Immobilization of Pd nanoparticles with functional ionic liquid grafted onto cross-linked polymer for solvent-free Heck reaction
Gang Liu, Minqiang Hou, Jiyuan Song, Tao Jiang, Honglei Fan, Zhaofu Zhang and Buxing Han*
Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100080, China. E-mail: hanbx@iccas.ac.cn; Fax: +86-10-62562821; Tel: +86-10-62562821
First published on 22nd October 2009
Abstract
1-Aminoethyl-3-vinylimidazolium bromide ([VAIM]Br) grafted on the cross-linked polymer polydivinylbenzene (PDVB) was synthesized. The copolymers were used as a support to immobilize palladium nanoparticles. The catalyst was characterized by Fourier transform infrared spectroscopy (FT-IR), thermogravimetric (TG) analysis, transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The catalytic performance of the copolymer-supported Pd nanoparticles for the Heck arylation of olefins with different aryl iodides was studied under solvent-free conditions. The results demonstrated that the catalyst was very active and stable under solvent-free conditions, and could be reused after simple separation. The reason for the high activity and stability of the catalyst is discussed.
Introduction
Metallic nanoparticles have attracted much attention in the field of catalysis because they can catalyze many organic reactions effectively due to the quantum size effect and high ratio of surface area to volume.1–3 However, the naked nanoparticles tend to aggregate to form bulk metal because of the impulse of high surface energy, resulting in a severe decease in catalytic activity and selectivity. An effective way to solve this problem is to immobilize the metallic nanoparticles on suitable supports, and many methods have been developed for this.4–7The Heck reaction is a very useful route for the formation of new C–C bonds in a single operational step.8–10 The wide functional group tolerance in both reactants allows convenient application in the total synthesis without protecting groups. The Heck reaction has mostly been catalyzed by palladium complexes combined with phosphine ligands in organic solvents under homogeneous conditions. It is known that the homogeneous systems suffer drawbacks in separation, and result in environmental problems, especially in the case of toxic ligands. Therefore, it is desirable to develop cheaper and environmentally benign heterogeneous catalytic systems. It is also known that palladium nanoparticles have high catalytic activity for the Heck reaction.11,12 Immobilization of Pd nanoparticles on solid supports to prepare active and stable catalytic systems for the Heck reaction is an interesting topic, and different supports have been used to stabilize the nanoparticles, such as carbon,13 hydroxyapatite,14 molecular sieves,7,15 and polymers.16,17
In recent years, ionic liquids (ILs) have been widely studied owing to their unique properties, such as negligible vapor pressure and high thermal stability.18 Some functionalized ILs have also been used to stabilize metal nanoparticles. For example, Safavi and coworkers reported highly efficient palladium nanocatalysts supported on a phosphorylated IL modified xerogel, which could be evenly coated on glass slides.19 Dyson and coworkers synthesized a nitrile-functionalized IL, [C3CNpy][Tf2N], which was an effective immobilization solvent for palladium-catalyzed Suzuki and Stille reactions; TEM analysis of the nanoparticles extracted from the catalysis solution showed the stabilizing effect of the IL.20
The design and preparation of active and stable catalysts for Heck reactions under solvent-free conditions is highly desirable, and consistent with the requirements of green chemistry. It is well known that amine groups bind strongly to palladium.21,22 It can be expected that the ILs with amine groups on solid supports should be effective for stabilizing palladium nanoparticles. Herein we describe the synthesis of a new type of imidazolium IL with an amine group, and its copolymerization with cross-linker divinylbenzene. The prepared copolymer was used for the immobilization of Pd nanoparticles for Heck reaction.
Results and discussion
Preparation and characterization of the catalyst
The route to synthesize the copolymers (PDVB-IL) is presented in Scheme 1, and the procedures were similar to those for the copolymerization of 1-vinyl-3-butylimidazolium chloride and DVB.23 In brief, the IL with amine group was first prepared by the reaction of 1-vinylimidazole and 2-bromoethylamine hydrobromide to give [VAIM]Br·HBr. This was then copolymerized with DVB using azobisisobutyronitrile (AIBN) as the initiator, and subsequent treatment of the copolymers with NaOH gave the cross-linked copolymer with IL (PDVB-IL). To prepare the copolymer-supported Pd nanoparticles (PDVB-IL-Pd), the copolymer was added to aqueous H2PdCl4solution with vigorous stirring, giving the catalyst after reduction of the Pd(II) on the PDVB-IL with NaBH4 and drying under vacuum. | ||
| Scheme 1 Synthesis of the cross-linked polymer-supported ionic liquid. | ||
The prepared copolymers (PDVB-IL) and neat PDVB were characterized by thermogravimetric analysis. The thermograms are shown in Fig. 1. The small weight loss of PDVB-IL before 220 °C resulted from the loss of the adsorbed water which also occurred in the case of neat PDVB. It can be seen from the two thermograms that the weight loss of PDVB-IL between 220 °C and 380 °C was due to the elimination of the ILs immobilized on the polymers, because there was no weight loss for the neat PDVB. Further weight loss at higher temperature (above 380 °C) was attributed to the decomposition of PDVB. The amount of the IL in the PDVB-IL obtained from the thermal analysis was about 7.0 wt%. The composition of PDVB-IL was also examined by elemental analysis, and the result indicated that the content of IL was 7.4 wt%, which was consistent with the result of thermal analysis. The results of element analysis also showed that the molar ratio of N/Br was 3, indicating that the ILs were copolymerized without hydrobromide, and the PDVB-IL had a structure shown in Scheme 1.
 | ||
| Fig. 1 The thermogram of PDVB-IL and pure PDVB. | ||
Fig. 2 shows the FT-IR spectra of the PDVB-IL and PDVB-IL-Pd. Both spectra showed an asymmetric broad band at around 3435 cm−1, which is attributed to the stretching vibration of the amine groups. It is clear that the band became narrow after the Pd nanoparticles were supported on the polymers, indicating the binding of Pd nanoparticles to the polymers through the amine groups.21 The small change in intensity of the band may result from the change of absorption coefficient of the amino groups after interaction with Pd particles.
 | ||
| Fig. 2 The FT-IR spectra of PDVB-IL and PDVB-IL-Pd. | ||
Fig. 3 shows the typical SEM and TEM images of the catalyst (PDVB-IL-Pd). The SEM image (Fig. 3A) shows that the catalyst was almost amorphous. The dark spots in the TEM image (Fig. 3B) indicate the presence of palladium nanoparticles that were bound to the copolymers. The diameter of palladium nanoparticles was in the range 7–8 nm and the size distribution was very narrow.
 | ||
| Fig. 3 SEM (A) and TEM (B) images of the PDVB-IL-Pd; the TEM image (C) of the recovered PDVB-IL-Pd after being reused three times. | ||
The catalyst (PDVB-IL-Pd) was also characterized by XPS before reaction, and the results are shown in Fig. 4. It can be seen that the Pd 3d spectrum could be resolved into two spin–orbit pairs with 3d5/2 binding energies of 335.9 eV and 337.1 eV, respectively. The peak binding energies of 335.9 eV (Pd 3d5/2) and 341.0 eV (Pd 3d3/2) correspond to fully reduced Pd nanoparticles, while the peak at 337.1 eV suggests the presence of the unreduced Pd2+ ions on the surface of the Pd particles.21,24 It can be seen from the XPS spectrum that most of Pd2+ was reduced to Pd(0) because the area of the peak of Pd2+ ion was relatively small. The content of Pd in the catalyst was 2.3 wt%, as determined by the ICP-AES method.
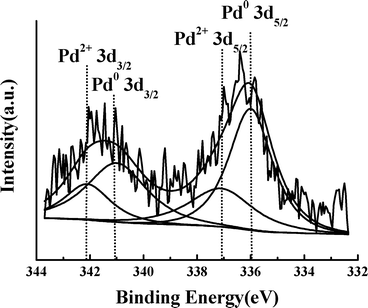 | ||
| Fig. 4 XPS spectrum of the Pd 3d edge of the PDVB-IL-Pd sample. | ||
Catalytic Heck reactions
The catalytic activity of the PDVB-IL-Pd for Heck reactions was investigated under solvent-free conditions. It is known that a base is required for Heck reactions. The effect of Et3N and some inorganic bases on the reaction of iodobenzene and methyl acrylate was studied at 120 °C. The results showed that the catalyst was very active and selective for the reaction in the presence of Et3N (entry 1, Table 1), while the reaction rate was very slow when inorganic bases were used (entries 2–4, Table 1). The main reason for this is that the solubility of the inorganic bases in the reaction system was very poor, while Et3N is miscible with the substrates. This indicates that the solubility of the bases in the reaction mixture is crucial for the Heck reaction. Using Et3N as the base, the effect of temperature on the reaction was studied. The results demonstrated that the activity of the catalyst increased with temperature as the temperature increased from 80 to 140 °C (entries 1 and 5–7 of Table 1), with the activity being very high at 120 °C and 140 °C.
| ||||
|---|---|---|---|---|
| Entry | Base | T/°C | Time/h | Yield (%) |
a The molar ratio of iodobenzene/methyl acrylate/base/Pd is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0002. 0.0002. | ||||
| 1 | Et3N | 120 | 4 | 97 |
| 2 | NaHCO3 | 120 | 4 | <2 |
| 3 | CH3COONa | 120 | 4 | <2 |
| 4 | NaOH | 120 | 4 | <2 |
| 5 | Et3N | 80 | 12 | <2 |
| 6 | Et3N | 100 | 12 | 73 |
| 7 | Et3N | 140 | 1 | 98 |
The catalytic activity of the catalyst for the arylation of different olefins with iodobenzene was tested under solvent-free conditions; the results are given in Table 2. The iodobenzene was very active with electron-poor olefins such as methyl, ethyl and butyl acrylate. As the size of the substituted group of acrylate ester increased from methyl to butyl, the reaction time needed for high conversion increased from 4 h to 6 h (entries 1–3, Table 2). This may be due to the steric hindrance effect that reduced the reaction rate.
| ||||
|---|---|---|---|---|
| Entry | R1 | R2 | Time/h | Yield (%) |
a The molar ratio of haloarene/olefin/Et3N/Pd is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0002.b The reaction was performed without solvent at 120 °C.c Entries 9, 10 and 11 are the results of reusing the catalyst for the second, third and fourth times under the reaction conditions of entry 1. 0.0002.b The reaction was performed without solvent at 120 °C.c Entries 9, 10 and 11 are the results of reusing the catalyst for the second, third and fourth times under the reaction conditions of entry 1. | ||||
| 1 | H | Me | 4 | 97 |
| 2 | H | Et | 5 | 94 |
| 3 | H | Bu | 6 | 95 |
| 4 | 4-F | Me | 3 | 93 |
| 5 | 4-Cl | Me | 2 | 94 |
| 6 | 4-OH | Me | 1 | 98 |
| 7 | 4-OMe | Me | 3 | 96 |
| 8 | 4-Me | Me | 6 | 93 |
| 9c | H | Me | 4 | 95 |
| 10c | H | Me | 4 | 95 |
| 11c | H | Me | 4 | 94 |
In order to investigate the effect of substituted groups of iodobenzene on the Heck reaction, various substituted iodobenzenes were studied. The coupling reaction of both electron-deficient and electron-rich iodobenzenes with olefins can proceed with high yields using the catalyst. Iodobenzenes with electron-donating groups, such as methyl, gave reduced reaction rates, and longer reaction times were required for a high yield. Groups with a lone electron pair (e.g. -OH, -OMe, -F, -Cl) in the iodobenzenes affect the electron cloud density of benzene ring and the coordination of Pd with the benzene ring, ultimately accelerating the reaction rate, as can be seen from entries 1 and 4–8 of Table 2.
The reusability of the catalyst was tested using iodobenzene and methyl acrylate as the substrates. After each run, the catalyst was recovered by filtration, followed by washing with ethanol (10 mL × 3). After drying, the catalyst was reused directly for the next run. The results for the three repeated runs are also presented in Table 2. The activity of the catalyst remained unchanged after it was reused three times, indicating that the catalyst was not only very active, but also very stable. There are several reasons for the excellent stability of the catalyst. First, the PDVB-IL was not soluble in the reactants and the product because it was a cross-linked polymer; second, it was thermally stable up to about 220 °C (Fig. 1), which was much higher than the reaction temperature; third, the amine groups in the PDVB-IL interacted strongly with Pd particles by coordination, which anchored the Pd nanoparticles stably on the support. The TEM image of the catalyst after being used four times is shown in Fig. 3C. The aggregation of the Pd particles in the used catalyst was not obvious, supporting the argument above.
The mechanism of the Heck reaction employing supported Pd nanoparticles as catalysts has been discussed in the literature.25 Many authors have reported the leaching of Pd during the reaction. In order to investigate the mechanism in our catalytic system, we conducted a filtration test for the Heck reaction between iodobenzene and methyl acrylate using PDVB-IL-Pd as catalyst. After 2 h (the reaction was completed in 4 h), the reaction was stopped and the reaction mixture was centrifuged at 16![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 rpm for 20 min. Then the mixture without the solid catalyst was allowed to continue under the same conditions for another 2 h, and the conversation found to increase from 60% to 73%. This suggests that the leaching of active Pd species from the solid supports occurred to some extent, but the dissolved Pd redeposited back onto the polymers after the iodobenzene was completely consumed. This argument was confirmed by our ICP-AES analysis, which showed that the content of Pd in the catalyst before and after reaction was 2.30% and 2.23%, respectively.
000 rpm for 20 min. Then the mixture without the solid catalyst was allowed to continue under the same conditions for another 2 h, and the conversation found to increase from 60% to 73%. This suggests that the leaching of active Pd species from the solid supports occurred to some extent, but the dissolved Pd redeposited back onto the polymers after the iodobenzene was completely consumed. This argument was confirmed by our ICP-AES analysis, which showed that the content of Pd in the catalyst before and after reaction was 2.30% and 2.23%, respectively.
Conclusion
We synthesized DVB cross-linked copolymer with chemically supported IL [VAIM]Br. The copolymer was proved to be an effective supporter for palladium nanoparticles. The nanocatalysts immobilized on the copolymers showed high catalytic activity in the Heck reaction for various substrates. The catalyst was very stable and could be easily separated from the products and reused because of the insoluble nature of the cross-linked copolymer and the coordination force between the amine group and the palladium nanoparticles. We believe that this route can be used to support some other metallic nanocatalysts on a highly cross-linked polymer matrix to prepare active, stable catalysts for different reactions.Experimental
Materials
Azobis(isobutyronitrile) (AIBN), methanol, acetone, acetonitrile, ethanol, PdCl2, tetrahydrofuran (THF), methyl acrylate, ethyl acrylate, n-butyl acrylate, sodium hydroxide, sodium bicarbonate, sodium acetate, triethylamine, iodobenzene, 4-fluoroiodobenzene, 4-chloro-1-iodobenzene, 4-iodophenyl methyl ether and 4-iodophenol were provided by Beijing Chemical Company. Divinylbenzene (DVB) was purchased from Fluka, and 1-vinylimidazole was provided by Aldrich. AIBN was recrystallized three times before use. Other chemicals were used as received. All the chemicals were A.R. grade.Preparation of [VAIM]Br·HBr
To prepare the basic IL [VAIM]Br·HBr, 1-vinylimidazole(9.42 g, 0.10 mol) and acetonitrile (50 mL) were added to a two-necked flask equipped with a magnetic stirrer. The mixture was refluxed at 78 °C under a nitrogen atmosphere. 2-Bromoethylamine hydrobromide (20.50 g, 0.10 mol) was added into the flask stepwise over 24 h. Then, the reaction mixture was cooled down, the liquid was poured out and the solid was washed with anhydrous ethanol three times to remove the unreacted starting materials. After drying under vacuum, 1-aminoethyl-3-vinylimidazolium bromide hydrobromide ([VAIM]Br·HBr) was obtained. 1H NMR (400 MHz, D2O) δ: 3.52 (m, 2H), 4.65 (m, 2H), 5.47 (dd, 1H), 5.84 (dd, 1H), 7.17 (dd, 1H), 7.71 (s, 1H), 7.87 (s, 1H), 9.23 (s, 1H). Positive ion ESI-MS: m/z 138.1. Anal. Calcd for C7H13N3Br2: N, 14.0; C, 28.1; H, 4.3; Br, 53.5. Found: C, 25.9; H, 4.4; N, 13.2; Br, 54.9.Our experiment showed that the isolated [VAIM]Br could be obtained by neutralization of [VAIM]Br·HBr by NaOH. In the experiment, [VAIM]Br·HBr (10 mmol) was dissolved in the water (10 mL) and then equimolar NaOH (10 mmol) was added. The solution was stirred for 6 h at r.t. followed by vaporization of the water under vacuum. Then the obtained viscous liquid was dissolved in MeOH (20 mL) with stirring for 1 h. The solution was filtered, and ionic liquid [VAIM]Br was obtained after the MeOH in the filtrate was evaporated. The identity of [VAIM]Br was confirmed by NMR. 1H NMR (300 MHz, DMSO) δ: 2.78 (m, 2H), 4.12 (m, 2H), 5.27 (dd, 1H), 5.88 (dd, 1H), 7.20 (dd, 1H), 7.79 (s, 1H), 8.11 (s, 1H), 9.47 (s, 1H).
Preparation of PDVB-IL
To prepare the DVB-cross-linked polymers with the supported IL (PDVB-IL), DVB (1.95 g, 15 mmol), AIBN (0.05 g) and [VAIM]Br·HBr (0.45 g, 1.5 mmol) were dissolved in ethanol/water (100 ml, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) under nitrogen. The mixture was refluxed at 100 °C with stirring. After 24 h, a white solid formed was filtrated and was washed with THF, acetone, and methanol. The final product was treated with equimolar NaOH and washed with water before being dried in vacuum at 60 °C for 12 h. Element analysis: C, 84.4; H, 7.8; N, 1.5; Br, 2.7. The PDVB without the ionic liquid was synthesized by direct polymerization of DVB under the same conditions.
1 v/v) under nitrogen. The mixture was refluxed at 100 °C with stirring. After 24 h, a white solid formed was filtrated and was washed with THF, acetone, and methanol. The final product was treated with equimolar NaOH and washed with water before being dried in vacuum at 60 °C for 12 h. Element analysis: C, 84.4; H, 7.8; N, 1.5; Br, 2.7. The PDVB without the ionic liquid was synthesized by direct polymerization of DVB under the same conditions.Preparation of PDVB-IL-Pd
PdCl2(2 mmol) was dissolved in the solution of HCl (0.04 M, 100 mL) with stirring for 24 h. PDVB-IL (1.00 g) was added to the aqueous solution of H2PdCl4 (0.02 M, 50 mL) in a 100 mL flask with vigorous stirring. The mixture was stirred for 12 h and then filtered. The solid catalysts were then dispersed in water (20 mL) and reduced by a fresh aqueous solution (10 mL) of NaBH4 (0.185 g, 5 mmol) at room temperature. The resulting grey catalysts were washed with water (10 mL × 3) and ethanol (10 mL × 3) respectively, then dried under vacuum at 60 °C for 24 h. The content of Pd in the catalyst was 2.30 wt%, which was determined using ICP-AES.Catalytic reaction
The Heck reaction was carried out in a 6 mL stainless steel reactor with a magnetic stirrer. In the experiment, aryl halide (1 mmol), vinylic substrate (1.5 mmol), triethylamine (1.5 mmol) and Pd catalyst (1.0 mg) were added into the reactor. Then the reactor was heated to the desired temperature in an air bath under stirring. After reaction, the reactor was cooled by a ice bath. The products were collected using DMF as solvent after centrifuging the catalysts from the suspension. The liquid phase was analyzed by GC (Agilent 4890 D) equipped with a flame-ionized detector, and biphenyl was used as the internal standard. The formation of product was confirmed by GC-MS (QP2010). All the reactions were conducted three times and the experimental errors were less than ±2%. In the catalyst recycling experiments, the catalyst was reused after washing with ethanol (10 mL × 3) and drying at 60 °C for 6 h.Characterization
The FT-IR spectra were collected on a Bruker Tensor 27 spectrometer in KBr pellet form. SEM examination was carried out on a scanning electron microscope (JEOL, JSM-4300) operated in a high-vacuum mode at 15 kV, which provided general textural information of the samples. The SEM sample was sputter-coated with gold before observation. TEM observation was performed on a transmission electron microscope (JEOL, JEM 1011) at an operating voltage of 100 kV, and the images were electronically captured using a CCD camera. X-ray photoelectron spectroscopy data of the as-prepared samples were obtained with an ESCALab220i-XL electron spectrometer from VG Scientific using 300 W MgKα radiation. The base pressure was about 3 × 10−9 mbar. The binding energies were referenced to the C 1s line at 284.8 eV from adventitious carbon. TG measurements were performed on a thermal analyzer (NETZSCH STA 409 PC/PG) with a heating rate of 3 °C min−1. The loading content of Pd in the catalysts was determined by ICP-AES (VISTAMPX); the samples were dissolved in aqua regia beforehand. The elemental composition of the polymer was determined using a Flash EA1112 analyzer.Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (20533010, 20773144) and the Chinese Academy of Sciences (KJCX2.YW.H16) for financial support.References
- M. Turner, V. B. Golovko, O. P. H. Vaughan, P. Abdulkin, A. Berenguer-Murcia, M. S. Tikhov, B. F. G. Johnson and R. M. Lambert, Nature, 2008, 454, 981–983 CrossRef CAS.
- A. A. Herzing, C. J. Kiely, A. F. Carley, P. Landon and G. J. Hutchings, Science, 2008, 321, 1331–1335 CrossRef CAS.
- V. Cimpeanu, M. Kocevar, V. I. Parvulescu and W. Leitner, Angew. Chem., Int. Ed., 2009, 48, 1085–1088 CrossRef CAS.
- D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852–7872 CrossRef CAS.
- R. W. J. Scott, O. M. Wilson and Richard M. Crooks, J. Phys. Chem. B, 2005, 109, 692–704 CrossRef CAS.
- Handbook of heterogeneous catalysis, 2nd edn, ed. G. Ertl, H. Knözinger and J. Weitkamp, Wiley-VCH, 2008 Search PubMed.
- V. L. Budarin, J. H. Clark, R. Luque, D. J. Macquarrie and R. J. White, Green Chem., 2008, 10, 382–387 RSC.
- M. T. Reetz and J. G. de Vries, Chem. Commun., 2004, 1559 RSC.
- C. Amatore and A. Jutand, Acc. Chem. Res., 2000, 33, 314–321 CrossRef CAS.
- M. Lombardo, M. Chiarucci and C. Trombini, Green Chem., 2009, 11, 574–579 RSC.
- Y. Wan, H. Y. Wang, Q. f. Zhao, M. Klingstedt, O. Terasaki and D. Y. Zhao, J. Am. Chem. Soc., 2009, 131, 4541–4550 CrossRef CAS.
- R. T. Tao, S. D. Miao, Z. M. Liu, Y. Xie, B. X. Han, G. M. An and K. L. Ding, Green Chem., 2009, 11, 96–101 RSC.
- T. Maegawa, Y. Kitamura, S. Sako, T. Udzu, A. Sakurai, A. Tanaka, Y. Kobayashi, K. Endo, U. Bora, T. Kurita, A. Kozaki, Y. Monguchi and Hironao Sajiki, Chem. Eur. J., 2007, 13, 5937–5943 CrossRef CAS.
- K. Mori, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2004, 126, 10657–10666 CrossRef CAS.
- L. Djakovitch and K. Koehler, J. Am. Chem. Soc., 2001, 123, 5990–5999 CrossRef CAS.
- S. Ko and J. Jang, Angew. Chem., Int. Ed., 2006, 45, 7564–7567 CrossRef CAS.
- B. Karimi, S. Abedi, J. H. Clark and V. Budarin, Angew. Chem., Int. Ed., 2006, 45, 4776–4779 CrossRef CAS.
- T. Welton, Coord. Chem. Rev., 2004, 248, 2459–2477 CrossRef CAS.
- A. Safavi, N. Maleki, N. Iranpoor, H. Firouzabadi, A. R. Banazadeh, R. Azadi and F. Sedaghati, Chem. Commun., 2008, 6155–6157 RSC.
- D. B. Zhao, Z. F. Fei, T. J. Geldbach, R. Scopelliti and P. J. Dyson, J. Am. Chem. Soc., 2004, 126, 15876–15882 CrossRef CAS.
- S. Mandal, D. Roy, R. V. Chaudhari and M. Sastry, Chem. Mater., 2004, 16, 3714–3724 CrossRef CAS.
- R. M. Crooks, M. Zhao, L. Sun, V. Chechnik and L. K. Yeung, Acc. Chem. Res., 2001, 34, 181 CrossRef CAS.
- Y. Xie, Z. F. Zhang, T. Jiang, J. L. He, B. X. Han, T. B. Wu and K. L. Ding, Angew. Chem., Int. Ed., 2007, 46, 7255–7258 CrossRef CAS.
- K. R. Priolkar, P. Bera, P. R. Sarode, M. S. Hegde, S. Emura, R. Kumashiro and N. P. Lalla, Chem. Mater., 2002, 14, 2120–2128 CrossRef CAS.
- X. M. Ma, Y. X. Zhou, J. C. Zhang, A. L. Zhu, T. Jiang and B. X. Han, Green Chem., 2008, 10, 59–66 RSC.
| This journal is © The Royal Society of Chemistry 2010 |


