Combining nutrition, food science and engineering in developing solutions to Inflammatory bowel diseases – omega-3 polyunsaturated fatty acids as an example
Lynnette R.
Ferguson
*ad,
Bronwen G.
Smith
bd and
Bryony J.
James
c
aDiscipline of Nutrition, FM&HS, The University of Auckland, Auckland, New Zealand
bFood Science Programmes, FoS, The University of Auckland, Auckland, New Zealand
cDepartment of Chemical and Materials Engineering, FoE, The University of Auckland, Auckland, New Zealand
dNutrigenomics New Zealand Web: http://www.nutrigenomics.org.nz
First published on 22nd September 2010
Abstract
The Inflammatory bowel diseases, Crohn's disease and ulcerative colitis, are debilitating conditions, characterised by lifelong sensitivity to certain foods, and often a need for surgery and life-long medication. The anti-inflammatory effects of long chain omega-3 polyunsaturated acids justify their inclusion in enteral nutrition formulas that have been associated with disease remission. However, there have been variable data in clinical trials to test supplementary omega-3 polyunsaturated fatty acids in inducing or maintaining remission in these diseases. Although variability in trial design has been suggested as a major factor, we suggest that variability in processing and presentation of the products may be equally or more important. The nature of the source, and rapidity of getting the fish or other food source to processing or to market, will affect the percentage of the various fatty acids, possible presence of heavy metal contaminants and oxidation status of the various fatty acids. For dietary supplements or fortified foods, whether the product is encapsulated or not, whether storage is under nitrogen or not, and length of time between harvest, processing and marketing will again profoundly affect the properties of the final product. Clinical trials to test efficacy of these products in IBD to date have utilised the relevant skills of pharmacology and gastroenterology. We suggest that knowledge from food science, nutrition and engineering will be essential to establish the true role of this important group of compounds in these diseases.
Introduction
Inflammatory bowel diseases (IBD) appear in two forms, Crohn's disease (CD) and ulcerative colitis (UC). Both are debilitating diseases, for which pharmaceutical treatments are moderately successful, albeit having significant side-effects. We have previously suggested that nutritional (or nutrigenomic) solutions may be appropriate,1,2 and there have been a number of clinical trials in this area. However, if nutritional therapies are to be successfully developed, it is essential that we move beyond pharmaceutical thinking, and put the full power of an integrated set of knowledge on effects of food science and engineering behind new developments. The potential of omega-3 polyunsaturated acids (n-3 PUFA) in IBD is reviewed in this light.Fish oil is a common source of n-3 PUFA, and there is reason to believe that increasing fish oils or other sources of n-3 PUFA may have health benefits in IBD.3,4 However, their role is controversial, with variable data among different studies and between individual responses. In all of these reviews and in specific studies identified therein and below, the wide variation in dosages and formulations used, and often lack of information on storage conditions and administration methods, makes it difficult to reach an informed opinion on potential benefits, or to recommend dosages for specific treatment goals.
The method of presentation of the n-3 PUFA could be expected to have a direct impact on the bioavailability and efficacy of the nutrient. It has been shown that with foods high in naturally occurring n-3 PUFA the cooking method has a direct impact on the amount of EPA and DHA retained in the product.5–7 The options for including PUFA into fortified foods include direct addition of a high n-3 PUFA source such as fish oil, or incorporation of stabilised emulsions or microcapsules. The impact of subsequent food processing steps, such as cooking, is not well reported in the literature. Although many commercial products such as breads and spreads incorporate these ingredients, much of the knowledge of their preparation is proprietary. Where the impact of heating is reported,8,9 the effect on EPA and DHA levels appears to be minimal. By far the greater concern is the oxidative stability of the PUFA during storage, and antioxidants are widely incorporated into PUFA ingredients. Alternatively the n-3 PUFA is incorporated in the form of microencapsulated particles, which have improved oxidation resistance and the potential to protect the PUFA from interaction with the food matrix.10
There are a number of currently available systematic and Cochrane reviews, to effects of enteral nutrition formulas containing n-3 PUFA or of high dose supplementation with either oils or capsules of various sorts on the induction or maintenance of remission in IBD.11–15 However, sample numbers are often small and results are variable. Bassaganya-Riera and Hontecillas16 concluded “there is an urgent need for placebo-controlled, large-scale, multicenter clinical trials”. We would contend that an equally urgent need is to integrate current knowledge of food science and engineering to ensure strict definitions of sample composition, storage and delivery to optimise the likely effects of these materials, before even planning such important trials.
Polyunsaturated fatty acids and common dietary sources
The chemistry of fatty acids has been well described elsewhere17 but a few characteristics are outlined here. Fatty acids are linear hydrocarbon molecules which vary in the length of their acyl chain and bond type, and this gives rise to their nomenclature. Chains which consist entirely of single bonds are referred to as being saturated, while those with one double bond are monounsaturated, and those with two or more are described as polyunsaturated fatty acids (PUFA).18 Each chain is further characterized by the presence of a methyl group and a carboxyl group at either end of the molecule. The designation omega, ω- or n- to PUFA refers to the position of the first double bond from the terminal methyl carbon Hence, n-3 PUFA have the first double bond in the third position, whereas for n-6 PUFA it is in the sixth position. Common names also exist for several. The n-3 PUFA group includes octadecatrienoic usually termed α-linolenic acid (18:3n-3), eicosopentaenoic acid (EPA) (20:5n-3) and docosahexaenoic acid (DHA) (22:6n-3). When inflammatory conditions are being considered, two n-6 PUFA should also be noted and these are linoleic acid (LA) (18:2n-6) and arachidonic acid (AA) (20:4n-6). Structures of some key compounds are illustrated on Fig. 1.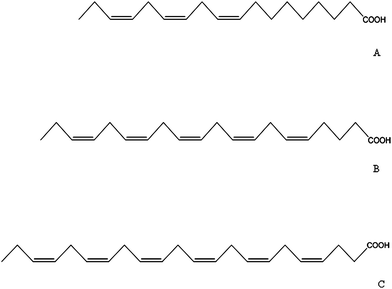 | ||
| Fig. 1 n-3 Fatty acids. A = α-linolenic acid, 18:3n-3; B = eicosapentaenoic, 20:5n-3; C = docosahexaenoic acid, 22:6n-3. | ||
Linoleic and α-linolenic acids participate in biochemical pathways. LA can undergo elongation and saturation to AA, and α-linolenic acid can be metabolized to EPA and DHA,4 although this process is less efficient in men than in women.19 AA has been associated with inflammation as this is the precursor for eicosanoids, whereas EPA and DHA have been shown to have ameliorating effects for inflammatory conditions.3 Thus, the relative amounts of LA and α-linolenic acid in a food have some biological relevance, but not all cell types may respond the same way. For example the infiltration of monocytes or macrophage cells in the terminal ileum of SAMP1/Yit mice was impaired by chows containing either fish oil or perilla oil, with the perilla oil being more effective. Unfortunately the complete fatty acid profile of the oils was not reported except to say that the fish oil contained 25–30% EPA and DHA, and the perilla oil was 55–60% α-linolenic acid with overall concentration of 8% w/w n-3 PUFA.20
Oily fish and fish oil usually contain more EPA and DHA than other foods, but not all extracted oils or oily foods are created equal. Moreover, compositional data can be confusing and unintentionally misleading, if not all the information is considered.21 Fish are biological entities and their tissue composition will reflect their genetics, diet, musculature, sea temperature and global location, season and spawning cycles. Fish store oil in their liver and tissues, primarily as a reserve for gonad development. Wild fish have more freedom to roam or migrate than farmed and as a consequence may have less fatty tissue. Thus, their lipid content and profile may be very different from those of farmed fish. However, the advantage of the farmed fish is that their diet can be controlled and may include supplementation with n-3 PUFA. Nevertheless, one cannot assume that dietary intake will always result in an equivalent change in tissue composition, and the responses may not always be as expected.22
Because of concerns of depletion of fish resources, attention is shifting towards more sustainable production of long chain n-3 PUFA. An oil rich in these products can be produced from microalgae such as Micromonas pusilla,23 and a diet enriched in such an oil shows similar protective properties to one enriched in fish oil, at least in experimental models.24 Other authors (e.g.25) are seeking a sustainable, land-based production system for long chain n-3 PUFA, including metabolic engineering of an artificial pathway that produces such compounds in plants.
Evidence for n-3 PUFA playing a role in human IBD
Where dietary supplements or fortified foods are advocated for use, this is usually to address a nutritional deficiency in a population group. However, there is no reason to believe that IBD patients are deficient in these essential fatty acids. For example, no significant differences were found between the long-chain n-3 PUFA status of IBD patients compared with controls, and data did not support the concept of EPA or DHA deficiency in patients with IBD.26 These observations would agree with other studies. However, there seems some reason to believe that IBD patients may show an excessive n-3:n-6 PUFA ratio. The fatty acid composition of plasma phospholipids, anthropometric characteristics, and dietary intake data were measured on 29 UC patients, 20 CD patients, and 31 healthy controls.27 The authors reported a significantly lower lipid intake in IBD patients as compared with controls, but proportionally higher levels of n-6 fatty acids, thereby implicating n-3:n-6 PUFA ratios rather than n-3 PUFA levels per se in the pathophysiology of the disease.The significance of n-3:n-6 PUFA ratios to human health have been highlighted by several authors, including Simopoulos28 and Calder;3,4. A high n-6/n-3 ratio, is found in current Western diets, although it was not a characteristic of traditional diets. There is some reason to believe that this high ratio promotes the pathogenesis of many chronic diseases, in addition to IBD. A high dietary LA intake not only leads to LDL oxidation, but also interferes with the incorporation of n-3 PUFA into cell membrane phopholipids. Both n-3 and n-6 PUFA influence gene expression, but whereas n-3 PUFA have anti-inflammatory effects, reducing the expression of TNF-α, IL-1b, IL-6 and IL-8, high levels of n-6 PUFA increase the expression of pro-inflammatory genes.
If n-3 PUFA do indeed have a beneficial effect in IBD, this might be expected to most effectively manifest itself in highly controlled studies such as those considering enteral nutrition. Under these circumstances, the entire diet is strictly controlled, and the formulae are unlikely to contain substances that may trigger disease symptoms. A Cochrane Database systematic review compared different enteral nutrition formulas in inducing or maintaining remission in active CD.12 While it was concluded that there was evidence for the nature and amount of fat affecting both outcomes, none of the trials included had comparable formulas with and without n-3 PUFA, or varied the ratios of n-3:n-6 PUFA. The closest was the report that compared an n-6 PUFA-containing formula with one containing monounsaturated fatty acids, and showed the latter to be superior.29 Two enteral supplements enriched with n-3 fatty acids and/or n-6 fatty acids were compared, with results suggestive of superior results for the n-3 enriched formula.30 However, remission was not an endpoint of that study, and both formulas improved clinical and biochemical markers during the course of the experiment.
Even in the absence of a deficiency, n-3 PUFA dietary supplements may be beneficial because of their known anti-inflammatory effects. Fig. 2 provides an illustration of some of the points at which n-3 PUFA might act to reduce chronic inflammation in IBD. A systematic review studied both published and unpublished trials on the effects of n-3 fatty acids on IBD between 1966 and 2003.4,11 The authors identified 13 controlled trials that assessed the effects on pathologically confirmed rates of induced remission or relapse, or requirements for steroids and other immunosuppressive agents in IBD. However, there was an enormous variability in considered end points across the various studies. Three studies (but only one reaching adequate statistical significance) suggested that n-3 PUFA reduced corticosteroid requirements, but there were no other consistent effects across studies. The therapeutic potential of n-3 PUFA in IBD was again reviewed.13 The authors reported that the most commonly reported adverse effects of fish oil supplements are a fishy aftertaste and gastrointestinal upsets. When recommending n-3 PUFA, the authors suggested that clinicians should be aware of any possible adverse effects. Neither review put a high emphasis on the form of the supplement and/or storage/administration conditions.
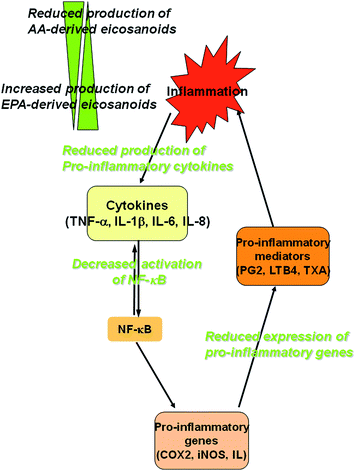 | ||
| Fig. 2 Schematic representation of some of the points at which n-3 PUFA may affect the development of chronic inflammation in IBD. Long chain n-3 fatty acids (EPA and DHA) are incorporated into cell membranes where they influence the production of eicosanoids, resolvins, and cytokines. A number of n-3 PUFA act as substrates for the synthesis of eicosanoids, which in turn may directly down-regulate inflammation. This biosynthetic process may also competitively reduce the formation of eicosanoids from n-6 PUFA, which again may reduce inflammation. Long chain n-3 PUFA can also down-regulate the activation of the expression of pro-inflammatory genes, including TNF-α, Cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS) or various interleukins (IL), possibly through their effects on transcription factors or through their conversion to resolvins. They also have effects on T cell reactivity and antigen presentation (not illustrated). | ||
Two sets of Cochrane Database systematic reviews are available on effects of n-3 PUFA supplementation on maintenance of remission in IBD. A focus on CD14 included data on patients of any age group, who were in remission at the time of recruitment, and were followed for at least six months. The intervention must have been fish oil or n-3 PUFA, given in a pre-defined dosage. The primary outcome was the rate of relapse, while secondary outcomes included changes in disease activity scores, time to first relapse and adverse events. Six studies met the criteria for eligibility, and showed a marginally significant benefit of n-3 PUFA therapy for maintaining remission. However, the studies were both clinically and statistically heterogeneous, and two large studies showed negative results. There was also evidence for publication bias. Although no serious adverse events were seen, a pooled analysis showed a significantly higher rate of diarrhoea and symptoms of the upper gastrointestinal tract in the n-3 PUFA treatment group. The authors concluded that existing data do not support routine maintenance treatment of CD with n-3 PUFA.15 A further analysis focused on the use of fish oil for induction of remission in UC. Six studies were included, 3 of cross-over design and 3 of parallel design. No data were pooled for analysis due to differences in outcomes and methodology among the included studies. One small study showed a positive benefit for induction of remission, while some of the other included studies showed some positive benefits for secondary outcomes. However, the authors cautioned that results were inconclusive due to small study size and poor study quality.
More conclusive evidence can be provided by considering individual clinical trials, examples of which are summarised in Table 1.31–40 An n-3 PUFA diet therapy was considered for IBD patients.31 Dietary guidance on increasing n-3 PUFA and decreasing n-6 PUFA led to a significant increase in the n-3:n-6 ratio in the erythrocyte membrane of IBD patients, and this ratio was significantly higher in the remission group, suggesting an influence on the fatty acid composition of the cell membrane and clinical activity in IBD patients.
| Population and numbers | Trial design | n-3 PUFA form and dose | Method of administration | Study endpoint | Result | Reference |
|---|---|---|---|---|---|---|
| The initial-onset Japanese patients were composed of 12 UC patients (3 males, 9 females, mean age 32.9 years and 8 CD patients (5 males, 3 females, mean age 29.0 years) who had not undergone any diet therapy before dietary intervention. The follow-up group was divided into 2 subgroups: the remission and relapse groups, and fatty acid composition was compared between the 2 subgroups. Among the UC patients, those in whom the severity was evaluated as mild were assigned to the remission group. The others were assigned to the relapse group. Among the CD patients, those in whom neither endoscopy nor contrast-enhanced radiography revealed an active lesion were assigned to the remission group. The others were assigned to the relapse group. | Non blinded, non crossover | Patients were prohibited from consuming the main sources of dietary n-6 PUFA: i.e., vegetable oil; seasonings such as margarine, dressings, and mayonnaise; foods cooked in vegetable oil; and snacks. The target for n-3 PUFA ingestion was a total of 5100 mg. The patients were able to confirm the n-3 and n-6 PUFA contents of each food using an “n-3 PUFA food exchange table” | About 3400 mg/day (2 units) of ALA were ingested from 7 mL/day of perilla (Egoma) oil in addition to a daily intake of 1700 mg from fish oil (EPA, DHA), taken as oil (no storage recommendation, n-3 PUFA food exchange table | Fatty acid composition of the erythrocyte membranes and disease activity after 12–18 months intervention | In a subset of 20 initial-onset patients, the mean n-3/n-6 ratio significantly increased after intervention. In the follow-up group the ratio in the remission group (n = 145) was significantly higher than that in the relapse group (n = 85). The ratio decreased significantly in those who suffered a relapse after the beginning of treatment. | 31Uchiyama et al. (2010) |
| 18 out-patients at Haukeland University Hospital (Bergen, Norway) between 18 and 75 years old with IBD, as assessed by a gastroenterologist, in combination with presence of joint pain. | Randomized, controlled, double blind pilot trial comparing whale oil with seal oil, but with no placebo group. | Seal Oil (purchased from JFM Sunile AS, Os, Norway) or Whale Oil (donated by Myklebust Trading AS, Myklebost, Norway). The oils were protected with nitrogen on top in bottles, and stored in a refrigerator during the study, otherwise in a −20 °C freezer. | Ten mL were self-adminis- tered through a naso-pharyngeal feeding tube for 10 days, three times daily before meals. | Plasma arachidonic acid to EPA ratio and prostaglandin E2 levels, decreased IBD-related joint pain and IBD-disease activity, and improved quality of life. | Significant and positive changes from baseline to study end were observed in both groups for all endpoints. There were no significant differences seen between seal oil or whale oil. | 32Bjørkkjær et al., 2009 |
| EPIC1: 363 patients total. 188 patients were assigned to receive n-3 PUFA and 186 patients to receive placebo. | Patients were eligible if they had experienced a disease exacerbation within the past year and were in remission for at least 3 months but not longer than 12 months. Patients were randomly assigned to receive either 4 × 1g/d of n-3 PUFA or placebo for 52 weeks. No other treatments for CD were permitted. | Epanova capsules consisting of 50% to 60% EPA and 15% to 25% DHA as a free fatty acid, The placebo capsule consisted of 1 g of medium-chain triglyceride oil. | 1-g of n-3 PUFA encapsulated in a delayed-release soft gelatin capsule (Epanova; Tillotts Pharma AG, Ziefen, Switzerland) | Clinical relapse, or initiation of treatment for active CD. | No significant difference in the relapse rate was found between the patients treated with n-3 or placebo. | 33Feagan et al. (2008) |
| EPIC2: 375 patients. 189 patients were randomised to n-3 PUFA and 190 patients to placebo. | Patients with active disease were treated with a standardized 16-week tapering course of either prednisone or budesonide. Eight weeks after the initiation of corticosteroid treatment, a disease was assessed. If this was less than an agreed cut off, the patient was eligible for randomization. Patients were randomly assigned to receive either 4 g/d of n-3 PUFA or placebo for 58 weeks. No other treatments for CD were permitted. | As for EPIC1. | As for EPIC1 | As for EPIC1 | The relapse rate was 84/187 (44.9%) and 94/188 (50%) in the n-3 and placebo groups, respectively | 33Feagan et al. (2008) |
| A total of 38 children (5 to 16 years of age, 53% male), 18 in the n-3 PUFA group and 20 in the controls. They were recruited from pediatric gastroenterology centers in Italy. At baseline, participants were in remission. | A double blind, placebo-controlled trial of one year duration. | TRIOLIP-SOFAR, Italy; 1.2 g/d of EPA and 0.6 g/day of DHA, as triglycerides, versus identical placebo of time dependent 5-ASA (50 mg/kg/d) + olive oil | Time dependent 5-ASA (50 mg/kg/d) + n-3 PUFA in GI-resistant capsules | Primary outcome was relapse rate within one year. Time to first relapse was also recorded, but not systematically presented. | A very high relapse rate was found in the placebo group of this study. The authors concluded that enteric coated n-3 PUFA in addition to 5-ASA are effective for maintenance of remission in pediatric CD. In this study, compliance was optimal, no patients were lost to follow-up and all patients were analyzed. The median time to first relapse was eight months in the intervention group compared to one month in the placebo group. | 34Romano et al., 2005 |
| A total of 78 adults (18 to 67 years of age, 50% men), 39 in each arm from outpatient clinics in Italy. At baseline, participants were in remission and high risk for relapse, as judged by an increase in at least one of three serum inflammatory markers. | A double blind, placebo-controlled trial of one year duration. Patients treated with 2.7 g/d of n-3 PUFA compared with placebo. | 1.8 g/day of EPA and 0.9 g/day of DHA, as free fatty acids, versus identical placebo of 500 mg Miglyol 812 (a mixed-acid triglyceride of fractionated short chain fatty acids made up of caprylic and capric acid). | Enteric coated fish oil hard gelatine capsule with 60 min delay timed release. Oral route of administration | Relapse rates over one year of follow-up. Adverse events and time to first relapse were also monitored. | Significantly fewer patients from the intervention group relapsed compared to placebo and it was concluded that enteric coated, timed release n-3 PUFA is highly effective in maintaining remission in CD. | 35Belluzzi et al., 1996 |
| A multicenter study in Germany. A total of 135 adults (17 to 65 years of age, 31% men) with CD, 70 in the n-3 PUFA arm and 65 in the control arm. At baseline, participants were in remission, induced by a course of corticosteroids. | A double blind, placebo-controlled trial of one year duration. Steroids were still administered during the first two months of the study, but no other co-interventions were allowed. Patients were instructed to consume a diet low in arachidonic acid and rich in fiber and were followed every three months until one year. | 5g/d of highly concentrated n-3 PUFA (3.3 g/day of EPA and 1.8 g/day of DHA, as ethyl Esther) versus identical placebo of corn oil. | Gelatin n-3 PUFA capsules. Oral route of administration | Primary outcome was the relapse- free time period after randomization. Relapse rate and adverse events were also monitored. | No difference in the relapse rate of the intervention group. The estimated 1-year relapse rate was 69% in both groups. The authors concluded that gelatin capsules of ethyl ester fish oil capsules are not effective for maintenance of remission in CD. | 36Lorenz-Meyer et al., 1996 |
| 18 out-patients (9 male, 9 female) with UC. All had distal procto-colitis. | A double-blind, placebo-controlled randomised study, and a parallel group, for a 6 month treatment period | Active group: 15 ml of fish oil extract that provided a total of 3.2g of EPA and 2.4g of DHA. Placebo group: 15 ml of sunflower oil. All oil supplements included 3% vitamin E. | Patients received either 15 ml of fish oil extract or 15 ml of sunflower oil as placebo, and were instructed to take 5 ml, 3 times a day. Oil preparations were supplied by Callanish Ltd (Isle of Lewis, Scotland). There were no special instructions for storage, although the material contained a small amount of an antioxidant. Oral route of administration, oil taken as a liquid. | Sigmoidoscopic score, histological score, NK cytotoxicity and flow cytometry. Assessments were done at monthly intervals. | After 3 months of supplementation, all the patients in the EFA group went into remission. After 6 months supplementation with EPA/DHA there was a significant improvement in disease activity as shown by the reduction of the clinical score, compared with baseline In the placebo group, there was no significant change in the clinical score, as compared with baseline. | 37Almallah et al., 1998 |
| 17 male patients with mild to moderate UC. Oral steroids (<20 mg per day) and sulfasalazine were allowed if the patient had taken them for more than 4 weeks. | A double-blind, placebo-controlled, crossover study. An 8 month treatment period (3 months per treatment arm followed by a two month washout period). | Active group: Max-EPA (15 capsules provided a total of 2.7g of EPA, 1.8g of DHA, and 135 kcal). Placebo group: Corn oil (provided a total of 10.3g of oleic acid, 2.1g of palmitic acid, 1.8g of linoleic acid, and 135 kcal). | Oral route of administration | Disease activity, clinical and laboratory evaluation, histology and mucosal LTB4 levels. Assessments were done at monthly intervals. | Significant reduction in disease activity index. The changes were determined from baseline data. | 38Aslan et al., 1992 |
| 10 patients (5 male, 5 female) with mild to moderate UC. The comparison was fish oil versus sulfasalazine. | Randomised, crossover study. A 2 month intervention period, followed by 2 month washout period and then 2 month cross-over intervention period. | Active group 1: 5.4 g/d fish oil (18 capsules, each of 180 mg of EPA and 120 mg of DHA. Active group 2: 2 g/d sulfasalazine. | Fish oil capsule (R.P. Scherer do Brasil Encapsulac, Sao Paulo, Brazil). Oral route of administration. No details of nature of capsule, purity, or storage. | Laboratory blood parameters, sigmoidoscopy score, histologic activity and protein metabolism evaluation. Assessments were done at monthly intervals. | Regression not assessed. CRP, ERS, and platelet count increased significantly during treatment with fish oil. The changes were determined from baseline data. The sigmoidoscopy Score after n-3 PUFA intake was significantly lower than at study entry. | 39Dichi et al., 2000 |
| 24 out-patients (10 male, 8 female), with active UC. | Multicenter, randomised, double-blind, placebo-controlled, crossover trial. The initial 4-month treatment period was followed by a 1-month washout period during which all patients received placebo. Patients then crossed over and began a second 4-month period during which the original treatment assignments were reversed. | Active group: Max-EPA (18 capsules provided a total of 3.24g of EPA, 2.16g of DHA, and 162 kcal.). Placebo group: vegetable oil (18 capsules provided 12.36g of oleic acid, 2.52g of palmitic acid, 2.16g of linoleic acid, and 162 kcal). | Fish oil (Max-CPA, R. P. Scherer, Clearwater, Florida) or placebo (vegetable oil capsules, provided by R. P. Scherer). Patients received either 18 fish oil capsules or 18 placebo capsules per day and were instructed to take 6 capsules 3 times a day. The Max-EPA and placebo capsules were identical in appearance. Oral route of administration. | Sigmoidoscopy scores, global assessment score, histology and rectal dialysis. Assessments were done at monthly intervals. | No significant changes in endoscopic score for both groups. | 40Stenson et al., 1992 |
The Epanova Program in Crohn's Study (EPIC) was a pair of randomized controlled trials, that were based over 98 centers in Canada, Europe, Israel, and the United States.33 Details of the trial design and data are described in Table 1. The commonly cited conclusion of these trials has been described as “treatment with n-3 PUFA was not effective for the prevention of relapse in CD”. However, consideration of the details in the table show differences in a number of events, including CD worsening (or relapse) where the n-3 PUFA group showed fewer subjects, but these individuals also showed an increase in abdominal pain, diarrhea, arthralgia, nasopharyngitis, nausea and fatigue. There were differences between the trials, not only in length of time they went, but also in the entry criteria. What is unclear is how this dose level was set, since it appears higher than commonly found in a dietary supplement.
De Ley15 published a Cochrane review on n-3 PUFA and UC. Of the 6 published trials that met their stringent trial design criteria, only 437–40 were full manuscripts, for which details are summarised in Table 1.31–40 Although most of these studies were small and underpowered, the results are generally positive.
Other marine sources may prove important for IBD. Liprinol, a preparation from New Zealand green-lipped mussels, is thought to be worth pursuing for anti-inflammatory effects after results indicating that there were benefits in C57BL/6 mice fed a preparation of this in olive oil.41 However, the authors conceded that it was unlikely that the n-3 PUFA were responsible for the ameliorating effects as the dosage was very low, < 1mg per day.
Despite optimism in some of the studies, it must be concluded that hard data, directly relevant to human IBD, is limited. The reasons for an apparent lack of activity in many of the studies may include pharmacokinetic limitations and intestinal degradation of the compounds, in particular insufficient sub-mucosal levels of the effective compounds, as well as the known general limitations of studies with free living humans. The studies are typically high dose, and the importance of dose response has not been generally stressed. Furthermore, with very few exceptions,32 there is no indication of storage conditions or measures taken to prevent oxidation of the products.
Possible reasons for failure of some of the n-3 PUFA supplementation trials in IBD
There are a considerable number of potential artifacts, if a clear understanding of genetics, nutrition, food science and engineering is not applied to trials of these fatty acids. These fall under several headings, as follows:Differences between individuals in response to n-3 PUFA
Simopoulos42 draws attention to the range of genes that affect the uptake, transport and metabolism of n-3 PUFA. The level and composition of n-3 PUFA in the human body is not only a function of the dietary intake, but also determined by endogenous metabolism, as fatty acid precursors are endogenously elongated and desaturated to physiologically active long chain compounds. D5 and D6 desaturases are the key enzymes involved in this conversion, and these enzymes are highly active in the liver, brain, heart and lung. The fatty acid desaturase 1 gene (FADS1), encodes saturase D5, while fatty acid desaturase 2 (FADS2), encodes saturase D6, both genes being located on chromosome 11 (11q12– 13.1). Genetic variants in FADS1 and FADS2 lead to a highly significant decrease in the endogenous production of arachidonic acid (AA) and its precursors.43,44 Given male/female differences in response to n-3 PUFA, it is of some interest that variants in the FADS1 and FADS2 genes are associated with altered levels of both n-6 and n-3 PUFA in plasma and erythrocyte phospholipids in women during pregnancy, and in breast milk during lactation.45High density lipoprotein (HDL) particles transport certain lipids through the properties of the apolipoprotein (apo) components, apoA-I and apoE. ApoA-I and apoE act to solubilize phospholipids and stabilize HDL particles, enabling these proteins to be partners with products of the multidrug transporter, ABCA1, in mediating the efflux of cellular phospholipids and cholesterol.46 Variants in the genes encoding these enzymes modulate the transport, and therefore effective functioning of n-3 PUFA. For example, there was evidence that the plasma n-3 fatty acid response to an n-3 PUFA acid supplement was modulated by the apoE epsilon4 variant.47 The authors considered the plasma fatty acid response to a dietary supplement of EPA + DHA in carriers of the E4 allele in a group of human volunteers. When the group was separated based on the presence of E4, the baseline EPA and DHA in plasma were 67 and 60% higher, respectively, in E4 carriers. Thus, highly significant gene x diet interactions were found, whereby only non-carriers of the E4 allele had increased levels of EPA and DHA in plasma in response to an n-3 PUFA dietary supplement.47
The effects of n-3 PUFA intake were considered in relation to effects on various genetic polymorphisms, as an example of nutrigenetics in CD.48 The study estimated seven SNPs in interleukin 1 (IL-1), tumor necrosis factor alpha (TNF-α), lymphotoxin alpha (LT-alpha), and IL-6 genes in 116 controls and 99 patients with CD, in relation to the nature and levels of fat intake. A high intake of total, saturated, and monounsaturated fats, and a higher ratio of n-3:n-6 PUFA, was associated with a more active phenotype. Additionally, low intakes of n-3 PUFA and high n-3:n-6 PUFA ratios in patients with the TNF alpha 857 polymorphism were associated with significantly higher disease activity.
Different disease characteristics of selected patient groups
Different stages of disease or patient characteristics might explain some of the data. For example, Belluzzi and coworkers35 only included patients at a high risk of relapse, as defined by the presence of an elevated serum concentration of inflammatory biomarkers. In contrast, patents recruited into the EPIC studies were at substantially lower risk and it is possible that the trials lacked sufficient statistical power to detect the required 15% benefit of treatment. However, the authors noted this could not have been true for EPIC-2, in which 51.2% of patients relapsed33Poor subject compliance
Although no beneficial effect was observed in prevention of relapse in the EPIC trials, the authors noted a statistically significant decrease in the serum concentration of triglycerides in the patients who received the n-3 PUFA supplement. Additionally, an analysis of capsule counts provided confidence that the patients consumed adequate amounts of the dietary supplements, suggesting that poor compliance could not account for the negative results.Different formulations used in the different studies
The formulation evaluated in the study by Belluzzi et al/35 was a hard gelatin capsule, whereas the EPIC trials incorporated a soft gelatin capsule.33 Although the authors claimed that a similar concentration of −3 PUFA is incorporated into cell membranes following administration of either preparation, there are some very substantial differences in the properties of these two capsule types. First, there is likely to be difference in the dissolution rate of the hard vs. soft gelatin once it is ingested.49 Secondly, there is a substantial difference in oxidation protection from hard vs. soft gelatin. This is reported for microcapsules,50 and would be equally true of full size capsules, although the surface area to volume ratio means the degradation rate would be faster for the microcapsules.Oxidation of trial products
As n-3 PUFA are highly unsaturated, they are very susceptible to oxidation.51,52 Literature reports on studies of oxidative stability of PUFA are contradictory and somewhat controversial, making the reported impact of antioxidants difficult to consistently quantify.53 This might be due to the range of test methods employed, as suggested by Frankel, but equally might reflect the impact of the food matrix itself on the action of antioxidants.54 What is clear is that as n-3 PUFA oxidise, the detection of “off flavours” increases and, with PUFA from marine sources, these manifest as a fishy flavour or odour.55–57 Oxidation also reduces the nutritional benefit of the PUFA and can lead to products that have an adverse effect of health.58,59To avoid or delay oxidation, PUFA are stabilised by antioxidants. These can be synthetic (for example butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA)60 or disodiumethylenediaminetetraacetic acid EDTA,61 or natural extracts such as ascorbic acid, rosemary, oregano or green tea extract.54,62–64 However, once incorporated into a food matrix the efficacy of these antioxidants may be compromised by factors such as pH, in some cases even acting to accelerate oxidation, as occurred in a study of omega 3 enriched mayonnaise54 where ascorbic acid was used as the antioxidant. In that study the proposed mechanism for oxidation acceleration was the based on the role of iron; in the presence of pH below 6.0 the iron ions become accessible and are reduced, by the ascorbic acid, from Fe3+ to Fe2+ which is a more efficient oxidation catalyst. Similarly, in a study of baked cereal, the antioxidant EDTA was added to an incorporated fish oil emulsion but actually resulted in increased oxidation.9 In this instance the proposed mechanism was again the reduction of Fe3+ to Fe2+ by EDTA chelates. Without information on the storage time, temperature and humidity of the capsules prior to ingestion the impact of oxidation cannot be quantified, although the hard versus soft gelatin would have had an impact if all else had been equal.
Bioavailability of n-3 PUFA in different formulations
Numerous studies from the food engineering perspective report levels of EPA and DHA potentially available, depending on method of incorporation. Studies from the nutritional perspective report on the bioavailability of these compounds in various food products or dietary supplements, with few details of the method of presentation of the PUFA. Very few studies report the links between bioavailability and method of presentation, though equivalence has been reported between traditional fish oil capsules and microencapsulated PUFA incorporated into food matrices.65–67The influence of oral breakdown of a food matrix containing PUFA additions, the impact of enzymes on the matrix and PUFA-food matrix interaction during transport through the GI tract have not yet been reported. However, this aspect of the nutritional fortification of foods by PUFA will need to receive attention in the near future and the tools and techniques are available or are being developed. For example the role of oral breakdown and interactions with the food matrix is now being studied in the context of bioavailability of nutrients68 and glycaemic index.69
Potential interactions with other components in the capsules or food matrix
n-3 PUFA are not usually found in isolation or in equal amounts among species, or their extracted oils, and this may give rise to equivocal results and make it difficult to attribute a clinical outcome. This was true in the studies by Brox70 and Brunborg71 who compared the effects of seal and cod liver oils. The seal oil had similar amounts of EPA, almost three times as much docosapentaenoic acid (DPA, 22:5n-3), and a third less DHA, than cod liver oil. When administered nasoduodenally for 10 days there was improvement in joint pain and overall disease activity in the seal oil group. However when administered orally for two weeks, despite improvements, there was no significant difference between the CD and UC groups taking either seal oil or cod liver oil.71 The seal oil used in that study had approximately 25% less thiobarbituric acid reactive substances, which are an indication of secondary oxidation products, and 80% less α-tocopherol, but the impact of the quantities of these components of the oils in the study is not known. The important point here is that n-3 PUFA do not occur in isolation, and that the contribution of other fatty acids or components in the oil or food should not be overlooked.Increasing the dietary intake of n-3 PUFA
If we assume that there is sufficient positive data to imply a significant benefit to either healthy humans or IBD patients, it is important to consider the best source of these. n-3 PUFA occur at naturally high levels in oily fish such as mackerel, salmon, tuna, herring etc.72 Fish is often regarded as being expensive, but the perceived high cost in terms of their n-3 PUFA may not always be valid because of the richness of the source.21 Although fresh farmed salmon, for example, may be of higher cost than other species, the concentration of n-3 PUFA (%/ g) should not be ignored. Nevertheless, in any local market, the full range of available species should be analysed to identify the species which may provide good sources of n-3 PUFA as well as a nutritious meal for a family.The low consumption of fish in a Western diet73 means that alternative means of delivery are being continually explored. Increasing the levels of PUFA in other meat based products by incorporating it into animal feed is one approach74–76 but dietary supplements (capsules) and fortification of foods are the two main routes to increasing intake of n-3 PUFA. Many commercial products are now available from speciality ingredient and nutritional supplement suppliers, and these are often described as stabilised and odourless, both necessary factors for inclusion in foods.
Despite the advances in aquaculture, marine-derived oils and fish stocks in general are under threat from diminishing resources. Hence the focus turns back to terrestrial sources and modifications of existing high producing oil crops for sustained supply of n-3 PUFAs. However, native plant oils and plant foods in general are not usually regarded as being such excellent sources of n-3 PUFAs as fish oils because of the lower concentrations of n-3 PUFAs in these. Nevertheless, one cannot discount the native forms entirely. For example, the leaves of purslane (Portulaca oleracea), which for a long time has featured in the diets of many communities, contain 4 mg/g wet weight α-linolenic acid.77,78
Cultivation and plant breeding techniques to enhance production of oil crops are well-established. However, Murphy points out that means of encouraging plants to produce desirable amounts of fatty acids in the organ which is most easy to harvest and process is often challenging.79 Nevertheless, advances in analytical techniques coupled with micro-dissection, have permitted rapid screening of high producing lines and the technical advances likely to arise from biofuel initiatives may well provide more.
Optimising the presentation of dietary omega-3 PUFA
Naturally present or direct addition
Adding n-3 PUFA, especially those sourced from marine oils, directly to foods has numerous implications in terms of sensory attributes (taste, odour and texture), shelf stability, nutritional quality and safety. One of the main challenges facing food producers is the consumer acceptability of long chain n-3 PUFA fortified products. Direct incorporation of commercial PUFA products into a range of foods, including fat based spreads, yoghurts and fruit juices, has been shown to decrease palatability in direct proportion to an increase in detectable fishy odour.59,80 Even when incorporated into fat based spreads a stabilised commercial fish oil described as “odourless” was detectable, and undesirable, at levels around 0.05% weight.81 Storage often emphasises the “off flavours” associated with rancidity, reported for both baked goods82 and fruit juice.80 This is a direct result of the oxidative instability of PUFA.Microencapsulation
Alternative methods of stabilising PUFA prior to addition to foods include emulsification83 and microencapsulation (an extension of emulsification where a stable emulsion is subsequently dried, often by spray drying.60 Microencapsulation is a very promising technology for encapsulating and protecting PUFA for incorporation into food matrices and for targeted delivery of nutrients.10 Microencapsulation has been used for protecting functional ingredients, such as flavours, for over 40 years84 The active ingredient is enclosed in a capsule that may be made of a variety of materials including polysaccharides, gums, proteins, lipids or synthetic polymers. The particles range between 5 and 1000 microns, and are produced by a number of techniques including, amongst others, spray drying, coacervation, extrusion and self-assembly of liposomes. There are many reviews of the various microencapsulation technologies including details of the capsule material, the particle size and the particle production step.60,84–86 Interestingly, despite widespread application in pharmaceuticals and cosmetics, microencapsulation in food engineering is still a relatively niche technology. This is driven, in part, by the lower margins typically associated with food ingredients; the influence of this aspect of food manufacturing is reviewed, in the context of microencapsulation.85 The advantages of the microencapsulation technique, for fortifying foods with PUFA and other bioactives, include protecting functional ingredients from degradation and concealing undesirable flavours, whilst maintaining acceptable product texture.10One of the primary advantages for microencapsulation of PUFA is the protection against oxidation (and subsequent detectable “off” flavours) during food storage. Microencapsulation can substantially delay, but not completely eliminate, oxidation.59,81,87 In fact the microencapsulation process can accelerate oxidation of the PUFA during the emulsification stage due to greatly increased surface area.88,89 Notwithstanding the possible oxidation during production of the microcapsules, these particles have been repeatedly shown to increase resistance to oxidation of PUFA when incorporated into foods. When encapsulated with gelatine, n-3 PUFA showed a 3-fold decrease in oxidation products after 4 weeks storage (storage conditions not reported).50 Oxidation resistance reported in this study was best when an additional hardening step (ethanol extraction of chemical cross-linking) was included for the capsule. However, a hardened microcapsule would possibly be discernible, as the capsules in this study were around 40μm diameter, and depending on hardness and shape, particles as small as 15μm can be detected as “grittiness” in foods.90,91 When incorporated into baked cereal snack bars as direct oil addition, oil emulsion and as microencapsulated particles the encapsulated particles showed no significant oxidation products after 11 weeks of storage.9
A further advantage of microencapsulation of n-3 PUFA is the possibility to reduce detectable flavours, such as the fishy flavour associated particularly with marine based fatty acids. Studies have shown the advantage of microencapsulation over direct addition of fish oil in terms of taste80,82 and, again, the influence of food matrix selection has been recognised. For example when adding PUFA to breads (considered an ideal delivery system as the CO2 generated during proofing and baking protect against oxidation, and bread products have an intrinsically short shelf-life60) lower additions are possible to white bread than brown due to the stronger flavours of brown bread.72 Selecting a microencapsulation system (capsule material, active ingredient formulation and encapsulation system) is dictated by the food matrix that is to be fortified.60 An optimum solution for minimising impact on flavour and texture, whilst maintaining the nutritional value of the n-3 PUFA, might not be the most technologically accessible. For example in a study examining addition to bread correct selection of the capsule material (soybean protein extract) ensured minimal impact on flavour of the baked bread or rheology of the dough. However, the authors recommended an alternative encapsulant (methylcellulose) for ease of production, despite a greater impact on taste and texture.92 Many reports of dietary studies list large numbers of commercially available foods that have been eaten by subjects with little or no adverse impact on the favour or texture profile of the product.72,93–95
Bioavailability affected by method of presentation
Linking the method of presentation of n-3 PUFA (naturally occurring, direct oil addition, emulsion or microencapsulated) with its bioavailability is a vexed question. Studies from the food engineering perspective tend to concentrate on the PUFA prior to consumption. These studies imply nutritional availability by reporting the amount of EPA and DHA provided in a serving of food.81,96–99 However, studies from the nutritional analysis point of view often do not include details of the method of incorporating PUFA into the food matrix73,93,94 nor controls comparing, for example, direct oil addition with microencapsulation.The impact of the method of presentation of PUFA in a fortified food matrix might be inferred from complementary studies, for example where n-3 PUFA has been incorporated into bread as direct oil addition100 and as microcapsules.101 In both cases, plasma levels of fatty acids indicated the n-3 PUFA were bioavailable. However, in the case of direct oil addition, detail is not provided as to how and when the addition was made. In the case of the microcapsules, whether the bread was served toasted or not and the impact of the toasting step is not reported. Incorporating n-3 PUFA microcapsules as part of a number of commercially available fortified food products has demonstrated good bioavailability (measured by plasma fatty acid profile).102 Moreover, the subjects reported good acceptability of the food products (milk, yoghurt and breads) with no gastrointestinal distress.
Some studies have compared the bioavailability of PUFA incorporated into food by direct oil addition and in the microencapsulated form. A study in rats103 found the same distribution of fatty acids in the livers of the animals, whether the PUFA was incorporated directly or in microcapsules. This study might be indicative for incorporation of PUFA into human foods that require no cooking step (such as fat based spreads). However, it is not informative about the potential impact of heat or long term storage, since the samples were stored for up to 14 days at −80 °C, substantially colder than commercial cold-store.
Three studies by separate groups have reported a comparison of bioavailability of n-3 PUFA from traditional gel encased fish oil capsules and microencapsulated fish oil incorporated into a food matrix. The food matrices studied were bread, biscuit and soup65 or milkshake.66,67 In the case of the milkshakes, the microencapsulated fish oil powder was stirred into the drink prior to consumption. In the case of the bread, biscuit and soup, specific details of how and when the microencapsulated fish oil was incorporated are not given. In all cases, the studies reported equivalent bioavailability of n-3 PUFA, as measured by plasma levels of fatty acids and cholesterol, whether administered as capsules or food incorporated microcapsules. These are exemplary studies for comparing delivery vehicles (capsules versus microcapsules) but do not tackle the question of the impact of the food matrix, heating effects or storage time on bioavailability of PUFA. A recent review104 of the influence of emulsion structure on lipid digestion includes an excellent overview of some of the structural analysis techniques that will be required to fully understand how the method of presentation of n-3 PUFA influences their bioavailability.
One of the most promising areas of development in microencapsulation is the potential for site-specific delivery of nutrients. Site specific drug delivery has received more attention than site specific nutrient delivery. For example, microencapsulation has been used for delivery of drugs directly to the colon.105–107 Similar approaches are now being taken to increase the intestinal delivery of probiotics,108–110 where encapsulation in alginate capsules has been show to protect the contents against degradation in the upper gastrointestinal (GI) tract. Very few studies are reported for site specific delivery of lipids, though animal studies have shown the feasibility of protecting dietary fats using lecithin-chitosan encapsulation.111 A very recent study has reported site specific delivery of n-3 PUFA to the GI tract in rats.112 The choice of encapsulating material dictated whether fish oil was delivered predominantly to the small intestine or to the large bowel.
Conclusions
There is reason to believe that increasing n-3 PUFA and decreasing n-6 PUFA in the diet of IBD patients could be beneficial. However, we suggest that there is an urgent need for consistency in design among clinical trials, and attention paid to method of presenting these to humans, whether as foods, fortified foods or dietary supplements. In particular, we believe that consideration needs to be paid to the source and form of n-3 PUFA. The best available evidence pertains to dietary sources rather than high dose supplements, and the approach using a combination of food exchange tables and lower dose supplements is of significant interest.31 We noted the evidence that feeding Atlantic salmon diets rich in n-3 PUFAs did not always have the expected outcome. Can something be learned from this for other animal or human intervention trials? The duration of the supplementation, stage of life and pre-existing medical conditions are all likely to affect the outcome of supplementation.If dietary supplements are to be recommended to IBD patients, it is essential to ensure that advice is based upon adequate trial design, that is well powered with different patient groups showing comparable disease activity. Stratifying according to genotype would be beneficial. The dietary supplement or n-3 PUFA-containing foods must be stored or manufactured so as to prevent oxidation, and administered in such as way as to optimise uptake and bioavailability, in order to be quite sure about the benefits that a micro-ingredient might provide to healthy individuals, or those compromised in some way such as the IBD group. Careful characterisation of the exact fatty acid composition of any food or oil supplement, together with knowledge of the target tissue and cell types, should be mandatory to assign with confidence the precise factors giving rise to a clinical outcome.
Abbreviations
| AA | arachidonic acid |
| Apo | apolipoprotein |
| CD | Crohn's disease |
| COX | cyclooxygenase |
| DHA | docosahexaenoic acid |
| EPA | eicosapentaenoic acid |
| FADS1 | fatty acid desaturase 1 gene |
| FADS2 | fatty acid desaturase 2 gene |
| HDL | high density lipoprotein |
| IBD | Inflammatory bowel disease |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| LA | linoleic acid |
| LDL | low-density lipoprotein |
| LT | leukotriene |
| LT-alpha | lymphotoxin alpha |
| NF-κB | nuclear factor kappa B |
| n-3 | omega-3 |
| PG | prostaglandin |
| PUFA | polyunsaturated fatty acid |
| TNF | tumor necrosis factor |
| UC | ulcerative colitis |
References
- L. R. Ferguson, M. Philpott and P. Dryland, Cell. Mol. Life Sci., 2007, 64, 3105–3118 CrossRef CAS.
- L. R. Ferguson, Expert Review of Clinical Immunology, 2010, 6, 573–583 Search PubMed.
- P. C. Calder, Mol. Nutr. Food Res., 2008, 52, 885–897 CrossRef CAS.
- P. C. Calder, Int. Rev. Immunol., 2009, 28, 506–534 Search PubMed.
- S. Mierke-Klemeyer, R. Larsen, J. Oehlenschläger, H. Maehre, E. O. Elvevoll, N. M. Bandarra, R. Parreira, A. M. Andrade, M. L. Nunes, E. Schram and J. Luten, Eur. Food Res. Technol., 2008, 227, 827–833 CrossRef CAS.
- B. Regulska-Ilow and R. Ilow, Nahrung, 2002, 46, 383–388 CrossRef CAS.
- S. Al-Saghir, K. Thurner, K.-H. Wagner, G. Frisch, W. Luf, E. Razzazi-Fazeli and I. Elmadfa, J. Agric. Food Chem., 2004, 1, 38–43.
- J. Alamed, D. J. McClements and E. A. Decker, Food Chem., 2006, 95, 585–590 CrossRef CAS.
- N. S. Nielsen and C. Jacobsen, Int. J. Food Sci. Technol., 2009, 44, 1536–1546 CrossRef CAS.
- C. P. Champagne and P. Fustier, Curr. Opin. Biotechnol., 2007, 18, 184–190 CrossRef CAS.
- C. H. MacLean, W. A. Mojica, S. J. Newberry, J. Pencharz, R. H. Garland, W. Tu, L. G. Hilton, I. M. Gralnek, S. Rhodes, P. Khanna and S. C. Morton, Am. J. Clin. Nutr., 2005, 82, 611–619 CAS.
- M. Zachos, M. Tondeur and A. M. Griffiths, Cochrane Database of Systematic Reviews Reviews, 2007 DOI:10.1002/14651858.CD000542.pub2 CD000542.
- J. W. Fetterman, Jr and M. M. Zdanowicz, Am. J. Health-Syst. Pharm., 2009, 66, 1169–1179 CrossRef.
- D. Turner, J. Hyams, J. Markowitz, T. Lerer, D. R. Mack, J. Evans, M. Pfefferkorn, J. Rosh, M. Kay, W. Crandall, D. Keljo, A. R. Otley, S. Kugathasan, R. Carvalho, M. Oliva-Hemker, C. Langton, P. Mamula, A. Bousvaros, N. LeLeiko and A. M. Griffiths, Inflammatory Bowel Dis., 2009, 15, 1218–1223 Search PubMed.
- M. De Ley, R. de Vos, D. W. Hommes and P. Stokkers, Cochrane Database of Systematic Reviews Reviews, 2007 DOI:10.1002/14651858.CD005986.pub2 CD005986.
- J. Bassaganya-Riera and R. Hontecillas, Curr. Opin. Clin. Nutr. Metab. Care, 2010, 13, 569 CrossRef CAS.
- T. P. Coultate, Food: the chemistry of its components, Royal Society of Chemistry, Cambridge, UK, 5th edn, 2009 Search PubMed.
- F. D. Gunstone, The chemistry of oils and fats: Sources, composition, properties, and uses, Blackwell Publications, Oxford, UK, 2004 Search PubMed.
- G. C. Burdge, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2006, 75, 161–168 CrossRef CAS.
- H. Matsunaga, R. Hokari, C. Kurihara, Y. Okada, K. Takebayashi, K. Okudaira, C. Watanabe, S. Komoto, M. Nakamura, Y. Tsuzuki, A. Kawaguchi, S. Nagao and S. Miura, Clin. Exp. Immunol., 2009, 158, 325–333 CrossRef CAS.
- C. J. O'Connor, S. R. Katvi and Q. Chen, in Handbook of Australasian edible oils, ed. C. J. O'Connor, Oils and Fats Specialist Group of the New Zealand Institute of Chemistry, Auckland, New Zealand, 2007, ch., pp. 109–119 Search PubMed.
- R. G. Ackman, in Fatty foods and their health implications, ed. C. K. Chow, CRC Press, Boca Raton, FL, 3rd edn, 2008, ch. 8, pp. 155–185 Search PubMed.
- D. Cannon, Explore (New York, N.Y.), 2009, 5, 299–303 Search PubMed.
- V. A. van Beelen, B. Spenkelink, H. Mooibroek, L. Sijtsma, D. Bosch, I. M. C. M. Rietjens and G. M. Alink, Food Chem. Toxicol., 2009, 47, 316–320 CrossRef CAS.
- J. R. Petrie, P. Shrestha, M. P. Mansour, P. D. Nichols, Q. Liu and S. P. Singh, Metab. Eng., 2010, 12, 233–240 CrossRef CAS.
- M. Figler, B. Gasztonyi, J. Cseh, G. Horvath, A. G. Kisbenedek, S. Bokor and T. Decsi, Br. J. Nutr., 2007, 97, 1154–1161 CrossRef CAS.
- Y. Ueda, Y. Kawakami, D. Kunii, H. Okada, M. Azuma, D. S. N. T. Le and S. Yamamoto, Nutr. Res., 2008, 28, 239–244 CrossRef CAS.
- A. P. Simopoulos, Exp. Biol. Med., 2008, 233, 674–688 Search PubMed.
- M. A. Gassull, F. Fernandez-Banares, E. Cabre, M. Papo, M. H. Giaffer, J. L. Sanchez-Lombrana, C. Richart, H. Malchow, F. Gonzalez-Huix and M. Esteve, Gut, 2002, 51, 164–168 CrossRef CAS.
- A. A. Nielsen, J. N. Nielsen, H. Grønbaek, M. Eivindson, I. Vind, P. Munkholm, I. Brandslund and H. Hey, Digestion, 2007, 75, 10–16 CAS.
- K. Uchiyama, M. Nakamura, S. Odahara, S. Koido, K. Katahira, H. Shiraishi, T. Ohkusa, K. Fujise and H. Tajiri, Inflammatory Bowel Dis., 2010 DOI:10.1002/ibd.21251.
- T. Bjorkkjaer, P. Araujo, T. M. Madland, A. Berstad and L. A. Froyland, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2009, 81, 425–432 CrossRef CAS.
- B. G. Feagan, W. J. Sandborn, U. Mittmann, S. Bar-Meir, G. D'Haens, M. Bradette, A. Cohen, C. Dallaire, T. P. Ponich, J. W. McDonald, X. Hebuterne, P. Pare, P. Klvana, Y. Niv, S. Ardizzone, O. Alexeeva, A. Rostom, G. Kiudelis, J. Spleiss, D. Gilgen, M. K. Vandervoort, C. J. Wong, G. Y. Zou, A. Donner and P. Rutgeerts, JAMA, J. Am. Med. Assoc., 2008, 299, 1690–1697 Search PubMed.
- C. Romano, S. Cucchiara, A. Barabino, V. Annese and C. Sferlazzas, World J. Gastroenterol., 2005, 11, 7118–7121 Search PubMed.
- A. Belluzzi, C. Brignola, M. Campieri, A. Pera, S. Boschi and M. Miglioli, N. Engl. J. Med., 1996, 334, 1557–1560 CrossRef CAS.
- H. Lorenz-Meyer, P. Bauer, C. Nicolay, B. Schulz, J. Purrmann, W. E. Fleig, C. Scheurlen, I. Koop, V. Pudel and L. Carr, Scand. J. Gastroenterol., 1996, 31, 778–785 CrossRef CAS.
- Y. Z. Almallah, S. Richardson, T. O'Hanrahan, N. A. Mowat, P. W. Brunt, T. S. Sinclair, S. Ewen, S. D. Heys and O. Eremin, Am. J. Gastroenterol., 1998, 93, 804–809 CrossRef CAS.
- A. Aslan and G. Triadafilopoulos, Am. J. Gastroenterol., 1992, 87, 432–437 CAS.
- I. Dichi, P. Frenhane, J. B. Dichi, C. R. Correa, A. Y. O. Angeleli, M. H. Bicudo, M. A. M. Rodrigues, C. R. Victória and R. C. Burini, Nutrition, 2000, 16, 87–90 CrossRef CAS.
- W. F. Stenson, D. Cort, J. Rodgers, R. Burakoff, K. DeSchryver-Kecskemeti, T. L. Gramlich and W. Beeken, Ann. Intern. Med., 1992, 116, 609–614 CAS.
- D. Tenikoff, K. J. Murphy, M. Le, P. R. Howe and G. S. Howarth, J. Gastroenterol., 2005, 40, 361–365 CrossRef CAS.
- A. P. Simopoulos, Journal of Nutrigenetics and Nutrigenomics, 2009, 2, 117–118 Search PubMed.
- E. Lattka, T. Illig, J. Heinrich and B. Koletzko, Clin. Nutr., 2010, 29, 277–287 CrossRef CAS.
- C. Molto-Puigmarti, J. Plat, R. P. Mensink, A. Muller, E. Jansen, M. P. Zeegers and C. Thijs, Am. J. Clin. Nutr., 2010, 91, 1368–1376 CrossRef CAS.
- L. Xie and S. M. Innis, J. Nutr., 2008, 138, 2222–2228 CrossRef CAS.
- S. Lund-Katz and M. C. Phillips, Subcell. Biochem., 2010, 51, 183–227 Search PubMed.
- M. Plourde, M. C. Vohl, M. Vandal, P. Couture, S. Lemieux and S. C. Cunnane, Br. J. Nutr., 2009, 102, 1121–1124 CrossRef CAS.
- C. S. Guerreiro, P. Ferreira, L. Tavares, P. M. Santos, M. Neves, M. Brito and M. Cravo, Am. J. Gastroenterol., 2009, 104, 2241–2249 CrossRef.
- C. B. Bottom, M. Clark and J. T. Carstensen, J. Pharm. Sci., 1997, 86, 1057–1061 CrossRef CAS.
- A. Lamprecht, U. Schafer and C. M. Lehr, J. Microencapsulation, 2001, 18, 347–357 CrossRef CAS.
- C. D. Nuchi, D. J. McClements and E. A. Decker, J. Agric. Food Chem., 2001, 49, 4912–4916 CrossRef CAS.
- A. Kamal-Eldin and N. V. Yanishlieva, Eur. J. Lipid Sci. Technol., 2002, 104, 825–836 CrossRef CAS.
- E. N. Frankel, T. Satue-Gracia, A. S. Meyer and J. B. German, J. Agric. Food Chem., 2002, 50, 2094–2099 CrossRef CAS.
- C. Jacobsen, M. Timm and A. S. Meyer, J. Agric. Food Chem., 2001, 49, 3947–3956 CrossRef CAS.
- C. Jacobsen, Fett/Lipid, 1999, 101, 484–492 CrossRef CAS.
- A. Ye, J. Cui, A. Taneja, X. Zhu and H. Singh, Food Res. Int., 2009, 42, 1093–1098 CrossRef CAS.
- W. Kolanowski, F. Świderski, E. Lis and S. Berger, Int. J. Food, 2001, 52, 469–476 Search PubMed.
- R. Turner, C. H. McLean and K. M. Silvers, Nutr. Res. Rev., 2006, 19, 53–62 Search PubMed.
- W. Kolanowski and G. Laufenberg, Eur. Food Res. Technol., 2006, 222, 472–477 CrossRef CAS.
- L. Sanguansri and M. A. Augustin, in Functional food ingredients and nutraceuticals processing technologies, ed. J. Shi, CRC/Taylor & Francis, Boca Raton, FL, 2006, ch. 12, pp. 297–327 Search PubMed.
- L. A. Shaw, D. J. McClements and E. A. Decker, J. Agric. Food Chem., 2007, 55, 3112–3119 CrossRef CAS.
- S. Mildner-Szkudlarz, R. Zawirska-Wojtasiak, W. Obuchowski and M. Goslinski, J. Food Sci., 2009, 74, S362–370 CrossRef CAS.
- M. Pérez-Mateos, M. C. Gómez-Guillén, J. L. Hurtadoa, M. T. Solas and P. Montero, Food Chem., 2002, 79, 1–8 CrossRef CAS.
- R. A. Trindade, J. Mancini-Filho and A. L. C. H. Villavicencio, LWT–Food Sci. Technol., 2010, 43, 98–104, DOI:10.1016/j.lwt.2009.06.013.
- J. M. W. Wallace, A. J. McCabe, P. J. Robson, M. K. Keogh, C. A. Murray, P. M. Kelly, G. Márquez-Ruiz, H. McGlynn, W. S. Gilmore and J. J. Strain, Ann. Nutr. Metab., 2000, 44, 157–162 CrossRef CAS.
- C. J. Barrow, C. Nolan and B. J. Holub, Journal of Functional Foods, 2009, 1, 38–43 Search PubMed.
- S. Higgins, Y. L. Carroll, N. M. O'Brien and P. A. Morrissey, Journal of Human Nutrition and Dietetics, 1999, 12, 265–271 Search PubMed.
- R. M. Faulks and S. Southon, Biochim. Biophys. Acta, 2005, 1740, 95–100 CAS.
- K. A. Germaine, S. Samman, C. G. Fryirs, P. J. Griffiths, S. K. Johnson and K. J. Quail, J. Sci. Food Agric., 2008, 88, 652–658 CrossRef CAS.
- J. Brox, K. Olaussen, B. Osterud, E. O. Elvevoll, E. Bjornstad, G. Brattebog and H. Iversen, Lipids, 2001, 36, 7–13 CrossRef CAS.
- L. A. Brunborg, T. M. Madland, R. A. Lind, G. Arslan, A. Berstad and L. Frøyland, Clin. Nutr., 2008, 27, 614–622 CrossRef CAS.
- E. A. Trautwein, Eur. J. Lipid Sci. Technol., 2001, 103, 45–55 CrossRef CAS.
- R. G. Metcalf, M. J. James, E. Mantzioris and L. G. Cleland, Eur. J. Clin. Nutr., 2003, 57, 1605–1612 CrossRef CAS.
- M. Betti, B. L. Schneider, W. V. Wismer, V. L. Carney, M. J. Zuidhof and R. A. Renema, Poult. Sci., 2009, 88, 1085–1095 Search PubMed.
- M. Juárez, A. Marco, N. Brunton, B. Lynch, D. J. Troy and A. M. Mullen, Food Chem., 2009, 117, 393–397 CrossRef CAS.
- M. D'Arrigo, L. Hoz, I. Cambero, C. J. Lopez-Bote, C. Pin and J. A. Ordonez, Lebensm. Wiss. Technol., 2004, 37, 585–591 CrossRef CAS.
- A. P. Simopoulos, Asia Pac. J. Clin. Nutr., 2002, 11, S163–S173 CrossRef CAS.
- A. P. Simopoulos, D. X. Tan, L. C. Manchester and R. J. Reiter, J. Pineal Res., 2005, 39, 331–332 CrossRef CAS.
- D. J. Murphy, Plant breeding to change lipid composition for use in food, Woodhead Publishing, Cambridge, UK, 2006 Search PubMed.
- W. Kolanowski, F. Swiderski and S. Berger, J. Sci. Food Agric., 1999, 50, 39–49 CAS.
- W. Kolanowski, F. Świderski, D. Jaworska and S. Berger, J. Sci. Food Agric., 2004, 84, 2135–2141 CrossRef CAS.
- S. O. Serna-Saldivar, R. Zorrilla, C. De La Parra, G. Stagnitti and R. Abril, Plant Foods Hum. Nutr., 2006, 61, 121–129 CrossRef CAS.
- D. J. McClements, E. A. Decker and J. Weiss, J. Food Sci., 2007, 72, R109–124 CrossRef CAS.
- B. F. Gibbs, S. Kermasha, I. Alli and C. N. Mulligan, Int. J. Food Sci. Nutr., 1999, 50, 213–224 CrossRef CAS.
- S. Gouin, Trends Food Sci. Technol., 2004, 15, 330–347 CrossRef CAS.
- F. Shahidi and X.-Q. Han, Crit. Rev. Food Sci. Nutr., 1993, 33, 501–7852 CrossRef CAS.
- W. Kolanowski, M. Ziolkowski, J. Weißbrodt, B. Kunz and G. Laufenberg, Eur. Food Res. Technol., 2006, 222, 336–342 CrossRef CAS.
- W. Kolanowski, D. Jaworska, J. Weißbrodt and B. Kunz, J. Am. Oil Chem. Soc., 2007, 84, 37–45 CrossRef CAS.
- K. Heinzelmann, J. Franke, J. Velasco and G. Márquez-Ruiz, Eur. Food Res. Technol., 2000, 211, 234–239 CrossRef CAS.
- A. J. Rosenthal, ed., Food texture - Measurement and perception, Aspen Publishers, Gaithersburg, MD, 1999 Search PubMed.
- J. M. Aguilera and P. J. Lillford, ed., Food materials science: Principles and practice, Springer, New York, 2007 Search PubMed.
- G. Davidov-Pardo, P. Roccia, D. Salgado, A. E. León and R. Pedroza-Islas, American Journal of Food Technology, 2008, 3, 384–393 Search PubMed.
- C. S. Patch, L. C. Tapsell, T. A. Mori, B. J. Meyer, K. J. Murphy, J. Mansour, M. Noakes, P. M. Clifton, I. B. Puddey, L. J. Beilin, G. Annison and P. R. Howe, J. Am. Diet. Assoc., 2005, 105, 46–1926 CrossRef.
- J. Whelan and R.C., Annu. Rev. Nutr., 2006, 26, 75–103 CrossRef CAS.
- R. A. Harrison, M. Sagara, A. Rajpura, L. Armitage, N. Birt, C. A. Birt and Y. Yamori, Nutr., Metab. Cardiovasc. Dis., 2004, 14, 344–350 Search PubMed.
- J. Kong, M. P. Dougherty, L. B. Perkins and M. E. Camire, J. Food Sci., 2008, 73, S118–123 CrossRef CAS.
- K. H. Lee, H. Joaquin and C. M. Lee, J. Food Sci., 2007, 72, S119–124 CrossRef CAS.
- L. Gouveia, A. P. Batista, A. Raymundo and N. Bandarra, Nutr. Food Sci., 2008, 38, 492–501 CrossRef.
- L. Gouveia, C. Coutinho, E. Mendonça, A. P. Batista, I. Sousa, N. Bandarra and A. Raymundo, J. Sci. Food Agric., 2008, 88, 891–896 CrossRef CAS.
- M. Liu, R. Wallin and T. Saldeen, Nutr. Res., 2001, 21, 1403–1410 CrossRef CAS.
- Y. L. Yep, D. Li, N. J. Mann, O. Bode and A. J. Sinclair, Asia Pac. J. Clin. Nutr., 2002, 11, 285–291 CrossRef CAS.
- C. P. Earnest, M. K. Hammar, M. Munsey, C. R. Mikus, R. M. David, J. A. Bralley and T. S. Church, Journal of the International Society of Sports Nutrition, 2009, 6, 12, DOI:10.1186/1550-2783-6-12.
- A. Rosenquist and G. Holmer, Zeitschrift für Ernährungswissenschaft, 1996, 35, 178–184 CrossRef CAS.
- M. Golding and T. J. Wooster, Curr. Opin. Colloid Interface Sci., 2010, 15, 90–101 CrossRef CAS.
- H. J. Lee, Arch. Pharmacal Res., 2002, 25, 572–584 Search PubMed.
- M. L. Lorenzo-Lamosa, C. Remunan-Lopez, J. L. Vila-Jato and M. J. Alonso, J. Controlled Release, 1998, 52, 109–118 CrossRef CAS.
- H. Tozaki, J. Komoike, C. Tada, T. Maruyama, A. Terabe, T. Suzuki, A. Yamamoto and S. Muranishi, J. Pharm. Sci., 1997, 86, 1016–1021 CrossRef CAS.
- S. Graff, S. Hussain, J.-C. Chaumeil and C. Charrueau, Pharm. Res., 2008, 25, 1290–1296 CrossRef CAS.
- P. Sriamornsak, Eur. J. Pharm. Sci., 1999, 8, 221–227 CrossRef CAS.
- V. Chandramoulia, K. Kailasapathy, P. Peiris and M. Jones, J. Microbiol. Methods, 2004, 56, 27–35 CrossRef.
- G. Y. Park, S. Mun, Y. Park, S. Rhee, E. A. Decker, J. Weiss, D. J. McClements and Y. Park, Food Chem., 2007, 104, 761–767 CrossRef CAS.
- G. S. Patten, M. A. Augustin, L. Sanguansri, R. J. Head and M. Y. Abeywardena, Dig. Dis. Sci., 2009, 54, 511–521 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
