Caffeoylquinic acid derived free radicals identified during antioxidant reactions of bitter tea (Ilex latifolia and Ilex kudincha)
Katharina Franziska
Pirker
a and
Bernard Albert
Goodman
*ab
aHealth and Environment Department, Environmental Resources & Technologies, Austrian Institute of Technology, A-2444 Seibersdorf, Austria. Fax: +(43) 50550 3520; Tel: +(86) 771 327 2475E-mail: bernard_a_goodman@yahoo.com; katharina.pirker@ait.ac.at
bGuangxi Key Laboratory of Subtropical Bioresource Conservation and Utilization, Guangxi University, Nanning, 520004, Guangxi, China. Fax: +(43) 50550 3434; Tel: +(86) 771 323 7873
First published on 18th November 2010
Abstract
In order to provide some insight into the chemical basis for the antioxidant behaviour of bitter tea, the Chinese medicinal beverage derived from leaves of Ilex kudincha or Ilex latifolia, free radicals generated during the oxidation of aqueous extracts of dried leaves have been investigated by electron paramagnetic resonance (EPR) spectroscopy. With both beverages, the major components in the EPR spectra after accelerated autoxidation under alkaline conditions or oxidation with the superoxide anion radical were comparable to those derived from reactions of caffeoylquinic acids. Thus these reaction products have sufficient stability for biological activity, and the present results suggest that such molecules contribute appreciably to the antioxidant chemistry of these beverages.
1. Introduction
“Kudingcha” or “bitter tea” is a traditional Chinese beverage. It is derived from leaves of the tree Ilex kudincha and has been consumed for centuries in southern China as an alternative to green tea. Similar products may be produced from other members of the Ilex family, e.g. bitter tea from I. latifolia, in southern China and Vietnam, and Yerba Maté from leaves and twigs of I. paraguariensis in several South American countries.In traditional Chinese medicine, several beneficial effects have been associated with kudingcha. These have been summarised by Dharmananda1 and include treatments for the common cold, rhinitis, itching eyes, red eyes, bronchitis, and headache, as well as alleviation of thirst associated with fever or severe diahorrea. Other beneficial effects include preventing the deterioration of the heart and brain functions, and improved digestion. Ahai2 reports uses of kudingcha for relief of hypertension and weight loss, as a diuretic, and as a remedy for sore throats.
Triterpene glycosides (saponins) are considered to be the main active components of extracts from Ilex species, and there have been considerable efforts to isolate and identify the various triterpenes and saponins from leaves of I. kudincha,3–6I. latifolia,7,8 and I. paraguariensis.9,10 However, in view of the wide range of medicinal properties associated with beverages derived from the Ilex species, it is unlikely that their beneficial effects are derived solely from one group of components, such as the saponins. Leaves of the Ilex species contain polyphenols,11,12 which might also be expected to contribute to their biological properties, analogous to the association of such molecules with the beneficial effects of green tea consumption.13 With green tea, it is generally assumed that these beneficial effects of the beverage are associated with the antioxidant (free radical scavenging) properties of its constituent polyphenols,14–17 and recent papers concerning polyphenols in kudingcha have focused on measurements of their overall antioxidant activity and the use of chemical assays to identify individual polyphenol molecules.11,12 However, although such measurements are of undoubted value, they represent just one step towards understanding the chemical behaviour of the beverages, which in turn is just one step towards understanding their biological properties.
Although antioxidant assays provide an overall measure of a particular chemical property, they give little information as to the chemical nature of the individual molecules responsible for the activity, and no information on the behaviour of oxidation products which are formed during antioxidant reactions. Identification of components that might feature in antioxidant reactions is provided by chemical analyses, but such measurements give no information on the chemical behaviour of such molecules in a complex mixture, i.e. a beverage, where reactions between individual components could have a major impact on the overall chemistry. Indeed it is one of the principles of traditional medicines that the activity is often dependent on the presence of many molecules, and not on a single molecular species as is the case with pharmaceutical products.
The biological properties of traditional medicinal products, however, are likely to be determined to a major extent by molecules with sufficient stability to be transported within the organism, but also with sufficient reactivity to react selectively with specific molecules, cells or tissue types. Thus the next logical step in developing an understanding of the chemical basis for the biological properties of beverages is to identify components or products derived from beverage components that possess such intermediate reactivity. Semiquinone radicals produced by the oxidation of polyphenols are candidates for such behaviour, and measurements made with green tea18 indicate that they have appreciable stability in the complex medium of the beverage. Indeed there have been several recent suggestions that epigallocatechin gallate, the major polyphenol in green tea, could be used as an individual medicine or nutritional supplement for treating a wide range of diseases or disorders, such as cancer,19 atherosclerosis,20 blood sugar control,21 and neurodegenerative diseases.22
The principal objective of the present paper was to gain further insight into the oxidative chemistry of components in bitter teas in order to develop further our knowledge of the chemical basis for the biological effects of the beverages. Therefore, we investigated the extent to which free radical generation analogous to that observed during oxidation of green tea might occur in aqueous extracts of I. kudincha and I. latifolia. These measurements were based on the use of electron paramagnetic resonance (EPR) spectroscopy, a physical technique that can specifically characterise paramagnetic molecules such as free radicals, and followed the experimental approach described by Ferreira Severino et al.18 to identify free radical products from leaves of I. kudincha and I. latifolia under accelerated (alkaline) autoxidation and oxidation by the superoxide anion radical (O2˙−). The reason for using alkaline conditions for investigation of autoxidation reactions is that autoxidation of polyphenols with O2 at neutral pH is a slow process, but proceeds much more rapidly at high pH. However, although there are many reports of the use of such conditions for accelerating the oxidation reactions of polyphenols, it is recognised that they are not relevant either to the chemistry of teas as beverages, or to the reactions of the phenols after the beverage has been consumed. Additional oxidation measurements using O2˙− radicals as the oxidising agent were, therefore, performed in order to establish to what extent results obtained from alkaline autoxidation can be extrapolated to other chemical conditions. Also, since the major antioxidant components in bitter tea are di- and mono-caffeoylquinic acids,12 additional measurements were made using chlorogenic acid (Fig. 1a) and cynarin (Fig. 1b) as representatives of these two groups of polyphenols.
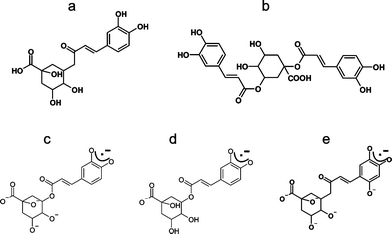 | ||
| Fig. 1 Molecular structure of (a) chlorogenic acid, (b) cynarin. Proposed structures for radicals derived from chlorogenic acid: (c) initial radical after alkaline autoxidation, (d) initial radical after oxidation by O2˙− radicals, (e) hydroxylised radical of chlorogenic acid after prolonged alkaline autoxidation. | ||
2. Experimental
Bitter tea samples used were a commercial Kudingcha purchased from a Chinese supermarket and tenderised leaves of I. latifolia from Cao Bang, Vietnam (kindly supplied by Prof. Pham Van Ho of the National University of Vietnam, Ho Chi Minh City). Dimethyl sulfoxide (DMSO, >99.9% purity) was bought from Sigma-Aldrich Handels GmbH (Vienna, Austria), chlorogenic acid (5-O-caffeoylquinic acid) (>97% purity) were purchased from Carl Roth GmbH & Co. KG (Karlsruhe, Germany) and cynarin (1,3-di-O-caffeoylquinic acid) (96% purity) was bought from LGC Standards GmbH (Wesel, Germany).For autoxidation investigations of bitter tea solutions, 200 mg of dried leaves were initially extracted into 10 ml distilled water at 100 °C for 10 min; the mixture was then filtered and allowed to cool to room temperature before use. Alternative measurements were made using similar tea extracts that were freeze dried and stored at room temperature in a vacuum desiccator. Stock solutions containing 20 mg ml−1 were prepared in DMSO, and used for oxidation experiments using O2˙− radicals, for which solutions of KO2 (50 mM) were prepared in DMSO under N2 atmosphere in the presence of the crown ether 18C623 in order to stabilise the O2˙− radical.
Alkaline autoxidation was carried out in two ways. In the first method, a pump-flow system was used and the aqueous tea extract (described above) was mixed with 0.1 M NaOH shortly before the entrance to a flow cell, which was already located inside the resonator of the spectrometer. The flow cell was basically a conventional quartz EPR flat cell, with two arms at the bottom into which reaction solutions could be pumped and mixed immediately below the flat cell. The flow of the two reaction solutions (tea extract and NaOH; stored in separate 50ml beakers) to the two arms of a flow cell was controlled by a peristaltic pump, and the flow rate was adjusted so as to obtain the maximum signal intensity (∼2ml min−1).
In the second method, the aqueous tea extract was mixed with an equal volume of 0.1 M NaOH in a tube outside the spectrometer and the resulting solution then transferred rapidly into a flat cell, which was then inserted into the spectrometer. Chlorogenic acid (10 mM) was also autoxidised under similar conditions to the tea samples described above. With cynarin (10 mM), the mixed solution containing equal volumes of the polyphenol and 0.1 M NaOH in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, was transferred into a 50μl capillary, sealed on both ends using Cristaseal, and this capillary was then placed inside a 3 mm i.d. quartz tube for the EPR measurements.
1, was transferred into a 50μl capillary, sealed on both ends using Cristaseal, and this capillary was then placed inside a 3 mm i.d. quartz tube for the EPR measurements.
Oxidation by O2˙− was carried out by gently mixing the stock solution of the freeze-dried tea in DMSO (20 mg ml−1) with the KO2/DMSO solution in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 outside the spectrometer, and then transferring the resulting solution rapidly to a flat cell for measurement of their EPR spectra. Similar measurements were carried out with the polyphenols chlorogenic acid or cynarin in DMSO (10 mM). In the case of cynarin, the final reaction solutions were transferred into a 50 μl capillary for recording their EPR spectra.
1 outside the spectrometer, and then transferring the resulting solution rapidly to a flat cell for measurement of their EPR spectra. Similar measurements were carried out with the polyphenols chlorogenic acid or cynarin in DMSO (10 mM). In the case of cynarin, the final reaction solutions were transferred into a 50 μl capillary for recording their EPR spectra.
EPR spectra were acquired at room temperature in 1024 points as first derivatives of the microwave absorption using a Bruker EMX CW spectrometer, operating at X-band frequencies (9 GHz), and equipped with a high sensitivity cavity. Microwaves were generated by a Gunn diode and the microwave frequency was recorded continuously with an in-line frequency counter. The measurements of cynarin were carried out on a Bruker EMX plus CW spectrometer using a super high Q cavity (1000 or 4000 points dependent on the modulation amplitude). All spectra were acquired using 2 mW microwave power, 2–20 μT modulation amplitude, and 10, 20 or 100 kHz modulation frequency, the actual choice being dependent on the signal intensity and spectrum of the sample.
With the exception of the measurements on the tea samples using the pump flow system which produced a steady state concentration of the untable free radicals, the first spectrum in each experiment was recorded immediately after tuning the spectrometer (i.e. about 2–3 min after starting the reaction).
Justification of the spectral interpretations and refinement of the parameters for the hyperfine coupling (hfc) constants were obtained by simulations using the Bruker WinSimfonia software.
3. Results
3.1 Alkaline autoxidation
The initial EPR spectra produced as a result of accelerated oxidation under alkaline conditions from aqueous extracts of leaves of I. kudincha and I. latifolia are shown in Fig. 2a and 2c along with the initial spectra from chlorogenic acid and cynarin obtained under similar conditions. These signals were unstable and the spectra of the tea samples and chlorogenic acid were acquired using a pump flow system to obtain more acceptable signal-to-noise ratios. The spectra from the two types of bitter tea are different in respect of both the numbers and magnitudes of their 1H hfc constants (see Table 1). The major component in the spectrum from the I. kudincha sample (Fig. 2a) contains six inequivalent 1H hfc constants and is similar to that obtained from autoxidised chlorogenic acid (Fig. 2e). The five largest values are similar to those reported previously for oxidation of chlorogenic acid,24,25 and the radical is assigned to a semiquinone with the unpaired electron located on the caffeic acid moiety (Fig. 1c). The initial spectrum observed with autoxidised cynarin (Fig. 2f) was very unstable and has not been identified. It could not be obtained with sufficient intensity for a reliable simulation, but seems to be different from that of chlorogenic acid. The spectrum obtained with I. latifolia (Fig. 1c) has not yet been identified, but it is also present as a minor component in the spectrum from the I. kudincha sample (Fig. 2a). Its hfc pattern indicates interaction of the unpaired electron with five 1H atoms, two of which are equivalent. Parameters for each of these radicals, refined by computer simulation (Fig. 2b and d), are presented in Table I along with relevant data from the literature.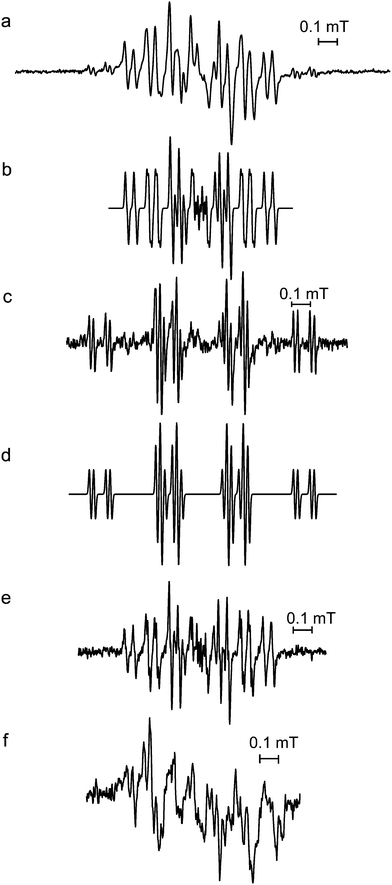 | ||
Fig. 2 Initial EPR spectra from solutions of (a) Ilex kudincha, (c) Ilex latifolia, (e) chlorogenic acid, and (f) cynarin during alkaline autoxidation using a pump-flow system for (a), (c), and (e). Simulations (using the parameters in Table 1) of the main spectra from the bitter tea samples are shown in (b) for I. kudincha, and (d) for I. latifolia. 0.1 M NaOH was used and mixed in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with either the tea extract (20 mg ml−1) or 10 mM of the polyphenol. All spectra were recorded using 2 mW microwave power. 100 kHz modulation frequency was used for (c) and (f), 20 kHz for (a) and 10 kHz for (e). Modulation amplitudes were 10 μT for (a) and (c), 2 μT for (e), and 20 μT for (f). 1 with either the tea extract (20 mg ml−1) or 10 mM of the polyphenol. All spectra were recorded using 2 mW microwave power. 100 kHz modulation frequency was used for (c) and (f), 20 kHz for (a) and 10 kHz for (e). Modulation amplitudes were 10 μT for (a) and (c), 2 μT for (e), and 20 μT for (f). | ||
| Figure/compound | a(1H) | a(1H) | a(1H) | a(1H) | a(1H) | a(1H) |
|---|---|---|---|---|---|---|
| 2b | 0.263 | 0.241 | 0.125 | 0.115 | 0.046 | 0.009 |
| Chlorogenic acid24 | 0.261 | 0.230 | 0.120 | 0.120 | 0.078 | |
| Chlorogenic acid25 | 0.260 | 0.232 | 0.117 | 0.117 | 0.065 | |
| 2d | 0.380 | 0.355 | 0.355 | 0.088 | 0.023 | |
| 3e | 0.286 | 0.183 | 0.133 | 0.041 | ||
| 2,4,5-Tri-OH-cinnamic acid24 | 0.292 | 0.180 | 0.137 | 0.040 | ||
| 2,4,5-Tri-OH-cinnamic acid26 | 0.283 | 0.175 | 0.132 | 0.044 | ||
| 4d | 0.498 | 0.090 | 0.034 | 0.034 | ||
| 5d | 0.238 | 0.220 | 0.195 | 0.149 | 0.086 | |
| 5f | 0.2528 | 0.2233 | 0.146 | 0.146 | 0.0997 | |
| 6b | 0.4202 | 0.0712 | 0.0563 | 0.0349 |
As was mentioned in the previous paragraph, the initial free radicals observed with the bitter tea and polyphenol samples were not sufficiently stable to record their EPR spectra under conventional conditions. When samples were prepared on the bench and then investigated as rapidly as possible, the spectra shown in Fig. 3 were obtained. The major component in the spectra from both bitter tea samples (I. kudincha and I. latifolia; Fig. 3a and b) is similar to the spectra that developed with time with both chlorogenic acid and cynarin (Fig. 3c and d). Its simulation using the parameters reported in Table 1 is presented in Fig. 3e; their similarity to those of the radical formed by autoxidation of 2,4,5-trihydroxy cinnamic acid24,26 suggests radical formation on the hydroxylised aromatic ring of caffeoylquinic acids (Fig. 1e).
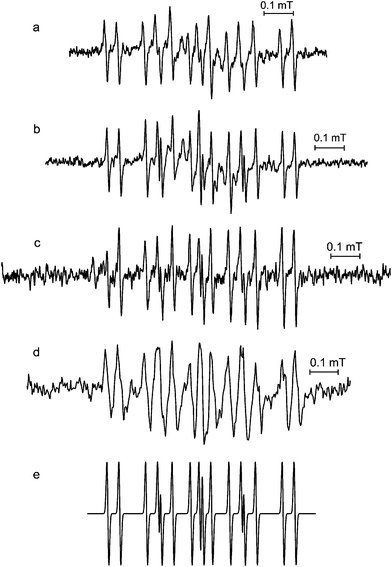 | ||
Fig. 3 EPR spectra of solutions of (a) Ilex kudincha, (b) Ilex latifolia, (d) cynarin after alkaline autoxidation outside the spectrometer, and (c) alkaline autoxidised chlorogenic acid using a pump-flow system (after the pump was stopped); (e) shows a simulation using the parameters from Table 1. 0.1 M NaOH was used and mixed in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with either the tea extract (20 mg ml−1) or 10 mM of the polyphenol. All spectra were recorded using 2 mW microwave power. 100 kHz modulation frequency was used for (a), (b) and (d), and 10 kHz for (c). Modulation amplitudes were 2 μT for (a) 5 μT for (b), 10 μT for (c), and 20 μT for (d). 1 with either the tea extract (20 mg ml−1) or 10 mM of the polyphenol. All spectra were recorded using 2 mW microwave power. 100 kHz modulation frequency was used for (a), (b) and (d), and 10 kHz for (c). Modulation amplitudes were 2 μT for (a) 5 μT for (b), 10 μT for (c), and 20 μT for (d). | ||
A number of additional spectra (Fig. 4) corresponding to intermediate radicals were observed in the pump flow measurements after the pump was switched off. The first intermediate signal (Fig. 4a and b) from both tea samples has 1H hfc constants similar to those from chlorogenic acid autoxidised outside the spectrometer (Fig. 4c, Table 1). Although it was not possible to assign this signal to a specific radical structure, it is almost certainly derived from one or more caffeoylquinic acids. This intermediate radical was unstable and its signal was quickly replaced by a spectrum (Fig. 4e and f), which was identical to the radical of hydroxylised caffeoylquinic acids (Fig. 1e), the main signal observed with the tea samples autoxidised outside the spectrometer (Fig. 3).
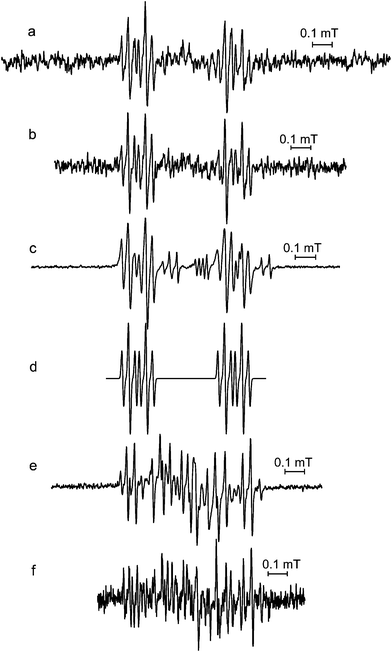 | ||
Fig. 4 Intermediate EPR spectra of alkaline autoxidised solutions of (a) Ilex kudincha, (b) Ilex latifolia, after the pump was stopped, and (c) chlorogenic acid after alkaline autoxidation outside the spectrometer. (d) is a simulation of these spectra using the parameters in Table 1. With increasing time spectra (a) and (b) were replaced by (e) Ilex kudincha and (f) Ilex latifolia. 0.1 M NaOH was used and mixed in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with either the tea extract (20 mg ml−1) or 10 mM of the polyphenol. All spectra were recorded using 2 mW microwave power and 100 kHz modulation frequency. Modulation amplitudes were 10 μT for (a), (b) and (f), 2 μT for (c), and 3 μT for (e). 1 with either the tea extract (20 mg ml−1) or 10 mM of the polyphenol. All spectra were recorded using 2 mW microwave power and 100 kHz modulation frequency. Modulation amplitudes were 10 μT for (a), (b) and (f), 2 μT for (c), and 3 μT for (e). | ||
3.2 Oxidation by superoxide anion radicals
Oxidation of lyophilised tea extracts of both I. kudincha and I. latifolia by O2˙− in DMSO solutions produced identical EPR spectra (Fig. 5a and b). These spectra could be simulated using five inequivalent 1H hfc constants (Table 1), and were similar to initial spectra from both chlorogenic acid and cynarin oxidised under similar conditions (Fig. 5c and e). This result strongly suggests the formation of a similar radical species with each tea sample, and it is assigned to oxidised caffeoylquinic acid (Fig. 1d) that was proposed for the initial spectrum from autoxidation of the bitter tea samples and chlorogenic acid. The differences in the magnitudes of these hfc constants (Fig. 4d and f, Table 1) and those from the radical obtained from alkaline autoxidation of kudingcha and chlorogenic acid (Fig. 2b) are probably the consequence of the use of different solvent systems (DMSO and water).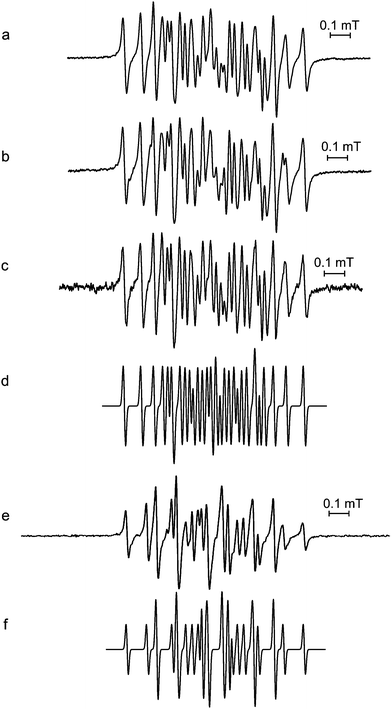 | ||
Fig. 5 Initial EPR spectra from DMSO solutions of (a) Ilex kudincha (20mg ml−1), (b) Ilex latifolia (20mg ml−1), (c) chlorogenic acid (10 mM), and (e) cynarin (10 mM) after oxidation by O2˙− radicals (50 mM), mixed in a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Simulations using the parameters in Table 1 are shown for the radicals from (d) chlorogenic acid, and (f) cynarin. All spectra were recorded using 2 mW microwave power. 100 kHz modulation frequency was used for (e), 20 kHz for (a) and (b), and 10 kHz for (c). Modulation amplitudes were 2 μT for (a) and (b), 10 μT for (c), and 5 μT for (e). 1. Simulations using the parameters in Table 1 are shown for the radicals from (d) chlorogenic acid, and (f) cynarin. All spectra were recorded using 2 mW microwave power. 100 kHz modulation frequency was used for (e), 20 kHz for (a) and (b), and 10 kHz for (c). Modulation amplitudes were 2 μT for (a) and (b), 10 μT for (c), and 5 μT for (e). | ||
With cynarin, the initial radical was very unstable and changed rapidly to produce other spectra (Fig. 6a and c). Although these radicals have not yet been identified, the 1st intermediate spectrum (Fig. 6a) is similar to the 1st intermediate spectrum obtained in bitter tea samples under alkaline autoxidation (Fig. 4a and b). The different values for the hfc constants are again most likely to the consequence of using different solvent systems. The 2nd intermediate spectrum showed at least two signals (Fig. 6c) which were not observed with either of the bitter tea samples, a result which suggests that if such radicals are formed in the bitter teas, they have much lower stability than in pure solutions of the polyphenol.
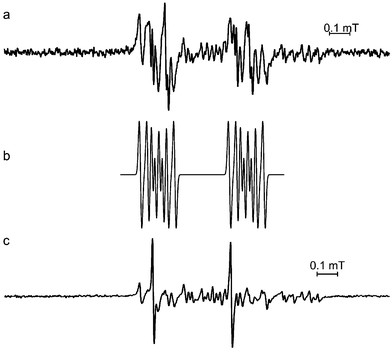 | ||
| Fig. 6 (a) 2nd and (c) 3rd EPR spectra observed from DMSO solutions of cynarin (5 mM final concentration) after oxidation by O2˙− radicals (25 mM final concentration) acquired approx. 4 min (a) and approx. 14 min after the start of the reaction. (b) is a simulation of spectrum (a) using the parameters in Table 1. Spectra were recorded using 2 mW microwave power, 100 kHz modulation frequency and 5 μT modulation amplitude. | ||
4. Discussion
Recent analytical investigations on the composition of bitter teas have identified di- and mono-caffeoylquinic acids as the major polyphenolic components.11,12 In I. kudingcha, Zhu et al.12 found that 67.4% of the total caffeoylquinic acids were accounted by two di-caffeoylquinic acids (3,5-di-O-caffeoylquinic acid and 4,5-di-O-caffeoylquinic acid) and 26.4% by three mono-caffeoylquinic acids, which included chlorogenic acid. The free radical species formed by oxidation of extracts from I. kudincha and I. latifolia samples that were identified by EPR in the present investigations are also consistent with originating largely from caffeoylquinic acids. The fact that these radicals were able to be identified indicates that the reactions of the semiquinone oxidation products with other molecules in the beverages are sufficiently slow for them to be observed in conventional EPR measurements. In the early stages of the autoxidation reaction of the bitter tea samples, these radicals were accompanied by another, as yet unidentified, free radical whose relative contribution differed greatly between the two samples. There may, therefore, be significant differences in the activity of the two beverages, but this would likely be dependent on whether or not this unstable radical contributes to the biological effects. At the present time, however, it has not been identified.In addition to these initial free radicals, spectra were observed from other unstable intermediates, which are probably also radical reaction products derived from caffeoylquinic acids, because of a resemblance between their spectra and intermediates in the oxidation of chlorogenic acid. Furthermore, after prolonged alkaline autoxidation, a radical compound was detected with similar hfc constants to those from 2,4,5-trihydroxycinnamic acid, indicating oxygenation of the aromatic ring of the caffeic acid moiety. However, both chlorogenic acid and cynarin produced similar spectra, so the EPR method was not able to distinguish between the relative importance of the mono- and di-caffeoylquinic acids in the formation of the relatively stable final radicals due to similar chemical environments of the unpaired electron in the various radical structures. Nevertheless, these results further strengthen the importance of the caffeoylquinic acids in the antioxidant chemistry of the two bitter tea samples.
Strong antioxidant activity has been demonstrated previously for aqueous extracts of I. paraguariensis,27,28 and I. kudincha,11,12 and it has been correlated with the total polyphenol contents.11,29 Anesini et al.29 also showed that caffeic and chlorogenic acids were two of the major polyphenols present in I. paraguariensis, and Liu et al.11 and Zhu et al.12 both reported that aqueous extracts of I. kudincha contain large quantities of caffeoylquinic acids, which account for much of the antioxidant activity of the beverages. However, as mentioned in the introduction of this paper, antioxidant assays simply provide an overall measure of a particular chemical property, and give no information on reactions involving oxidation products which are formed during their antioxidant reactions. Also, although chemical analyses provide identification of components that might feature in antioxidant reactions, they give no information on how they might behave in a complex mixture, such as a beverage, where reactions between individual components could have a major impact on the overall chemistry. Furthermore, biological properties are likely to be determined to a major extent by molecules with sufficient stability to be transported within an organism, but with sufficient reactivity to react selectively with specific cells or tissue type. On the basis of the present measurements, the caffeoylquinic acids would appear to be precursors for such molecules in kudingcha and related beverages, and it seems that these molecules show little reactivity with other components in the beverage, at least on a timescale of several minutes. This conclusion is also consistent with recent work with green tea,18 where it was concluded that polyphenol-derived free radicals have appreciable stability in the complex medium of the beverage.
Caffeic and chlorogenic acids have also been shown to have antioxidant activity in other tests,30,31 and the chlorogenic acids are reported to be effective scavengers of the superoxide anion radical.31 In their work with I. paraguariensis, Anesini et al.29 concluded that the contents of caffeic and chlorogenic acids were insufficient to account for all of the peroxidase activity in the leaf extracts, and proposed contributions from other polyphenols, such as quercetin and rutin that are also present in such extracts.32 However, in the present measurements with extracts of I. kudincha and I. latifolia, no evidence was found for the generation of free radicals that correspond to either oxidised quercetin or rutin, although some of the EPR spectral intensity has not yet been assigned, and we suspect that an appreciable fraction of the unidentified activity in the work of Anesini et al.29 is derived from other caffeoylquinic acids.
It should also be pointed out that EPR spectroscopy is most sensitive to the detection of free radicals that are stable or have moderate stability, and one of its greatest strengths is its ability to distinguish between individual radicals from closely related structures. However, very unstable free radicals are difficult to detect with conventional EPR techniques, and their formation might not be seen in experiments such as those reported here. Such radicals can be detected using spin trapping techniques, and Goodman et al.33 have shown results from such investigations on “bitter tea” samples, although these radicals could not be identified beyond the fact that there were O- and C-centred radical species. However, it is free radicals with moderate stability, such as those identified in the present work, that are able to react specifically in biological systems and their parent molecules would thus be expected to be major candidates for medicinal properties. Thus the identification of free radicals derived from caffeoylquinic acids during the oxidation of extracts of I. kudincha and I. latifolia strongly suggests that these molecules may play important roles in at least some of the biological properties of these beverages, especially where the ˙OH or O2˙− free radicals are involved.
5. Conclusions
The EPR spectra from samples of I. kudincha and I. latifolia demonstrate the importance of free radicals derived from caffeoylquinic acids in the oxidation chemistry of these bitter tea beverages; no spectra were observed that could be assigned specifically to saponins or other groups of polyphenol, although some components have not yet been assigned. It is also possible that some active components are not detected, because they do not form radical products with sufficient stability. Nevertheless, caffeoylquinic acids make important contributions to the antioxidant chemistry of various teas from extracts from Ilex species, and products from such reactions are now detected in the present paper. Thus it is important that contributions from these polyphenols are recognised in any attempts to identify quality issues associated with kudingcha and related beverages.Acknowledgements
This work was supported by the Austrian Ministry of Traffic, Innovation and Technology (BMVIT) and the Austrian Science Fund (FWF). We would like to thank Dr Chris Kay (University College London, UK) for providing use of the EMX plus spectrometer for measurements of the cynarin sample.References
- S. Dharmananda, http://www.itmonline.org/arts/kudingcha.htm.
- R. K. Ahai, Iconographia Cormophytorum Sinicoru, Beijing Science, 1985, 641 Search PubMed.
- K. Nishimura, T. Miyase and H. Noguchi, J. Nat. Prod., 1999, 62, 1128–1133 CrossRef CAS.
- M. A. Ouyang, C. R. Yang, Z. L. Chen and H. Q. Wang, Phytochemistry, 1996, 41, 871–877 CrossRef CAS.
- M. A. Ouyang, H. Q. Wang, Z. L. Chen and C. R. Yang, Phytochemistry, 1996, 43, 443–445 CrossRef CAS.
- M. A. Ouyang, C. R. Yang and Z. J. Wu, J. Asian Nat. Prod. Res., 2001, 3, 31–42 CrossRef CAS.
- M. A. Ouyang, Y. Q. Liu, H. Q. Wang and C. R. Yang, Phytochemistry, 1998, 49, 2483–2486 CrossRef CAS.
- J. Huang, X. Wang, Y. Ogihara, N. Shimizu, T. Akiyama and T. Takeda, Chem. Pharm. Bull., 2001, 49, 765–767 CrossRef CAS.
- A. T. C. Taketa, E. Breitmaier and E. P. Schenkel, J. Braz. Chem. Soc., 2004, 15, 205–211 CAS.
- S. C. B. Gnoatto, E. P. Schenkel and V. L. Bassani, J. Braz. Chem. Soc., 2005, 16, 723–726 CAS.
- L. Liu, Y. Sun, L. Tanguy, X. Liang, H. Ye and X. Zeng, Food Chem., 2009, 112, 35–41 CrossRef CAS.
- F. Zhu, Y.-Z. Cai, M. Sun, J. Ke, D. Lu and H. Corke, J. Agric. Food Chem., 2009, 57, 6082–6089 CrossRef CAS.
- Q. Guo, B. Zhao, M. Li, S. Shen and W. Xin, Biochim. Biophys. Acta, Lipids Lipid Metab., 1996, 1304, 210–222 Search PubMed.
- P. T. Gardner, D. B. McPhail and G. Duthie, J. Sci. Food Agric., 1998, 76, 257–262 CrossRef CAS.
- M. A. Morsy and M. M. Khaled, Spectrochim. Acta, Part A, 2002, 58, 1271–1277 CrossRef CAS.
- S. Valcic, A. Muders, N. E. Jacobsen, D. C. Liebler and B. N. Timmermann, Chem. Res. Toxicol., 1999, 12, 382–386 CrossRef.
- F. Nanjo, K. Goto, R. Seto, M. Suzuki, M. Sakai and Y. Hara, Free Radical Biol. Med., 1996, 21, 895–902 CrossRef CAS.
- J. Ferreira Severino, B. A. Goodman, C. W. M. Kay, K. Stolze, D. Tunega, T. G. Reichenauer and K. F. Pirker, Free Radical Biol. Med., 2009, 46, 1076–1088 CrossRef.
- S. Shankar, S. Ganapathy, S. R. Hingorani and R. K. Srivastava, Front. Biosci., 2008, 13, 440–452 CrossRef.
- R. Sheng, Z. L. Gu, M. L. Xie, W. X. Zhou and C. Y. Guo, Planta Med., 2009, 75, 113–120 CrossRef CAS.
- S. Anton, L. Melville and G. Rena, Cell. Signalling, 2007, 19, 378–383 CrossRef CAS.
- S. H. Koh, S. M. Lee, H. Y. Kim, K. Y. Lee, Y. J. Lee, H. T. Kim, J. Kim, M. H. Kim, M. S. Hwang, C. Song, K. W. Yang, K. W. Lee, S. H. Kim and O. H. Kim, Neurosci. Lett., 2006, 395, 103–107 CrossRef CAS.
- J. S. Valentine, A. R. Miksztal and D. T. Sawyer, Methods Enzymol., 1984, 105, 71–81 Search PubMed.
- J. A. Pedersen and B. Ollgaard, Biochem. Syst. Ecol., 1982, 10, 3–9 CrossRef CAS.
- J. A. Pedersen, Biochem. Syst. Ecol., 2000, 28, 229–253 CrossRef CAS.
- P. Ashworth, J. Org. Chem., 1976, 41, 2920–2927 CrossRef CAS.
- R. Filip, S. B. Lotito, G. E. Ferraro and C. G. Fraga, Nutr. Res., 2000, 20, 1437–1446 CrossRef CAS.
- N. Bracesco, M. Dell, A. Rocha, S. Behtash, T. Menini, A. Gigliucci and E. Nunes, J. Altern. Complement. Med., 2003, 9, 379–387 CrossRef.
- C. Anesini, G. Ferraro and R. Filip, Food Chem., 2006, 97, 459–464 CrossRef CAS.
- J. M. Lekse, L. Xia, J. Stark, J. D. Morrow and J. M. May, Mol. Cell. Biochem., 2001, 226, 89–95 CrossRef CAS.
- N. Nakatani, S.-I. Kayano, H. Kikuzaki, K. Sumino, K. Katagiri and T. Mitani, J. Agric. Food Chem., 2000, 48, 5512–5516 CrossRef CAS.
- R. Filip, P. López, G. Giberti, J. Coussio and G. E. Ferraro, Fitoterapia, 2001, 72, 774–778 CrossRef CAS.
- B. A. Goodman, K. F. Pirker, T. G. Reichenauer, P. T. Ho and T. D. Ho Huynh, in Natural Antioxidants and Micronutrients, ed. B. Zhao, G. Liu and L. Packer, Medimond, Bologna, 2005, pp. 139–143 Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
