermostatted at 25–30 °C and ascorbic acid (0.075 mL, 0.1 M) was added (colour changed from orange to colourless, indicating reduction of Au3+ into Au+). Finally, selected volumes of the seed solution were added to the growth solution, typically followed by a colour change (within 1 h) to purple or red, depending on the amounts of reagents, due to reduction of Au+ to Au0 and formation of the core-shell structure. This colour change is immediately apparent in the UV-visible spectra shown in Fig. 1E. Upon reduction of gold, an intense absorbance band, with a maximum around 530–540 nm, can be clearly seen which can be readily assigned to the surface plasmon resonance of spherical gold nanocrystals, but slightly red-shifted, as expected for larger particles or if they deviate from the spherical shape. However, this result is not conclusive of the formation of core-shell structures, since similar optical effects could be expected from the nucleation of free gold nanoparticles in solution or the formation of hybrid nanoparticles, as previously reported.5
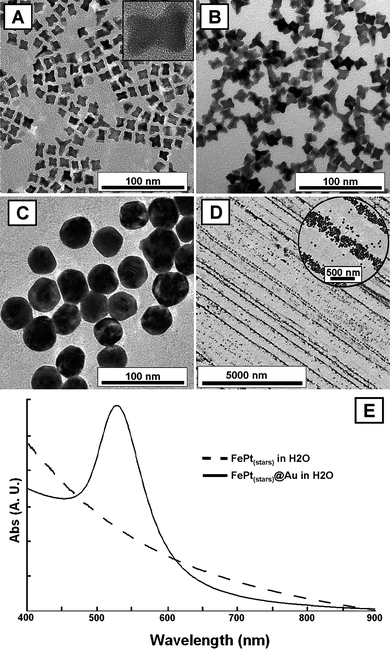 | ||
| Fig. 1 A, B: TEM images of FePt star-shaped nanoparticles (∼13 nm), before (A) and after (B) transfer from CHCl3 into aqueous CTAB. The inset in A is a HRTEM image of a single FePt nanocrystal. C: TEM image of FePt@Au nanoparticles produced from star-shaped FePt seeds. D: TEM image of FePt@Au 1D structures, organized by drying under an external magnetic field. E: UV-vis spectra of aqueous solutions of FePt stars (dashed line) and FePt@Au core-shells (continuous line). | ||
Thus, the morphological evolution of the nanoparticles during the process was characterized by transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HRTEM). Shown in Fig. 1(A, B) are TEM and HRTEM images of FePt star-shaped nanoparticles (∼14 nm), before and after transfer from organic into aqueous solution, without noticeable changes in their morphology. The images clearly reveal that the FePt nanocrystals are rather monodisperse and well separated on the TEM grid, which is an indication of their high stability in solution. The HRTEM image shows that they are single crystals and XEDS analysis (Fig. S1 in the ESI†) indicates an approximate composition of Fe1.2Pt8.8. The high Pt content of the seeds is crucial to allow uniform growth of the Au shell. On the other hand, the TEM images in Fig. 1(C, D) correspond to the particles obtained after reduction of the gold salt in the presence of FePt nanostars. Although a core-shell structure is not obvious, the size of the particles seems to indicate that the original nanostars were homogeneously coated giving rise to more spherical, FePt@Au composite particles (average diameter 37.3 ± 4.6 nm), while the original star-shaped particles are not to be found in the entire TEM grid (particle size distributions are shown in Fig. S2 and S3 in the ESI†). HRTEM analysis of the coated particles (Fig. S4 in the ESI†) reveals contrast differences in the areas near the FePt core, as well as polycrystallinity of the shell. While a more detailed characterization of the composition and core-shell morphology was carried out by X-ray energy dispersion spectroscopy (XEDS) and scanning transmission electron microscopy (STEM) elemental mapping (see below), the magnetic response of the obtained particles was evidenced through their one-dimensional (1D) alignment under external magnetic fields (preliminary magnetic characterization is shown in the ESI† (Fig. S5)). In order to do this, the excess surfactant was removed by centrifugation (twice) and redispersion in pure water, and then placing a drop (10 µL) of the particle solution on a carbon-coated copper grid and allowing it to dry at room temperature between two permanent magnets separated by ∼1 cm and producing a magnetic field of ∼1 T (magnetophoretic deposition20). The resulting nanostructures are shown in Fig. 1D, where it is apparent that the FePt@Au particles did organize into one-dimensional structures along the magnetic field lines, over very long distances (up to 0.175 mm in length). At higher magnification (Fig. 1D, inset), it becomes clear that the observed lines are formed by individual nanoparticles with a mean linewidth ∼160 nm, but varying between ∼40 and 300 nm, depending on the number of particles forming the lines (1–8 nanoparticles across each line). This is a definitive indication of the magnetic nature of the particles, thus suggesting complete incorporation of FePt into the Au spheroids.
The actual composition of the final particles was investigated through STEM-XEDS elemental mapping on individual core-shell nanoparticles (see a representative example in Fig. 2). In this particular case, the structural characterization was performed on 2 different points within the same core-shell nanoparticle, at the edge and at the particle center. As depicted from the TEM images and their corresponding XEDS spectra, it is clear that platinum (as representative of the magnetic FePt alloy) is placed exclusively at the centre of the particle, which agrees with the expected core-shell structure. Moreover, the structural defects observed in the crystallographic structure are found to originate from the particle centre, thus suggesting that they originate due to the presence of the rough FePt at the core, inside the outer gold shell. In addition, the relative intensities from the Au energy spectra related to the Cu signal (arising from the Cu grid used during TEM investigations, which can be used as an internal standard) was observed to remain constant at all points investigated, except the centre of the particle, where the intensity is lower, whereas the Pt signal becomes clearly visible (see ESI,† Fig. S4). A similar analysis was carried out with several particles, consistently yielding similar results.
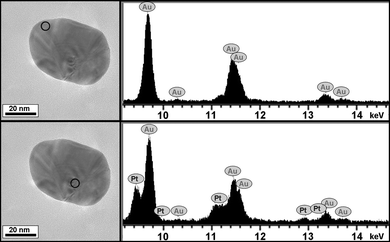 | ||
| Fig. 2 TEM images (left panel) corresponding to an individual FePt@Au core-shell nanoparticle from which various energy dispersion spectra (right panel) were acquired at different points. The Pt peaks (from FePt) are only visible at the particle centre. | ||
The rather irregular morphology that we often encountered during HRTEM and STEM analysis suggests that the FePt star seeds are gradually encapsulated during the growth of gold at different spots of the surface. Additional evidence for this is provided by additional analysis carried out at an intermediate stage during the formation of these core-shell nanoparticles. One example is shown in Fig. 3, which contains a TEM image and its corresponding energy spectrum, at an edge, where part of the initial star-like nanoparticle is sticking out of the encapsulating gold shell. At this edge, the spectrum is clearly dominated by Pt, though Au is already visible because the spot also covers part of the shell next to it. It should be noted that Fe was not clearly identified in these analyses because of its low concentration in the particles. We have thus demonstrated that star-like FePt nanoparticles can be readily obtained in organic solvent, transferred into water without loss of colloidal stability and homogeneously covered with a gold shell, giving rise to a novel system that displays optical and magnetic properties, allowing mesoscopic organization by simple application of a small external magnetic field.
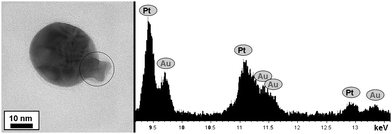 | ||
| Fig. 3 TEM image and the EDS analysis of an intermediate state during the formation of FePt@Au core-shell nanoparticles. The analysis was performed on the area where the FePt star is observed to stick out of the shell. | ||
An additional advantage of having a gold shell on the magnetic cores is that the coated particles can be additionally covered by an external shell of silica, which can be readily biofunctionalized and provides high colloidal stability,21 since several methods have been optimized for silica coating of gold colloids with different surface capping agents. In the particular case of CTAB capped gold nanocrystals, an efficient method based on initial wrapping with polyelectrolytes and poly(vinylpyrrolidone) (PVP) was recently reported.22 This method also proved extremely useful for homogeneous coating of our FePt@Au nanoparticles. The process comprised washing of the freshly made FePt@Au to remove excess CTAB, redispersion in water and dropwise addition to an aqueous poly(styrene sulfonate) (PSS) solution (2 g/L PSS, 6 mM NaCl) previously sonicated for at least 30 min, additional cleaning to remove excess polyelectrolyte (two-fold centrifugation at 5000 rpm, 20 min) and redispersion in water. After PSS coating the same process was repeated using poly(allylamine hydrochloride) (PAH). The solution obtained from the previous step was then added to an aqueous PVP solution (Mw 30000, 4g/L) and stirred for at least 12 h, washed and diluted with isopropanol, where silica coating could be carried out by addition of a solution of ammonia in isopropanol followed by addition of tetraethyl orthosilicate (TEOS) in isopropanol under gentle stirring. The resulting silica coated FePt@Au nanoparticles were characterized by TEM, which demonstrated the complete and homogeneous coverage with a smooth silica shell as shown in Fig. 4. From this figure (and others from the same sample), the thickness of the silica shell was estimated to be ∼25 nm. If required, the shell thickness could be increased by additional TEOS hydrolysis and condensation as previously reported.22
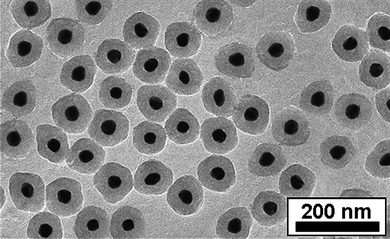 | ||
| Fig. 4 TEM image of silica coated FePt@Au core-shell nanoparticles. | ||
In summary, we have presented a novel procedure that allows complete encapsulation of magnetic star-shaped FePt nanocrystals within a gold shell, while preserving the identity of the original nanocrystals in the core. We expect that the present approach can be readily extended to coating of other alloy particles, as long as: (i) they contain platinum at a relatively high concentration; and, (ii) they display a star-shaped morphology which can allow nucleation and growth of gold at different points to ensure encapsulation, rather than formation of dimers, as previously reported. These magnetic/plasmonic nanoparticles can be organized into 1D mesoscopic assemblies, which may be additionally interesting for applications in wave guiding or biosensing applications. Finally, the further manipulation of these core-shell nanoparticles was achieved through coating with an additional, external shell of silica, which may facilitate bioconjugation with certain biomolecules and prevent agglomeration during handling.
Acknowledgements
The authors thank Izabela Firkowska for useful comments, Dmitry Baranov for assistance in FePt synthesis and Prof. M. Farle and Dr M. Spasova for magnetic measurements. This work was supported by the EU (Marie Curie Research Training Network SyntOrbMag, contract MRTN-CT-2004-005567) and the Spanish MiCInn (Consolider-Ingenio project Nanobiomed). MG would like to specially thank the Helmholtz Zentrum Berlin, for financial support in the realization of this project.References
- S. Sun, Adv. Mater., 2006, 18, 393 CrossRef CAS.
- G. Schmid, Chem. Soc. Rev., 2008, 37, 1909 RSC.
- R. P. Andres, J. D. Bielefeld, J. I. Henderson, D. B. Janes, V. R. Kolagunta, C. P. Kubiak, W. J. Mahoney and R. G. Osifchin, Science, 1996, 273, 1690 CrossRef CAS.
- D. G. Castner and B. D. Ratner, Surf. Sci., 2002, 500, 28 CrossRef CAS.
- N. Pazos-Pérez, Y. Gao, M. Hilgendorff, S. Irsen, J. Pérez-Juste, M. Spasova, M. Farle, L. M. Liz-Marzán and M. Giersig, Chem. Mater., 2007, 19, 4415 CrossRef CAS.
- T. Pellegrino, A. Fiore, E. Carlino, C. Giannini, P. D. Cozzoli, G. Ciccarella, M. Respaud, L. Palmirotta, R. Cingolani and L. Manna, J. Am. Chem. Soc., 2006, 128, 6690 CrossRef CAS.
- C. Xu, J. Xie, D. Ho, C. Wang, N. Kohler, E. G. Walsh, J. R. Morgan, Y. E. Chin and S. Sun, Angew. Chem., Int. Ed., 2007, 47, 173.
- J. Jeong, T. H. Ha and B. H. Chung, Anal. Chim. Acta, 2006, 569, 203 CrossRef CAS.
- S. J. Cho, J. C. Idrobo, J. Olamit, K. Liu, N. D. Browning and S. M. Kauzlarich, Chem. Mater., 2005, 17, 3181 CrossRef CAS.
- N. S. Sobal, M. Hilgendorff, H. Möhwald, M. Giersig, M. Spasova, T. Radetic and M. Farle, Nano Lett., 2002, 2, 621 CrossRef CAS.
- M. Grzelczak, B. Rodríguez-González, J. Pérez-Juste and L. M. Liz-Marzán, Adv. Mater., 2007, 19, 2262 CrossRef CAS.
- L. M. Liz-Marzán, Langmuir, 2006, 22, 32 CrossRef CAS.
- A. K. Salem, P. C. Searson and K. W. Leong, Nat. Mater., 2003, 2, 668 CrossRef CAS.
- X. H. Huang, P. K. Jain, I. H. El-Sayed and M. A. El-Sayed, Nanomedicine, 2007, 2, 681 CrossRef CAS.
- M. Chen, J. P. Liu and S. Sun, J. Am. Chem. Soc., 2004, 126, 8394 CrossRef CAS.
- C. Wang, Y. L. Hou, J. M. Kim and S. H. Sun, Angew. Chem., Int. Ed., 2007, 46, 6333 CrossRef CAS.
- H. Y. Fan, K. Yang, D. M. Boye, T. Sigmon, K. J. Malloy, H. F. Xu, G. P. Lopez and C. J. Brinker, Science, 2004, 304, 567 CrossRef CAS.
- S. H. Sun, C. B. Murray, D. Weller, L. Folks and A. Moser, Science, 2000, 287, 1989 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Chem. Commun., 2001, 617 RSC.
- M. Giersig and M. Hilgendorff, Eur. J. Inorg. Chem., 2005, 3571 CrossRef CAS.
- L. M. Liz-Marzán and P. Mulvaney, J. Phys. Chem. B, 2003, 107, 7312 CrossRef CAS.
- I. Pastoriza-Santos, J. Pérez-Juste and L. M. Liz-Marzán, Chem. Mater., 2006, 18, 2465 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: HRTEM images; XEDS spectra; particle size distributions; magnetic characterization data. See DOI: 10.1039/b911175a |
| This journal is © The Royal Society of Chemistry 2010 |
