Toxicity of imidazolium ionic liquids towards algae. Influence of salinity variations
Adam
Latała
a,
Marcin
Nędzi
a and
Piotr
Stepnowski
*b
aFaculty of Oceanography and Geography, University of Gdańsk, Piłsudskiego 46, 81–378, Gdynia, Poland
bFaculty of Chemistry, University of Gdańsk, Sobieskiego 18/19, 80–952, Gdańsk, Poland. E-mail: sox@chem.univ.gda.pl
First published on 15th October 2009
Abstract
This paper reports on a detailed study of the influence of salinity on the biological activity of 1-alkyl-3-methylimidazolium chlorides on two green algae Oocystis submarina and Chlorella vulgaris, one diatom Cyclotella meneghiniana and one blue-green alga Geitlerinema amphibium. All these organisms inhabit the Baltic Sea, an environment naturally varying greatly in salinity. The toxicity effects of ILs towards cyanobacterial and algal organisms were tested in fresh water and in water of four different salinities—8, 16, 24 and 32 PSU—reflecting the whole range encountered in the Baltic Sea. Increasing the salinity was found to exert a significant influence on ionic liquid toxicity in all cases. The lower toxicity is probably due to the reduced permeability of ionic liquid cations through the algalcell walls. Higher chloride concentrations offer a good ion-pairing environment for imidazolium cations, which therefore compete with hydroxyl or silanol functional groups in cell-wall structures. The results of this work indicate that at higher salinities algalgrowth is inhibited to a significantly lesser extent. With the same IL concentration, the toxicity decreases by eight–ten times in the algae or about three times in the cyanobacterium in the 0–32 PSU salinity range.
1. Introduction
First two parts of this series reported on acute toxicity of alkylimidazolium ionic liquids to various algal taxa. Part I concerned planktonic algae, green algae (Chlorella vulgaris and Oocystis submarina) characteristic for freshwater and brackish environment as well as two marine diatoms (Cyclotella meneghiniana and Skeletonema marinoi).1 Part II, diatom Bacillaria paxillifer and cyanobacteriumGeitlerinema amphibium, species characteristic for benthic environment.2 For all the organisms studied we have confirmed pronounced alkyl chain effect. The acquired results indicate that sensitivity to ionic liquids decrease in the following order cyanobacterium > diatoms > green algae. It seems that the most important determinant of the observed difference is the cell wall structure; peptidoglycan cell wall in cyanobacterium, silica based cell wall in the case of diatoms and cellulose in the case of green algae. The size of the cells also plays important role in intoxication process; 10 times difference in the cell size resulted in a double more sensitive reaction to ionic liquids within green algae as well as diatom taxonomic groups.The first report on the toxicity of ionic liquids to algae published by our group demonstrated that growth of two Baltic algae (Oocsytis submarina and Cyclotella meneghiniana) was effectively inhibited even by very low concentrations of alkylimidazolium ionic liquids.3 That study also gave an indication of the moderating influence of salinity on the biological activity of the 1-hexyl-3-methylimidazolium salt in the Oocystis submarina viability assay: the acute toxicity of this compound weakened with increasing ambient salinity.
In the present study we therefore decided to study this effect in detail using two green algae (Oocystis submarina and Chlorella vulgaris), one diatom (Cyclotella meneghiniana) and one blue–green alga (Geitlerinema amphibium). All these organisms inhabit the Baltic Sea, an environment that naturally varies greatly in salinity—from about 2–4 PSU (Practical Salinity Unit expressing parts per thousand) in the Bothnian Sea, up to 6–8 PSU in the Baltic Proper and in the Gulf of Gdańsk. In the Baltic straits (the Kattegat and Skagerrak), salinities are from 20 to 30 PSU.
In this study the toxicity effects towards algal organisms were tested in the presence of fresh water and water of four salinities—8, 16, 24 and 32 PSU—reflecting the whole range encountered in the Baltic Sea. In order to precisely assess modifying influence of salinity onto toxicity of ionic liquids, concentrations of compounds used in these experiments were near identical (rounded to decimal values) to those, which in two previous studies of ours1,2 were found to cause 50% growth inhibition of organism under investigation.
Results
In order to gain the insight into the salinity effect on toxicity of particular compounds we have chosen concentrations of ionic liquids reflecting their EC50 values for tested species and salinity 8 PSU, found in our two previous studies.1,2 Chosen concentrations were rounded to decimal values that made particular dissolutions easier. Our main aim therefore was to firstly clearly recognized the salinity effect rather than establish EC50 values for each compound in each salinity point.Tables 1–4 set out the influence of salinity expressed as a percentage of the growth inhibition of particular algae exposed to 1-ethyl-, 1-butyl-, 1-hexyl-, 1-octyl- and 1-decyl-3-methylimidazolium chlorides, and Fig. 1–4 depict graphically the observed reduction in growth inhibition. The concentrations of ionic liquids used represent the orders of magnitude of the EC50 values previously calculated for these species in salinity 8 PSU.1,2 The salinity impact on IL toxicity was tested in the range from 0 to 32 PSU. An increase in salinity reduced the toxic effect of ILs to a significant extent in all cases, as found previously.3 With the lowest salinity (8 PSU), growth inhibition in all four algal organisms fell from 60–70% noted in fresh water to c. 50%; this was achieved with all the compounds under investigation. An increase in salinity to 16 PSU further reduced the toxic effect, but in this case the reduction was especially conspicuous in the case of O. submarina, the growth of whose cultures was inhibited by only c. 20–30%. The other three species were more strongly affected by the presence of ILs in the culture media, the levels of growth inhibition being c. 30–40% in C. vulgaris and C. meneghiniana, and c. 40–50% in G. amphibium. The last-mentioned species was the most resistant to toxicity changes also in the higher salinity ranges, its growth being inhibited by c. 25–30% at 32 PSU. In the remaining species (C. vulgaris, C. meneghiniana and O. submarina) the highest salinity caused a drop in growth inhibition to 10% and less.
| Chlorella vulgaris | Growth inhibition [I%] in different salinities [PSU] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance | concentration [μM] | 0 | 8 | 16 | 24 | 32 | |||||
| %I | SD [%] | %I | SD [%] | %I | SD [%] | %I | SD [%] | %I | SD [%] | ||
| EMIMCl | 10000 | 55.91 | 1.29 | 38.94 | 1.19 | 35.74 | 2.26 | 29.79 | 1.82 | 5.31 | 1.35 |
| BMIMCl | 1000 | 66.22 | 2.77 | 48.56 | 4.64 | 36.36 | 4.35 | 8.79 | 1.98 | 7.51 | 1.26 |
| HMIMCl | 50 | 61.47 | 3.35 | 51.74 | 1.63 | 36.57 | 2.86 | 17.65 | 3.08 | 6.03 | 1.93 |
| OMIMCl | 5 | 63.84 | 1.75 | 51.76 | 2.03 | 25.54 | 2.34 | 16.43 | 2.03 | 5.41 | 1.34 |
| DMIMCl | 1 | 68.68 | 3.93 | 49.26 | 1.89 | 28.85 | 3.98 | 34.04 | 2.77 | 9.39 | 1.98 |
| Oocystis submarina | Growth inhibition [I%] in different salinities [PSU] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance | concentration [μM] | 0 | 8 | 16 | 24 | 32 | |||||
| %I | SD [%] | %I | SD [%] | %I | SD [%] | %I | SD [%] | %I | SD [%] | ||
| EMIMCl | 12 500 | 70.54 | 3.20 | 53.89 | 0.84 | 29.21 | 1.86 | 15.47 | 0.82 | 8.69 | 1.44 |
| BMIMCl | 1 500 | 67.00 | 1.65 | 50.45 | 0.94 | 25.73 | 2.52 | 11.86 | 1.55 | 7.93 | 0.43 |
| HMIMCl | 500 | 69.55 | 1.04 | 53.30 | 0.48 | 17.84 | 0.43 | 9.99 | 1.62 | 8.27 | 1.62 |
| OMIMCl | 75 | 65.14 | 1.48 | 48.65 | 4.06 | 19.54 | 2.06 | 10.25 | 1.12 | 7.92 | 1.99 |
| DMIMCl | 10 | 63.58 | 1.86 | 50.23 | 0.91 | 25.21 | 1.67 | 13.65 | 0.89 | 6.54 | 2.41 |
| Cyclotella meneghiniana | Growth inhibition [I%] in different salinities [PSU] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance | concentration [μM] | 0 | 8 | 16 | 24 | 32 | |||||
| %I | SD [%] | %I | SD [%] | %I | SD [%] | %I | SD [%] | %I | SD [%] | ||
| EMIMCl | 100 | 66.23 | 2.06 | 51.25 | 1.42 | 35.21 | 1.88 | 16.47 | 2.04 | 8.25 | 1.90 |
| BMIMCl | 10 | 68.21 | 4.00 | 52.69 | 3.03 | 37.25 | 0.54 | 13.68 | 0.46 | 7.69 | 1.88 |
| HMIMCl | 1.5 | 70.30 | 1.94 | 49.63 | 2.20 | 33.47 | 0.55 | 18.65 | 0.94 | 8.24 | 2.17 |
| OMIMCl | 0.5 | 72.23 | 2.78 | 50.87 | 2.92 | 40.78 | 1.49 | 12.58 | 0.48 | 7.65 | 2.49 |
| DMIMCl | 0.1 | 71.61 | 1.51 | 50.69 | 2.11 | 38.52 | 3.76 | 11.69 | 4.06 | 6.84 | 1.58 |
| Geitlerinema amphibium | Growth inhibition [I%] in different salinities [PSU] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance | concentration [μM] | 0 | 8 | 16 | 24 | 32 | |||||
| %I | SD [%] | %I | SD [%] | %I | SD [%] | %I | SD [%] | %I | SD [%] | ||
| EMIMCl | 25 | 70.59 | 1.55 | 54.34 | 2.47 | 48.94 | 0.94 | 39.25 | 2.65 | 28.72 | 0.94 |
| BMIMCl | 5 | 69.75 | 1.62 | 53.58 | 2.36 | 46.45 | 0.48 | 37.46 | 1.88 | 26.18 | 0.48 |
| HMIMCl | 1.5 | 71.43 | 1.12 | 53.07 | 2.06 | 47.49 | 1.06 | 38.06 | 0.54 | 27.70 | 2.06 |
| OMIMCl | 0.1 | 63.91 | 0.89 | 50.75 | 2.00 | 47.75 | 2.65 | 43.37 | 0.55 | 28.57 | 1.85 |
| DMIMCl | 0.01 | 65.97 | 2.54 | 53.30 | 1.94 | 42.75 | 2.96 | 39.23 | 2.84 | 24.49 | 2.54 |
 | ||
| Fig. 1 Influence of salinity on IL toxicity to the green alga Chlorella vulgaris. | ||
 | ||
| Fig. 2 Influence of salinity on IL toxicity to the green alga Oocystis submarina. | ||
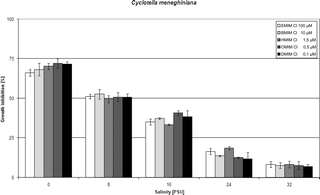 | ||
| Fig. 3 Influence of salinity on IL toxicity to the diatom Cyclotella meneghiniana. | ||
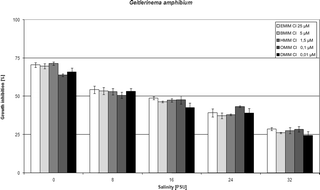 | ||
| Fig. 4 Influence of salinity on IL toxicity to the blue-green alga Geitlerinema amphibium. | ||
Discussion
The results supply positive evidence that the toxic effects of ILs on algae can be moderated by environmental conditions like salinity. The discovery that the toxicity of ionic liquids is reduced in more saline waters is of major importance for the assessment of the fate of these compounds in marine environments. We had previously hypothesised that the lower toxicity in more saline waters was probably due to the reduced permeability of ionic liquid cations through the algalcell walls.3 It is therefore likely that higher chloride concentrations offer a competitive ion-pairing environment for alkylimidazolium cations, the part of the ionic liquid composition responsible for the toxic mode of action. Paired (or complexed) cations are completely or partially prevented from interacting with peptidoglycan (blue–green alga), silanol (diatom) or cellulose (green alga) functional groups in the cell wall structures. Moreover, a higher salinity implies an elevated concentration of inorganic monovalent cations that are also capable of competing with alkylimidazolium cations for the countercharges available on the algal surfaces.The results also indicate that there are no significant differences between particular compounds. This very likely provides proof of the identical mode of toxic action of alkylimidazolium ionic liquids regardless of alkyl chain length. It seems, moreover, that the first step in the intoxication process, i.e. the interionic interaction between the IL cation and the negatively charged biological membrane component, is as important as the deeper penetration of the membrane by the alkyl moiety of the molecule.
The recorded trend of reduced IL toxicity in conditions of elevated salinity corroborates similar observations made recently by Kulacki and Lamberti.4 While studying the effects of imidazolium ILs towards the freshwater alga Scenedesmus quadricauda, these authors found that the presence of nutrient media had a mitigating effect on the toxicity of the compounds under investigation. Their explanation of this effect appears, however, to be of an ecological rather than a molecular character: differences in nutrient levels could enable a taxon to cope better with the higher stress levels associated with a particular ionic liquid. They suggest, moreover, that ecotoxicological protocols should be standardised to take into account the abiotic conditions under which an organism is likely to be exposed to a toxic substance. Our study has provided ample evidence that this postulate is absolutely justified if we are to fully understand and predict the real effects of chemicals (including ionic liquids) on the aquatic environment.
The influence of IL toxicity on the limnic green alga Scenedesmus vacuolatus has also been studied in the context of mixture and combination effects, when in addition to ionic liquids, these organisms were exposed to cadmium ions.5 It was found that when cadmium was present as a background pollutant, the toxic effects were much smaller than those of the fixed ratio mixtures of ILs. This can be explained by the possible complexation of the ILs with the heavy metal, since all these compounds are ionic and have the potential to interact with each other.
Returning to salinity effects, it is well known in the ecotoxicology of heavy metals that the salt content has the potential to modify the toxicity of metallic ions to algalcells.6,10 By analogy therefore, we can assume that to some extent at least we observed a similar effect in our studies, although with much larger cations. The main effect is attributed to the presence of chlorides, which possess a strong complexation potential. Chloride ions are known to be very mobile and capable of stabilising complexes with cationic microelements, thereby diminishing their bioavailability in water.
Conclusions
Obtained results using four different species and five ionic liquids differing in their toxic potential clearly shows a significant impact of salinity variations on algal toxicity. The highest toxicity towards all studied organisms is observed in freshwater, subsequently being reduced while salinity is increasing. All the organisms used in the present study (the green algae Oocystis submarina and Chlorella vulgaris, the diatom Cyclotella meneghiniana and the blue–green alga Geitlerinema amphibium) inhabit the Baltic Sea, an environment that naturally varies a great deal in salinity. They therefore constitute a reliable test kit for tracking the differences in toxic responses when exposed to potential contaminants (e.g. ionic liquids) in the presence of various salinities. The results show clearly that the toxic effect of ILs on algae can be moderated by environmental conditions such as salinity. With the same concentration of ILs the toxicity decreased by eight–ten times in the algae and by about three times in the cyanobacterium over a salinity range from 0 to 32 PSU. We therefore assumed that a potential ion-pairing or complexing environment comes into existence with elevated concentrations of chlorides and inorganic cations that prevents alkylimidazolium cations from interacting with the negatively charged biological membranes of algal organisms. This raises a number of issues, the most important of which is a general revision of the approximate data on the toxicity of ILs in various biological test systems, which have so far failed to take into account the composition of the abiotic environment and its variations.Therefore the main result of this paper presents a significant breakthrough in our understanding of ILs toxicity. For the first time we can see that environment can modulate biological effects of these compounds and often presented threats in regard to ionic liquids should now be deeply refined.
We have compared our results with those obtained for heavy metals; however, this may well be an oversimplification, and the mode of toxic action in the presence of various salinities, pH, and other organic and inorganic toxicants must be studied in detail if we are to gain a full understanding of the prospective fate of ionic liquids in the environment.
Material and methods
Tested organisms
Four taxonomically different algal species were used in this study: the green algae Oocystis submarina (BA-0001), Chlorella vulgaris BA-0002, diatom Cyclotella meneghiniana, BA-0010 and blue-green alga Geitlerinema amphibium (BA-0013) They were isolated from coastal waters of the Baltic Sea and maintained as unialgal cultures in the Culture Collection of Baltic Algae (CCBA) (http://ocean.univ.gda.pl/~ccba/) at the Institute of Oceanography of the University of Gdańsk.11The species chosen for study were of euryhaline nature. Therefore, they were not stressed by the salinity range (0–32 PSU) used in the experiments.12 Moreover, the algae were acclimated to each salinity before testing.
Ionic liquids
All ionic liquids were obtained from Merck (Darmstadt, Germany). They were: 1-ethyl-3-methylimidazolium chloride (EMIM Cl), 1-butyl-3-methylimidazolium chloride (BMIM Cl), 1-hexyl-3-methylimidazolium chloride (HMIM Cl), 1-octyl-3-methylimidazolium chloride (OMIM Cl) and 1-decyl-3-methylimidazolium chloride (DMIM Cl). Applied concentrations for green algae were in the range: EMIM Cl 10000–12500 μM, BMIM Cl 1000–1500 μM, HMIM Cl 50–500 μM, OMIM Cl 5–75 μM, DMIM Cl 1–10 μM and for diatom and cyanobacterium in the range: EMIM Cl 25–100 μM, BMIM Cl 5–10 μM, HMIM Cl 1.5 μM, OMIM Cl 0.1–0.5 μM, DMIM Cl 0.01–0.1 μM.Batch cultures and toxicity tests
The test algae were batch-cultured in f/2 medium13 prepared on distilled water. In cultures salinities of 8, 16, 24, 32 PSU were made on the base of sea salt TropicMarin® (Tropic Marin, Wartenberg, Germany).Stock cultures of the tested organisms were acclimatized in the same salinity media as the experimental salinity for 10 days at 20 °C and illumination by 25 μmol photons m−2 s−1 from daylight type fluorescent lamps using an L:D photoperiod of 16:8. Irradiance was measured using a quantum-meter LiCor (LI-189) (LiCor, Lincoln, Nebraska, USA). The data obtained for treated cultures of the tested organisms were normalized to control growth curves obtained for the same salinity environments.
The algal acute toxicity tests of IL's were carried out using modified versions of the methods recommended in the European Committee for Standardization's guidelines.14,15 The main modifications were use of f/2 medium, photoperiod and choice of the test strains. The final batch cultures used in the experiments were obtained by mixing a known amount of cells in the log growth phase with sterile medium. The initial cell number was constant and was spectrophotometrically measured as Optical Density (OD) at wavelengths of 665 nm. Density of green algae C. vulgaris and O. submarina were approximately 0.022/665 nm, diatom C. meneghiniana 0.062/665 nm, and G. amphibium 0.032/665 nm.
The volumes of 9.5 cm3 of algal suspension were transferred into glass Erlenmeyer flasks (25 ml). To each of these 0.5 cm3 of different concentrations of an aqueous ionic liquid solution or distilled water (control cultures) was added. The concentrations of ionic liquids used reflected their EC50 values registered for particular algal organisms exposed to these compounds.6,7 After 72 h of incubation the number of cells in cultures was determined by OD measurement. All experiments were run in triplicate and the variability of the results did not exceed 5% on the inhibition scale.
Acknowledgements
Financial support was provided by the Polish Ministry of Research and Higher Education under grants: 2P04G 118 29, 2P04F 036 30 and DS 8200–4-0085–9.References
- A. Latała, N. Nędzi and P. Stepnowski, Green Chem., 2009, 11, 580–588 RSC.
- A. Latała, N. Nędzi and P. Stepnowski, Green Chem. , 2009, 11, 1371–1376 RSC.
- A. Latała, P. Stepnowski, M. Nędzi and W. Mrozik, Aquat. Toxicol., 2005, 73, 91–98 CrossRef CAS.
- K. J. Kulacki and G. A. Lamberti, Green Chem., 2008, 10, 104–110 RSC.
- M. Matzke, S. Stolte, A. Boshen and J. Filser, Green Chem., 2008, 10, 784–792 RSC.
- A. Latała and W. Surosz, Biologia, 1998, 53, 547–555 CAS.
- HCh. Hahne and W. Kroontje, J. Environ. Qual., 1973, 2, 444–450 CAS.
- T. M. Florence and J. L. Stauber, Aquat. Toxicol., 1986, 8, 11–26 CrossRef CAS.
- D. S. McLusky, V. Bryant and R. Campbell, Oceanogr. Mar. Biol. Ann. Rev., 1986, 24, 481–520 Search PubMed.
- D. Y. Lee, C. Fortin and P. G. C. Campbell, Aquat. Toxicol., 2005, 75, 127–135 CrossRef CAS.
- A. Latała in Biological Resource Centers and the Use of Microbes, ed. N. Lima and D. Smith, Micoteca da Universidade do Minho, Braga, Portugal, 2003, pp. 323–345 Search PubMed.
- A. Latała, S. Jodłowska and F. Pniewski, Algological Studies, 2006, 122, 137–154 Search PubMed.
- R. R. L. Guillard in Culture of marine invertebrate animals, ed. W. L. Smith and M. N. Chanle, Plenum Press, New York, 1975, pp. 29–60 Search PubMed.
- EN. Water quality–Fresh water algal growth inhibition test with Scenedesmus subspicatus and Selenastrum capricornutum (ISO 8692:1993). European Committee for Standardization, Brussels, 1993.
- EN ISO. Water quality–Marine algalgrowth inhibition test with Skeletonema costatum and Phaeodactylum tricornutum (ISO 10253:1995). European Committee for Standardization, Brussels, 1995.
| This journal is © The Royal Society of Chemistry 2010 |
