Comparison of the polyphenolic composition and antioxidant activity of European commercial fruit juices
Gina
Borges
a,
William
Mullen
b and
Alan
Crozier
*a
aPlant Products and Human Nutrition Group, Division of Developmental Medicine, Faculty of Medicine, University of Glasgow, Graham Kerr Building, Glasgow, G12 8QQ, United Kingdom. E-mail: a.crozier@bio.gla.ac.uk; Tel: +44 141 330 4613
bDivision of Ecology and Evolutionary Biology, Faculty of Biomedical and Life Sciences, University of Glasgow, Graham Kerr Building, Glasgow, G12 8QQ, United Kingdom
First published on 13th September 2010
Abstract
Thirty six commercial European fruit juices were tested to ascertain their antioxidant capacity and polyphenolic composition. Six of the products were labelled 100% pomegranate juice, the others included 20 brands of diluted pomegranate juice or pomegranate blended with other fruit juices and 10 different non-pomegranate fruit juices. The antioxidant capacity of all the juices was determined while anthocyanin, ellagitannin and ellagic acid profiles of the 26 pomegranate juices and pomegranate juice blends were obtained using HPLC-PDA-MS2. Additional analysis was conducted on seven of the juices using HPLC with an on-line antioxidant detection system. Three of the “pure” pomegranate juices had the highest ellagitannin content and the highest antioxidant capacity. Only one of these three juices was rich in anthocyanins. The other “pure juices” had differences in their HPLC “pomegranate” fingerprint and also had a lower antioxidant capacity, in some cases lower than that of some of the blended juices. Vitamin C rather than phenolic compounds was the major contributor to the antioxidant capacity for some of the juices. Statistical analysis of both the antioxidant assay and the HPLC on-line antioxidant data demonstrated that the ellagitannins were the major antioxidants in the pomegranate juices. The complexity of the polyphenolic profile of pomegranates necessitates the use of HPLC-PDA-MS2 for a thorough evaluation of juice composition and authenticity.
1 Introduction
The evidence that diets rich in fruits and vegetables provide a reduced risk of chronic diseases is compelling.1 Flavonoids and related phenolic compounds that occur in plant-derived foods have been associated with these protective effects. As a consequence of the substantial research in this area, fruit juices are being used increasingly by people who are looking for healthy options as part of the WHO 5-a-day dietary recommendations. This is reflected in a steady global rise in fruit juice consumption. Western Europe is the second largest regional market.2 Of the ten countries with the highest per capita consumption, six are found within this region with a consumption of more that 28 liters/person/year. Interest in pomegranate (Punica granatum L.) juice and its products has also increased markedly in recent years with a growing number of reports on their potential health benefits. These include pomegranate juice consumption being associated with inhibition of prostrate cancer in men,3 a reduction in serum oxidative stress in plasma of type-2 diabetes mellitus patients,4 reduced atherosclerosis in diabetic patients,5 and potential protection against colon cancer.6There is enormous variability in antioxidant (AOX) activity and phenolic compounds present in different commercial fruit juices.7,8 Some products were of questionable authenticity with the actual ingredients not matching what was claimed on the label. Pomegranates are characterized by the presence of ellagitannins and anthocyanins. However, the levels vary in juices prepared from different pomegranate cultivars,9 maturity stage,9,10 and they are even absent in some commercial products.7,11 Zhang et al.12 used a combination of analytical procedures to develop an “International Multidimensional Authenticity Specification” (IMAS) algorithm to detect a diversity of adulterants of pomegranate juices and drinks.
This paper compares 36 European commercial juices derived from pomegranates, and in some instances other fruits, by measuring their total AOX capacity. HPLC-PDA-MS2 was used to obtain fingerprints of pure pomegranate juices and blended pomegranate products to ascertain their composition. In addition, HPLC with on-line AOX detection was used to assess the relationship between the ellagitannin, ellagic acid and anthocyanin content of pomegranate juices and their AOX capacity.
2 Results and discussion
2.1 AOX capacity and vitamin C levels
Thirty six juices (Table 1) were investigated initially using the Folin-Ciocalteu assay for total phenols (TP)13 and three different AOX assays. The FRAP14 and TEAC15 AOX assays are simple colorimetric methods based on a single electron transfer reaction and it is assumed that the antioxidant activity is equal to the reducing capacity. The ORAC assay quantifies the peroxyl radical scavenging capacity.16 In terms of overall ranking of the AOX capacity and TP content of the individual juices the four assays yielded very similar results (Table 2). However, for comparative purposes an AOX index was calculated using procedures described by Seeram et al.8 (AOX index = [(sample score/best score) × 100]) which gives a composite score taking into account the results obtained with the different methods. The AOX index was very high (>95) for three of the ‘pure’ juices, PG01, PG02, PG03 while values of <54 were obtained for the remainder of the juices including the other three ‘pure’ pomegranate (PG04, PG05, and PG06) (Table 2).| Code | Name | Ingredients (as per label) |
|---|---|---|
| a + 100% Pomegranate juices, * reconstituted or blended pomegranate juices, # non-pomegranate fruit juices. | ||
| PG01+ | BIONA Organic Pomegranate | Pomegranate (100%) |
| PG02+ | POM Wonderful | Pomegranate (100%) |
| PG03+ | Rabenshorst Granatapfel | Pomegranate (100%) |
| PG04+ | Pomegreat Pure | Pomegranate (100%) |
| PG05+ | Marks & Spencer Pure Pomegranate Juice | Pomegranate (100%) |
| PG06+ | gn & r, Pur Jus de Grenade | Pomegranate (100%) |
| PG07* | Sainsbury's Pomegranate & Blueberry | Pomegranate (25%), blueberry (5%) |
| PG08* | Pomegreat Ruby | Pomegranate (32%), aronia (5%) |
| PG09* | Pomegreat de Originale | Pomegranate (30%), grapes (2%), fruit extract, vitamins C & E |
| PG10* | Pomegreat Sapphire | Pomegranate (28%), blueberry (4%), aronia (4%) |
| PG11* | Chiquita | Pomegranate (7%), raspberry (18.5%), banana, orange, lemon, grapes |
| PG12# | Welch's Purple Grape | Purple grape |
| PG13* | Breaking Wave Pomegranate Juice (Aldi) | Pomegranate, grape juice, aonia, berry juice |
| PG14* | Rubicon Pomegranate | Pomegranate (29%), aronia (7%) |
| PG15* | Pomegreat | Pomegranate (21%), white grapes (3%), elderberry (3%), acai (1.9%), grapefruit (0.5%), lime, vitamins C & E |
| PG16* | Healthy People | Pomegranate (30%), aronia (7%), vitamins C and E |
| PG17* | Pomegreat | Pomegranate (30%), red grape (7%), vitamins A, C and E, folic acid |
| PG18* | Pomegreat Granatapfel and Orange | Pomegranate (20%), mandarin (5%), orange juice (2%), elderberry (3.6%), red grape (0.5%), vitamins C & E |
| PG19# | Becker's Bester Roter Traubensaft | Red grape |
| PG20* | Sainsbury's Pomegranate Juice | Pomegranate (37%), vitamin C |
| PG21* | Amecke | Pomegranate, red and white grape, apple, red currant, cranberry, lemon |
| PG22# | Healthy People | Apple, acai, raspberries, red grapes, lemon |
| PG23# | Innocent Smoothie | Cranberry, yumberry, blackcurrant, orange |
| PG24* | Innocent Smoothie | Pomegranate (15%), blueberry (4%), acai (3%), banana, orange, grapes, lemon |
| PG25# | Eckes Roter Traubensaft | Red grape |
| PG26* | Ocean Spray Cranberry and Pomegranate | Pomegranate (14%), cranberry (10.5%), apple (6.5%), vitamin C |
| PG27* | Rauch Happy Day | Pomegranate (22%), aronia, apple, elderberry, vitamin C |
| PG28# | Innocent Smoothie | Guava, mango, goji, orange, apple |
| PG29* | Fruity King | Pomegranate (5%), grapes (55%) |
| PG30* | Applesientje Super Fruit | Pomegranate (9%), raspberries (3%), black currant (1.7%) cranberry (1%), strawberry (0.7%), apple, white grapes, vitamins C & E |
| PG31* | Coolbest Pomegranate | Pomegranate, raspberry, apple, lemon, blackcurrant |
| PG32# | Coolbest SeaBuckthorn | Kiwi, goji, orange |
| PG33* | Ribena (Really Light) Raspberry & Pomegranate | Pomegranate/raspberry (8%), vitamin C |
| PG34# | Healthy People | Goji, passionfruit, white grapes, pineapple |
| PG35# | Guanabana and appel | Soursop, apple, soy |
| PG36# | VIFIT yogurt | Passionfruit, goji |
| Code | Vit C (mg/100ml) | TP (mmol/L) | FRAP (mmol/L) | FRAP-VitC (mmol/L) | Vit C (%) Contribution | ORAC (mmol/L) | TEAC (mmol/L) | AOX index | Labelled pomegranate content |
|---|---|---|---|---|---|---|---|---|---|
| a The AOX index was calculated according to Seeram et al.8 TP in gallic acid equivalents, FRAP in Fe+2 eq., ORAC and TEAC in trolox equivalents. n.d., - not detected; n.a. - not analysed; n.s. - not stated. + 100% Pomegranate juices, * reconstituted or blended pomegranate juices, # non-pomegranate fruit juices. | |||||||||
| PG01+ | n.d. | 20.1 ± 0.1 | 55.3 ± 0.2 | n.a. | n.a. | 83.7 ± 0.5 | 40.5 ± 1.7 | 98 | 100% |
| PG02+ | n.d. | 20.7 ± 0.1 | 52.4 ± 0.6 | n.a. | n.a. | 85.8 ± 1.3 | 39.7 ± 1.8 | 98 | 100% |
| PG03+ | n.d. | 19.9 ± 0.6 | 51.8 ± 0.3 | n.a. | n.a. | 82.7 ± 0.4 | 41.3 ± 2.7 | 96 | 100% |
| PG04+ | n.d. | 10.8 ± 0.4 | 25.4 ± 0.3 | n.a. | n.a. | 40.7 ± 0.5 | 19.5 ± 0.4 | 49 | 100% |
| PG05+ | 2.0 ± 0.0 | 10.7 ± 0.1 | 25.7 ± 0.3 | 24.5 ± 0.1 | 4.3 ± 2.1 | 34.5 ± 4.4 | 21.4 ± 2.3 | 47 | 100% |
| PG06+ | n.d. | 10.3 ± 0.2 | 24.1 ± 1.1 | n.a. | n.a. | 35.2 ± 1.6 | 17.9 ± 2.1 | 44 | 100% |
| PG07* | 2.0 ± 0.0 | 13.3 ± 0.2 | 34.4 ± 0.5 | 30.7 ± 0.0 | 10.6 ± 0.1 | 30.6 ± 1.2 | 25.9 ± 1.8 | 54 | 25% |
| PG08* | n.d. | 11.9 ± 0.0 | 27.8 ± 0.7 | n.a. | n.a. | 34.2 ± 1.7 | 23.6 ± 1.6 | 51 | 32% |
| PG09* | 6.1 ± 0.0 | 9.7 ± 0.1 | 24.7 ± 0.2 | 23.4 ± 0.1 | 5.4 ± 2.3 | 18.2 ± 2.2 | 20.5 ± 1.7 | 40 | 30% |
| PG10* | n.d. | 9.1 ± 0.2 | 21.9 ± 0.3 | n.a. | n.a. | 28.2 ± 0.7 | 18.5 ± 2.0 | 40 | 28% |
| PG11* | 1.4 ± 0.0 | 8.0 ± 0.2 | 18.3 ± 0.1 | 16.6 ± 0.4 | 9.3 ± 0.0 | 46.5 ± 4.3 | 13.2 ± 2.0 | 39 | 7% |
| PG12# | n.d. | 8.8 ± 0.0 | 14.1 ± 0.4 | 14.1 ± 0.4 | 0 | 33.2 ± 4.9 | 10.6 ± 1.0 | 38 | 0% |
| PG13* | 1.1 ± 0.1 | 6.7 ± 0.1 | 16.5 ± 0.1 | 16.0 ± 0.1 | 3.0 ± 0.3 | 37.8 ± 6.4 | 10.9 ± 1.4 | 33 | n.s |
| PG14* | 38.0 ± 0.1 | 9.3 ± 0.1 | 19.7 ± 0.2 | 15.5 ± 0.1 | 21.5 ± 1.3 | 17.6 ± 0.4 | 14.1 ± 1.3 | 32 | 29% |
| PG15* | 4.9 ± 0.1 | 7.7 ± 0.0 | 18.5 ± 0.3 | 17.3 ± 0.1 | 6.7 ± 1.6 | 16.2 ± 3.6 | 13.5 ± 1.6 | 30 | 21% |
| PG16* | 11.9 ± 0.1 | 7.5 ± 0.0 | 15.8 ± 0.4 | 14.2 ± 0.2 | 10.0 ± 0.7 | 22.5 ± 2.4 | 12.8 ± 1.7 | 30 | 30% |
| PG17* | 1.5 ± 0.0 | 5.6 ± 0.1 | 13.4 ± 0.4 | 12.7 ± 0.0 | 5.7 ± 2.3 | 41.9 ± 1.0 | 8.5 ± 0.4 | 30 | 30% |
| PG18* | 1.6 ± 0.0 | 7.5 ± 0.2 | 18.7 ± 0.2 | 18.0 ± 0.1 | 3.7 ± 0.0 | 15.5 ± 0.4 | 13.4 ± 1.5 | 30 | 20% |
| PG19# | n.d. | 6.3 ± 0.0 | 9.9 ± 0.2 | 9.9 ± 0.2 | 0 | 26.5 ± 1.2 | 7.0 ± 0.4 | 28 | 0% |
| PG20* | 7.1 ± 0.1 | 6.7 ± 0.0 | 17.4 ± 0.1 | 16.1 ± 0.6 | 7.9 ± 0.6 | 17.8 ± 1.1 | 11.5 ± 1.5 | 27 | 37% |
| PG21* | n.d. | 7.7 ± 0.0 | 12.8 ± 0.2 | n.a. | n.a. | 20.6 ± 1.6 | 9.4 ± 0.8 | 27 | n.s. |
| PG22# | n.d. | 6.3 ± 0.0 | 11.6 ± 0.1 | 11.6 ± 0.1 | 0 | 20.1 ± 0.6 | 9.0 ± 0.5 | 27 | 0% |
| PG23# | 26.4 ± 0.3 | 7.6 ± 0.1 | 12.7 ± 0.2 | 9.9 ± 0.1 | 22.4 ± 1.5 | 16.0 ± 3.4 | 8.6 ± 1.1 | 26 | 0% |
| PG24* | n.d. | 6.8 ± 0.1 | 10.7 ± 0.4 | n.a. | n.a. | 22.5 ± 8.0 | 7.9 ± 0.8 | 24 | 15% |
| PG25# | n.d. | 5.9 ± 0.0 | 9.5 ± 0.1 | 9.5 ± 0.1 | 0 | 19.1 ± 0.2 | 7.6 ± 0.7 | 24 | 0% |
| PG26* | 48.2 ± 0.6 | 6.2 ± 0.0 | 14.8 ± 0.1 | 10.9 ± 0.0 | 26.7 ± 0.8 | 16.9 ± 0.4 | 8.4 ± 0.4 | 22 | 14% |
| PG27* | 24.6 ± 0.2 | 6.0 ± 0.1 | 14.7 ± 0.1 | 11.6 ± 0.2 | 21.3 ± 0.7 | 16.7 ± 0.9 | 7.4 ± 0.3 | 22 | 22% |
| PG28# | 31.2 ± 0.1 | 5.7 ± 0.2 | 10.3 ± 0.1 | 7.0 ± 0.0 | 32.1 ± 1.4 | 20.0 ± 0.5 | 6.3 ± 0.1 | 22 | 0% |
| PG29* | n.d | 5.6 ± 0.0 | 8.7 ± 0.1 | n.a. | n.a. | 21.8 ± 0.8 | 7.2 ± 0.4 | 21 | 5% |
| PG30* | 38.5 ± 0.2 | 5.7 ± 0.0 | 12.5 ± 0.1 | 7.9 ± 0.0 | 36.7 ± 1.4 | 16.9 ± 2.0 | 7.2 ± 0.5 | 20 | 9% |
| PG31* | 21.4 ± 0.1 | 5.9 ± 0.0 | 13.3 ± 0.3 | 11.1 ± 0.2 | 16.6 ± 2.8 | 10.3 ± 1.6 | 8.1 ± 0.5 | 20 | n.s. |
| PG32# | 21.6 ± 0.1 | 3.3 ± 0.1 | 4.8 ± 0.0 | 2.8 ± 0.1 | 41.4 ± 0.2 | 16.0 ± 0.4 | 3.0 ± 0.2 | 14 | 0% |
| PG33* | 58.0 ± 0.2 | 3.8 ± 0.0 | 10.5 ± 0.0 | 3.9 ± 0.0 | 63.0 ± 1.0 | 7.4 ± 0.8 | 5.3 ± 0.1 | 12 | 8% |
| PG34# | 14.7 ± 0.2 | 2.4 ± 0.0 | 3.4 ± 0.0 | 1.7 ± 0.0 | 42.3 ± 1.0 | 14.2 ± 0.2 | 2.5 ± 0.2 | 11 | 0% |
| PG35# | 16.9 ± 0.1 | 1.8 ± 0.0 | 2.8 ± 0.0 | 1.0 ± 0.1 | 65.5 ± 0.4 | 6.0 ± 0.5 | 1.6 ± 0.1 | 6 | 0% |
| PG36# | 12.8 ± 0.0 | 1.2 ± 0.0 | 1.8 ± 0.0 | 0.5 ± 0.0 | 67.7 ± 0.0 | 4.4 ± 0.2 | 0.9 ± 0.2 | 4 | 0% |
The AOX capacity showed great variability not only among the “pure” pomegranate juices but also the “blended” group of samples. Two of the blended juices, PG07 and PG08, containing 25% and 32% of pomegranate, respectively, scored slightly higher than three of the 100% pomegranate juices (PG04, PG05, and PG06). Also of interest was PG20 which contained 37% pomegranate and had an AOX index of 27 which was lower than that of several juices including PG09 and PG10 which contained less pomegranate (Table 2). This may reflect dilution, adulteration and/or reconstitution factors associated with manufacture. It is, however, more difficult to explain in the context of juices from the same label company like PG04 (100% pomegranate; AOX index 49) vs. PG08 (32% pomegranate, 5% aronia; AOX index 51%) both from Pomegreat. The same applies for PG07 (25% pomegranate and 5% blueberry; AOX index 54) and PG20 (37% pomegranate; AOX index 27) from Sainsbury's. The levels of vitamin C in the blended products ranged from zero to 58 mg/100 ml in PG33. Vitamin C can influence the AOX activity as observed in the FRAP assay where removal of vitamin C with ascorbate oxidase resulted in a marked reduction in the AOX capacity of some of the blended products (Table 2). Most notable were PG33 where there was a 63% decline following treatment with ascorbate oxidase and PG14 where vitamin C made a 21.5% contribution to the FRAP AOX capacity (Table 2). It would appear that vitamin C is added to several of the juices during processing after pasteurisation and it is this supplementation, rather than the polyphenolic constituents of the fruit that boost the AOX capacity of the juice.
Very similar AOX profiles were detected with all four assays and when the data were analyzed statistically highly significant correlation values were obtained with the TP, FRAP, TEAC and ORAC assays (Table 3).
The analysis of the AOX capacity of juices for comparative purposes using simple colorimetric assays such as FRAP, TP and TEAC, as well as the more complex ORAC method, is of value as the data are well correlated (Table 3). However, more detailed HPLC-PDA-MS2 analysis is required to investigate quality issues and the great variability shown between supposedly similar juices.
2.2 Qualitative HPLC-PDA-MS2-on-line AOX analysis of pomegranate juices
Polyphenolic compounds in the 100% pomegranate juices as well as the other pomegranate juices were analysed by HPLC-PDA-MS2 and the identifications used to compile a “pomegranate fingerprint” to compare to those obtained from the blended drinks. At the same time, the AOX capacity contribution of the peaks were measured by an on-line ABTS system.17Fig. 1–7 show the 520, 280 and 720 nm traces for PG01- PG04, PG06, PG14 and PG33. The anthocyanin profile can be seen at 520 nm and the ellagitannins/ellagic acids at 280 nm while the 720 nm trace depicts the AOX activity associated with each peak.![Gradient reversed phase HPLC-PDA-AOX analysis of juice PG01 [BIONA Organic Pomegranate] (see Table 1) with detection at 520 nm (anthocyanins), 280 nm (ellagitannins and ellagic acid derivatives) and 720 nm (AOX activity). Peak 1 - punicalagin-like, peak 2 - punicalin A, peak 3 - punicalin B, peak 4 - delphinidin-3,5-O-diglucoside, peak 5 - 2-O-galloylpunicalagin, peak 6 - punicalagin A, peak 7 - punicalagin B, peak 8 - cyanidin-3,5-O-diglucoside, peak 9 - granatin A, peak 10 - pelargonidin-3,5-O-diglucoside, peak 11 - granatin B, peak 12 - pelargonidin-3,5-O-diglucoside, peak 13 - punicalagin isomer, peak 14 - cyanidin-3-O-glucoside, peak 15 - pelargonidin-3-O-glucoside, peak 16 - ellagic acid-O-hexoside and peak 17 - ellagic acid. For identification of peaks see Table 4.](/image/article/2010/FO/c0fo00008f/c0fo00008f-f1.gif) | ||
| Fig. 1 Gradient reversed phase HPLC-PDA-AOX analysis of juice PG01 [BIONA Organic Pomegranate] (see Table 1) with detection at 520 nm (anthocyanins), 280 nm (ellagitannins and ellagic acid derivatives) and 720 nm (AOX activity). Peak 1 - punicalagin-like, peak 2 - punicalin A, peak 3 - punicalin B, peak 4 - delphinidin-3,5-O-diglucoside, peak 5 - 2-O-galloylpunicalagin, peak 6 - punicalagin A, peak 7 - punicalagin B, peak 8 - cyanidin-3,5-O-diglucoside, peak 9 - granatin A, peak 10 - pelargonidin-3,5-O-diglucoside, peak 11 - granatin B, peak 12 - pelargonidin-3,5-O-diglucoside, peak 13 - punicalagin isomer, peak 14 - cyanidin-3-O-glucoside, peak 15 - pelargonidin-3-O-glucoside, peak 16 - ellagic acid-O-hexoside and peak 17 - ellagic acid. For identification of peaks see Table 4. | ||
![Gradient reversed phase HPLC-PDA-AOX analysis of juice PG02 [POM Wonderful] (see Table 1). For peak identification see legend to Fig. 1 and Table 4.](/image/article/2010/FO/c0fo00008f/c0fo00008f-f2.gif) | ||
| Fig. 2 Gradient reversed phase HPLC-PDA-AOX analysis of juice PG02 [POM Wonderful] (see Table 1). For peak identification see legend to Fig. 1 and Table 4. | ||
![Gradient reversed phase HPLC-PDA-AOX analysis of juice PG03 [Rabenshorst Granatapfel] (see Table 1). For details and peak identification see legend to Fig. 1 and Table 4.](/image/article/2010/FO/c0fo00008f/c0fo00008f-f3.gif) | ||
| Fig. 3 Gradient reversed phase HPLC-PDA-AOX analysis of juice PG03 [Rabenshorst Granatapfel] (see Table 1). For details and peak identification see legend to Fig. 1 and Table 4. | ||
![Gradient reversed phase HPLC-PDA-AOX analysis of juice PG04 [100% Pomegreat] (see Table 1). For details and peak identification see legend to Fig. 1 and Table 4.](/image/article/2010/FO/c0fo00008f/c0fo00008f-f4.gif) | ||
| Fig. 4 Gradient reversed phase HPLC-PDA-AOX analysis of juice PG04 [100% Pomegreat] (see Table 1). For details and peak identification see legend to Fig. 1 and Table 4. | ||
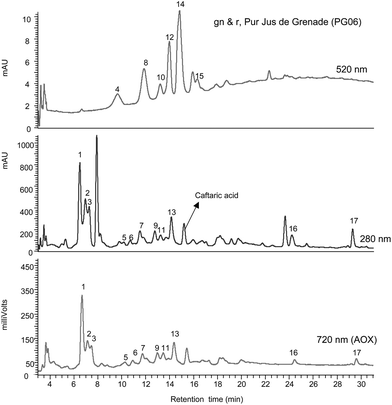 | ||
| Fig. 5 Gradient reversed phase HPLC-PDA-AOX analysis of juice PG06 [gn & r 100% Pur, Jus de Grenade (see Table 1). For details and peak identification see legend to Fig. 1 and Table 4. | ||
![Gradient reversed phase HPLC-PDA-AOX analysis of juice PG14 [Rubicon Pomegranate] (see Table 1). For details and peak identification see legend to Fig. 1 and Table 4.](/image/article/2010/FO/c0fo00008f/c0fo00008f-f6.gif) | ||
| Fig. 6 Gradient reversed phase HPLC-PDA-AOX analysis of juice PG14 [Rubicon Pomegranate] (see Table 1). For details and peak identification see legend to Fig. 1 and Table 4. | ||
![Gradient reversed phase HPLC-PDA-AOX analysis of juice PG33 [Ribena Raspberry and Pomegranate] (see Table 1). For details and peak identification see legend to Fig. 1 and Table 4.](/image/article/2010/FO/c0fo00008f/c0fo00008f-f7.gif) | ||
| Fig. 7 Gradient reversed phase HPLC-PDA-AOX analysis of juice PG33 [Ribena Raspberry and Pomegranate] (see Table 1). For details and peak identification see legend to Fig. 1 and Table 4. | ||
A total of 17 compounds were identified in all 100% juices. The identities of the peaks numbered in the traces (Fig. 1–7) are summarised in Table 4, and their contribution to the ABTS AOX content is evaluated in Table 5.
| Peak No. | Rt (min) | [M − H]− (m/z)* | MS2 daughter ions | Compound |
|---|---|---|---|---|
| a [M − H]− negatively charged molecular ion; + indicates positively charged molecular ion. | ||||
| 1 | 6.6 | 1101 | 781, 601, 301 | Punicalagin-like |
| 2 | 7.2 | 781 | 601, 301 | Punicalin A |
| 3 | 7.5 | 781 | 601, 301 | Punicalin B |
| 4 | 9.9 | 627+ | 465, 303 | Delphinidin-3,5-O-diglucoside |
| 5 | 10.5 | 933 | 781, 721, 601, 301 | 2-O-Galloylpunicalagin |
| 6 | 11.1 | 1083 | 781, 721, 601, 301 | Punicalagin A |
| 7 | 12.1 | 1083 | 781, 721, 601, 301 | Punicalagin B |
| 8 | 12.2 | 611+ | 449, 287 | Cyanidin-3,5-O-diglucoside |
| 9 | 13.2 | 783 | Granatin A | |
| 10 | 13.4 | 465+ | 303 | Delphinidin-3-O-glucoside |
| 11 | 13.7 | 951 | Granatin B | |
| 12 | 14.2 | 595+ | 433, 271 | Pelargonidin-3,5-O-diglucoside |
| 13 | 14.7 | 1083 | 781, 721, 601, 301 | Punicalagin isomer |
| 14 | 15.2 | 449+ | 287 | Cyanidin-3-O-glucoside |
| 15 | 17.0 | 433+ | 271 | Pelargonidin-3-O-glucoside |
| 16 | 24.8 | 463 | 301 | Ellagic acid-O-hexoside |
| 17 | 29.9 | 301 | Ellagic acid |
| PG01+ | PG02+ | PG03+ | PG04+ | PG06+ | PG14* | PG33* | |
|---|---|---|---|---|---|---|---|
| a + 100% Pomegranate juices, * reconstituted or blended pomegranate juices. | |||||||
| Punicalagin-like | 12.8 | 18.1 | 10.8 | 15 | 24.7 | 5.6 | 2.8 |
| Punicalins A–B | 19.8 | 11.7 | 13.5 | 20.9 | 17.6 | 11.5 | 3.1 |
| 2-O-Galloylpunicalagin | 3.2 | 2.6 | 3.8 | 2.9 | 2.5 | 0 | 0 |
| Punicalagin A + B + isomer | 12 | 19.4 | 20.4 | 13.2 | 14.3 | 7.4 | 3.2 |
| Granatin A | 5.5 | 7.6 | 5.1 | 9.8 | 3.9 | 1 | 0 |
| Granatin B | 4.9 | 4.9 | 4.7 | 6.8 | 3.4 | 1.9 | 0 |
| Total ellagitannins | 58.2 | 64.3 | 58.3 | 68.6 | 66.4 | 27.4 | 9.1 |
| Ellagic acid-O-hexoside | 1.6 | 1.3 | 1.6 | 1.2 | 2.1 | 0.5 | 0 |
| Ellagic acid | 2.1 | 4.8 | 3.7 | 2.9 | 2.4 | 0.8 | 0 |
| Total ellagic acids | 3.7 | 6.1 | 5.3 | 4.1 | 4.5 | 1.3 | 0 |
| Anthocyanins | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vitamin C | 0 | 0 | 0 | 0 | 0 | 62.3 | 81 |
| Caftaric acid | 0 | 0 | 0 | 0 | 5.2 | 0 | 0 |
| Unidentified compounds | 38.2 | 29.7 | 36.5 | 27.5 | 23.9 | 9.2 | 9.9 |
Peak 1 (retention time [Rt] - 6.6 min) had a [M − H]− at m/z 1101 and, like peaks 6 and 7, produced MS2 ions at m/z 781, 721, 601 and 301. On the basis of this fragmentation pattern, peak 1 is identified as a punicalagin-like compound. This type of compound has not been described before in pomegranate. This peak was the second major contributor to the ABTS AOX of the ‘pure’ juices ranging between 10.8% and 24.7% in PG03 and PG06 respectively.
Peaks 2 and 3 (Rts - 7.2 and 7.5 min) had a negatively charged molecular ion ([M − H]−) at m/z 781 which fragmented, yielding a base peak at m/z 721 and other ions at m/z 601 and m/z 301, which are from gallagic acid and ellagic acid moieties. Based on the report of Tanaka et al.,18 the fragmentation pattern and elution order identified these compounds as punicalins A and B. This is one of the typical ellagitannins in pomegranate and one of the major contributors to the AOX capacity with values between 11.7% to 20.9% (Table 5).
Peak 4 (Rt - 9.9 min, λmax - 520 nm) was characterized by a positively charged molecular ion ([M − H]+) at m/z 627 which produced two MS2 fragment ions at m/z 465 and 303. This fragmentation pattern and the absorbance spectrum identified this compound as delphinidin-3,5-O-diglucoside, a known pomegranate component.19
Peak 5 (Rt - 10.5 min) had a [M − H]− at m/z 933 which yielded daughter ions at m/z 781, 721, 601 and 301. This fragmentation pattern in keeping with published data19 identified this compound as 2-O-galloylpunicalagin. Its contribution to the AOX capacity is minor (2.5% and 3.8%) (Table 5).
Peaks 6 and 7 (Rts - 11.1 and 12.1 min) both had a [M − H]− at m/z 1083 which produced identical MS2 fragments at m/z 781, 721, and 601. This fragmentation pattern identifies these compounds as punicalagins.19
Peak 8 (Rt - 12.2 min, λmax - 520 nm) produced a [M − H]+ at m/z 611 and daughter ions at m/z 449 and 287. This fragmentation pattern and the absorbance spectrum identified this compound as cyanidin-3,5-O-diglucoside, another known pomegranate anthocyanin.19
Peaks 9 and 11 (Rts - 13.2 and 13.7 min) had a [M − H]− at m/z 783 and m/z 951 respectively. No fragmentation information was obtained. This is in keeping with the presence of granatin A and B, known constituents of pomegranate.20
Peak 10 (Rt - 13.4 min, λmax - 520 nm) produced a [M − H]+ at m/z 465 and a single MS2 fragment ion at m/z 303. This fragmentation pattern, absorbance spectrum and co-chromatography identified this compound as delphinidin-3-O-glucoside, a known constituent of pomegranates.19
Peak 12 (Rt - 14.2 min, λmax - 520 nm) had a [M − H]+ at m/z 595 and MS2 ions at m/z 433 and 271. This fragmentation pattern and the absorbance spectrum identified this compound as pelargonidin-3,5-O-diglucoside, a minor pomegranate anthocyanin.19
Peaks 13 (Rt - 14.7 min) had a [M − H]− at m/z 1083 and, like peaks 6 and 7, produced MS2 ions at m/z 781, 721, 601 and 301. On the basis of this fragmentation pattern, peak 13 is identified as a punicalagin-like compound. There are two known punicalagins, A and B, however, additional isomers occur in pomegranate.18
Peak 14 (Rt - 15.2 min, λmax - 520 nm) yielded a [M − H]+ at m/z 449 and a single MS2 fragment at m/z 287. This fragmentation pattern, absorbance spectrum and co-chromatography identified this compound as cyanidin-3-O-glucoside.19
Peak 15 (Rt - 17.0 min, λmax - 520 nm) was characterised by a [M − H]+ at m/z 433 which yields a daughter ion at m/z 271. This fragmentation pattern, absorbance spectrum and co-chromatography identified this compound as pelargonidin-3-O-glucoside.19 None of the anthocyanins seems to have any effect on the on-line AOX activity, as they are not reflected in the 720 nm ABTS profile.
Peak 16 (Rt - 24.8 min) had a [M − H]− at m/z 463 and a single MS2 fragment at m/z 301. This is in keeping with the presence of an ellagic acid-O-hexoside conjugate which has previously been reported to occur in pomegranates.19
Peak 17 (Rt - 29.9 min) had a [M − H]− at m/z 301 that yielded no MS2 fragment ions. Co-chromatography and the identical fragmentation of a reference compound identified this component as ellagic acid. Both ellagic acid and conjugate were minor contributors to the AOX capacity (Table 5).
In addition to these 17 peaks, the 280 nm trace of the PG01 juice (Fig. 1) had a peak with a retention time of 7.9 min that is also present in PG06 (Fig. 5) and appeared in smaller amounts in PG03 (Fig. 3) and PG14 (Fig. 6). This peak did not ionise, so no MS data were obtained to assist identification, nor did it exhibit on-line AOX activity.
Overall these results show that the two main groups of polyphenolic compounds in pomegranate juice are anthocyanins and ellagitannins. The spectrum of anthocyanins comprising principally of cyanidin-3,5-O-diglucoside, cyanidin-3-O-glucoside, delphinidin-3,5-O-diglucoside and delphinidin-3-O-glucoside together with smaller amounts of pelargonidin-3,5-O-diglucoside and pelargonidin-3-O-glucoside is in agreement with earlier reports.10,21 This can be used as a convenient fingerprint of pomegranate authenticity. The other potential diagnostic components are the ellagitannins in the form of punicalagins and punicalagin-like (peaks 1, 2, 3 and 13), 2-O-galloylpunicalagin (peak 5), punicalin A and B (peaks 6 and 7) and granatin A and B (peaks 9 and 11) which are the main contributors to the AOX capacity. Ellagic acid and an ellagic acid-hexose conjugate also occur but their presence is not specific to pomegranate as they can be derived from raspberries and other sources.22–24
The 720 nm traces for all the juices tested in the on-line AOX detector is almost identical to the 280 nm fingerprint of ellagitannins/ellagic acids (Fig. 1–7). Thus, as outlined in Table 5, the ellagitannins are the main antioxidants in the five pure pomegranate juices, PGO1–PGO4 and PGO6. The major contributors were the punicalins, punicalagins, and galloylpunicalagin which accounted for 58% to 69% of the total AOX of the juices. Around 30% of the AOX activity was due to an increased background probably due to unresolved oligomeric ellagitannins or proanthocyanidins25 with AOX activity. None of the anthocyanin peaks were associated with the 720 nm AOX peaks.
In Fig. 6 and 7, the profiles for juices PG14 and PG33, it can be seen that the predominant AOX is vitamin C, which is responsible for 62.3 and 81%, respectively, of the total AOX activity, with negligible contributions from pomegranate constituents. This confirms the observation made when the juices were analysed in the FRAP assay before and after treatment with ascorbate oxidase (Table 2).
2.3 Quantification of the phenolic pomegranate markers in the 26 juices analyzed
The results for the quantification of ellagitannins and anthocyanins and the overall HPLC total phenolics for the 26 pomegranate juices and pomegranate juice blends are presented in Table 6. For the anthocyanin quantification all the peaks appearing in 520 nm traces were quantified in cyanidin-3-O-glucoside equivalents. As expected, the levels of the total HPLC phenolics quantified for the three top samples (PG01, PG02, PG03) were of the order of 2 mmol/L, much higher than the other 23 samples (Table 6), in agreement with the AOX results in Table 2.| Punicalagin-like | Punicalins A and B | 2-O-Galloyl punicalagin | Punicalagin A | Punicalagin B | Punicalagin isomer | Granatin A | Granatin B | Total ellagitannins | Ellagic acid hexose | Ellagic acid | Total ellagic Acid | Total anthocyanins | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Data expressed as mean values in μmol/L. The standard error (n = 3) values (not shown), were less than 10% of the mean values. + 100% pomegranate juices, * reconstituted or blended pomegranate juices. | ||||||||||||||
| PG01+ | 178 | 972 | 22 | 30 | 58 | 181 | 123 | 47 | 1611 | 68 | 214 | 282 | 68 | 1961 |
| PG02+ | 261 | 345 | 14 | 18 | 63 | 196 | 136 | 35 | 1068 | 85 | 408 | 493 | 344 | 1905 |
| PG03+ | 158 | 512 | 26 | 34 | 125 | 382 | 116 | 48 | 1401 | 116 | 515 | 631 | 11 | 2043 |
| PG04+ | 62 | 236 | 5 | 7 | 14 | 48 | 68 | 20 | 460 | 19 | 104 | 123 | 115 | 698 |
| PG05+ | 58 | 167 | 3 | 7 | 18 | 66 | 78 | 18 | 415 | 23 | 160 | 183 | 155 | 753 |
| PG06+ | 123 | 230 | 8 | 9 | 21 | 61 | 34 | 11 | 497 | 33 | 96 | 129 | 9 | 635 |
| PG07* | 81 | 543 | 12 | 24 | 58 | 169 | 44 | 32 | 963 | 36 | 98 | 134 | 18 | 1115 |
| PG08* | 78 | 331 | 9 | 16 | 33 | 97 | 37 | 21 | 622 | 37 | 199 | 236 | 30 | 888 |
| PG09* | 53 | 375 | 12 | 14 | 23 | 70 | 33 | 21 | 601 | 28 | 65 | 93 | 62 | 756 |
| PG10* | 48 | 287 | 7 | 13 | 20 | 58 | 22 | 13 | 468 | 29 | 102 | 131 | 26 | 625 |
| PG11* | 0 | 8 | 0 | 0 | 0 | 2 | 0 | 1 | 11 | 3 | 21 | 24 | 288 | 323 |
| PG13* | 41 | 248 | 5 | 9 | 5 | 16 | 13 | 17 | 354 | 7 | 32 | 39 | 23 | 416 |
| PG14* | 32 | 172 | 2 | 5 | 5 | 33 | 15 | 9 | 273 | 9 | 39 | 48 | 34 | 355 |
| PG15* | 25 | 250 | 5 | 10 | 8 | 26 | 18 | 14 | 356 | 16 | 122 | 138 | 52 | 546 |
| PG16* | 14 | 63 | 3 | 5 | 20 | 55 | 28 | 10 | 198 | 17 | 34 | 51 | 159 | 408 |
| PG17* | 45 | 237 | 2 | 4 | 3 | 11 | 15 | 7 | 324 | 5 | 16 | 21 | 1 | 346 |
| PG18* | 29 | 263 | 4 | 9 | 10 | 28 | 16 | 12 | 371 | 17 | 42 | 59 | 46 | 476 |
| PG20* | 62 | 199 | 5 | 9 | 18 | 56 | 27 | 9 | 385 | 15 | 52 | 68 | 35 | 488 |
| PG21* | 7 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 10 | 10 | 20 | 222 | 254 |
| PG24* | 16 | 32 | 0 | 0 | 0 | 0 | 0 | 4 | 52 | 4 | 7 | 11 | 101 | 164 |
| PG26* | 22 | 121 | 3 | 4 | 6 | 15 | 13 | 7 | 191 | 13 | 26 | 39 | 3 | 233 |
| PG27* | 26 | 137 | 3 | 4 | 5 | 13 | 17 | 7 | 212 | 14 | 50 | 64 | 74 | 350 |
| PG29* | 25 | 18 | 0 | 0 | 0 | 1 | 13 | 8 | 65 | 4 | 20 | 24 | 151 | 240 |
| PG30* | 13 | 37 | 1 | 2 | 2 | 8 | 6 | 0 | 69 | 5 | 25 | 30 | 77 | 176 |
| PG31* | 11 | 39 | 2 | 3 | 7 | 19 | 13 | 7 | 101 | 8 | 28 | 36 | 112 | 249 |
| PG33* | 6 | 36 | 0 | 0 | 1 | 3 | 2 | 1 | 49 | 3 | 14 | 18 | 14 | 81 |
Although the total quantities of anthocyanins in the 100% pomegranate juices varied substantially, as discussed above, the 520 nm anthocyanin HPLC profiles were similar with only slight differences in the relative amounts of cyanidin-3,5-O-diglucoside (peak 8) and cyandin-3-O-glucoside (peak 14) (Fig. 1–3). Likewise, a similar profile was obtained with PG04 (Fig. 4) and also PG06 (Fig. 5). In both these juices, however, peak 12, pelargonidin-3,5-O-diglucoside, was much more prominent. PG04 also contained an anthocyanin peak (marked *) which was not detected in the other 100% pomegranate juices. This peak had a [M − H]+ at m/z 949 which produced MS2 fragments at m/z 611, 449 and 287 indicating a cyanidin-based compound. The unusual mass spectrum and the relatively late elution of this component suggest that it might be a cyanidin-O-feruloyl-triglucoside.
Among the blended pomegranate products, PG14, a 29% pomegranate, 7% aronia mixture, had an anthocyanin HPLC profile dominated by aronia anthocyanins26 principally in the form of cyanidin-3-O-galatoside and cyanidin-3-O-arabinoside, rather than pomegranate anthocyanins (Fig. 6). PG14 did, however, contain ellagitannins, suggesting that the pomegranate components might be derived from rind rather than arils which, as noted earlier, are the principal source of pomegranate anthocyanins.9 PG33, which is a 8% raspberry/pomegranate blend had a raspberry rather than a pomegranate anthocyanin fingerprint with cyanidin-3-O-sophoroside being the main component24 (Fig. 7).
The Pearson's correlation coefficients (Table 7) confirmed the significant relationship between the total ellagitannin and ellagic acid contents and the in vitro AOX capacity of the juices measured by TP, FRAP, ORAC and TEAC of the juices. In contrast in vitro AOX capacity was not associated with anthocyanin levels. This in agreement with earlier observations9,19 that anthocyanins make, at best, a very minor contribution to the AOX capacity of pomegranates.
| Assay | Total ellagitannins | Total ellagic acid | Total anthocyanins |
|---|---|---|---|
| a *** Correlation is significant at p < 0.001. NS, not significant. | |||
| TP | 0.879(***) | 0.899(***) | NS |
| FRAP | 0.918(***) | 0.895(***) | NS |
| ORAC | 0.730(***) | 0.815(***) | NS |
| TEAC | 0.919(***) | 0.906(***) | NS |
3 Experimental
3.1 Chemicals
5-O-Caffeoylquinic acid, potassium persulfate, dipotassium hydrogen phosphate, potassium dihydrogen phosphate, ascorbate oxidase (EC 1.10.3.3) and 2′,2-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid (ABTS) were purchased from Sigma-Aldrich (Poole, UK). Ellagic acid was obtained from AASC Ltd (Southampton, UK). Cyanidin-3-O-glucoside and pelargonidin-3-O-glucoside were purchased from Extrasynthese (Genay, France), and methanol was obtained from Rathburn Chemicals (Walkerburn, Scotland). Formic acid and acetic acid were supplied by Fisher Scientific (Loughborough, UK). Punicalagin was purchased from LGC Standards (Teddington, Middlesex, UK).3.2 Juices
Thirty six European juices were procured commercially. The brands and ingredients described on the label are shown in Table 1. The first six juices were labelled as “100% pomegranate juices”, and the remainder included 20 brands of diluted pomegranate juice or pomegranate blended with other fruit juices and 10 different non-pomegranate fruit juices.3.3 Extraction of juices
A 500 μL aliquot of juice was added to 500 μL of methanol and shaken for 3 min. The mixture was then centrifuged at 13000 g at 4 °C for 5 min and the supernatant stored at −80 °C prior to analysis.3.4 Analysis of vitamin C
The vitamin C (ascorbic acid) content of the juices was assessed using HPLC-PDA as described by Ross28 with a Surveyor HPLC system (Thermo-Fisher, Scientific, Waltham, MA). Separation was carried out using a 5 μm 250 × 4.6 mm i.d. Nucleosil C18 column (Phenomenex, Macclesfield, UK) fitted with a C18 guard cartridge. The column was eluted isocratically with a mobile phase comprising 0.05 mM sodium hydroxide, 25 mM myristyltrimethylammonium bromide, 0.06 M acetic acid, 7.5% acetonitrile mobile phase containing 100 mg/L homocysteine and 200 mg/L EDTA. The system was operated at 40 °C with a flow-rate of 0.6 mL/min and absorbance detection at 265 nm. The amount of ascorbic acid was calculated by reference to 0–200 μM vitamin C calibration curve.3.5 Total phenol content
The TP content of the juices was determined in triplicate, in diluted samples, using the Folin-Ciocalteu assay.13 The data were recorded in gallic acid equivalents (GAE).3.6 Ferric-reducing antioxidant power assay
The FRAP assay was used to estimate the AOX capacity of the juices. It measures the ability of a solution to reduce a ferric-tripyridyl-triazine complex (Fe3+-TPTZ) to the ferrous form, Fe2+, producing a blue color with absorption at 593 nm. One and a half mL of freshly prepared FRAP reagent (containing the Fe3+-TPTZ in excess at pH 3.6), was added to 50 μL of juice and 150 μL water. The absorbance at 593 nm, measured after a 4 min reaction period, was compared to a 0 to 1 mM Fe2+ standard curve.143.7 Contribution of vitamin C to FRAP antioxidant activity
Vitamin C reacts almost instantaneously in the FRAP assay. To determine the contribution of vitamin C to the antioxidant activity of the juices, it was selectively destroyed by the addition of ascorbate oxidase. Twenty μL of a 4 U/mL enzyme solution was added to one of a pair of juice aliquots before the FRAP reaction. Standard solutions of ascorbic acid (0–0.5 mM), in the presence and absence of the enzyme, were tested.3.8 Oxygen radical absorbance capacity assay
The principle of the ORAC assay is to monitor the capability of a test antioxidant to quench the fluorescent signal obtained when fluoroscein is exposed to an oxygen radical generator (2,2′-azobis-2-methyl-propionamide). The standard means of “normalising” the data requires comparison of the inhibitory effect of the test agent with that of Trolox, a water-soluble analogue of α-tocopherol. Comparing the area under the curve for 60 min incubations is the conventional means of analysing data obtained with this method.163.9 Trolox equivalent antioxidant capacity assay
After 12 h in darkness, a stock solution of 7 mM ABTS and 2.45 mM potassium persulfate was diluted with ethanol to an absorbance of 0.70 at 734 nm. Diluted juice samples were mixed with 1 mL of the ABTS solution and after 5 min, absorbance determined at 734 nm. TEAC values were calculated by reference to a Trolox standard curve.15 The same basic procedure was applied with the HPLC on-line antioxidant detection system described below.3.10 HPLC with PDA, MS2 and AOX detection
Analysis was carried out on a Surveyor HPLC system comprising of an autosampler with sampler cooler maintained at 4 °C, a PDA detector (Thermo Fisher Scientific), scanning from 200–600 nm. Samples were analysed on a 250 × 4.6 mm Gemini C6 phenyl column (Phenomenex, Macclesfield, UK), maintained at 40 °C using a 40 min mobile phase gradient of 5 to 60% methanol in 0.1% aqueous formic acid at a flow rate of 1 ml/min. After passing through the flow cell of the PDA detector, the eluate was split and 200 μL directed to a LCQ Advantage ion trap mass spectrometer fitted with an electrospray interface (Thermo Fisher Scientific). Capillary temperature was 300 °C, sheath gas and auxiliary gas were 60 and 20 units respectively, the source voltage was 4 kV. Samples were analysed using full scan in both positive and negative ionisation modes, the scan range was from 150–2000 m/z for negative ion and 190–1000 m/z for positive ion. Identifications are based on co-chromatography with authentic standards, where available. Absorbance spectra and mass spectra, using MS2, were used to identify compounds reported previously in the literature.For the detection of components with AOX activity, the remaining 800 μL/min of the HPLC eluate was mixed with an ABTS solution flowing at 0.5 mL/min and the resultant mixture passed through a holding coil before being directed to a P2000 absorbance detector (Nemphlar Bioscience, Lanark, UK) operating at 720 nm.17
3.11 Statistical analysis
The data were analyzed by SPSS software ver.14.0 to calculate Pearson correlation coefficients.4 Conclusions
Although consumption of dietary flavonoids and polyphenolics has been increasingly implicated in health benefits it is remains unclear as to whether or not they function in vivo by directly modulating the body's AOX network.29 There is growing evidence that in the body, they function in more subtle ways, at low concentrations, by regulating processes such as signal transduction pathways.30 None-the-less, monitoring the AOX and/or total phenolic content of plant-derived foods, including fruit juices, provides a useful initial guide to their potential protective effects as polyphenol-rich products, such as PG01–PG03, are more likely to have a beneficial effects on health effects than the more dilute blended juices (Table 2). It is also necessary to identify at this stage, juices such PG14 and PG33, that contain relatively low levels of fruit but have their AOX capacity boosted by the presence of substantial amounts of vitamin C (Table 2, Fig. 1F and 1G).The results of this study have provided an insight into the differences in both AOX activity and the concentrations of the main phenolic compounds in pure or blended pomegranate juices sold in Europe. While the ellagitannin profile can be used as a fingerprint for confirmation of the origin of the juice, it cannot on its own be used to judge purity or quality. It was, for instance, evident that the PG01 and PG03 juices, both of which had a very high AOX index, were authentic pomegranate juices from their ellagitannin profiles. However, the anthocyanin content of these juices was low compared to PG02 suggesting that juice from the arils had been diluted by more extensive extraction of the rind of the pomegranates. This may have resulted in PG01 and PG03 having an astringent taste which, arguably, may be masked by the addition of sweetener. The dark brown colour of these juices is in keeping with their low anthocyanin content.
The anthocyanins, although not associated with AOX activity, readily provide an additional specific fingerprint of pomegranate juice authenticity. The concentration of anthocyanins, along with the ellagitannin profile, can be used as indicators of both authenticity and quality of pomegranate juices. The HPLC-PDA-MS methodology utilised in this study provides a means of assessing, not just potential adulteration of pomegranate juices and drinks, but also that of a diversity of other fruit-based beverages.
5 Acknowledgements
This study was funded by a donation from POM Wonderful LLC, Los Angeles, CA, USA.6 References
- M. G. L. Hertog, E. J. M. Feskens, P. C. H. Hollman, M. B. Katan and D. Kromhout, Lancet, 1993, 342, 1007–1011 CrossRef CAS; B. Margetts, in Plants: Diet and Health, ed. G. Goldberg, Blackwell Publishing, Oxford, 2003, pp. 49–64 Search PubMed.
- http://www.foodanddrinkeurope.com/ .
- A. J. Pantuck, J. T. Leppert, N. Zomorodian, W. Aronson, J. Hong, R. J. Barnard, N. Seeram, H. Liker, H. Wang, R. Elashoff, D. Heber, M. Aviram, L. Ignarro and A. Belldegrun, Clin. Cancer Res., 2006, 12, 4018–4026 CrossRef CAS.
- M. Rosenblat, T. Hayek and M. Aviram, Atherosclerosis, 2006, 187, 363–371 CrossRef CAS.
- W. Rock, M. Rosenblat, R. Miller-Lotan, A. P. Levy, M. Elias and M. Aviram, J. Agric. Food Chem., 2008, 56, 8704–8713 CrossRef CAS.
- S. G. Kasimsetty, D. Bialonska, M. K. Reddy, G. Ma, S. I. Khan and D. Ferreira, J. Agric. Food Chem., 2010, 58, 2180–2187 CrossRef CAS.
- W. Mullen, S. Marks and A. Crozier, J. Agric. Food Chem., 2007, 55, 3148–3157 CrossRef CAS.
- N. P. Seeram, M. Aviram, Y. Zhang, S. M. Henning, L. Feng, M. Dreher and D. Huber, J. Agric. Food Chem., 2008, 56, 1415–1422 CrossRef CAS.
- R. Tzulker, I. Glazer, I. Bar-Ilan, D. Holland, M. Aviram and R. Amir, J. Agric. Food Chem., 2007, 55, 9559–9570 CrossRef CAS.
- M. I. Gil, J. Cherif, N. Ayed, F. Artes and F. A. Tomás-Barberán, Z. Lebens. Unter. Forschung, 1995, 201, 361–364 Search PubMed; E. Shwartz, I. Glazer, I. Bar-Ya'akov, I. Matityahu, I. Bar-Ilan, D. Holland and R. Amir, Food Chem., 2009, 115, 965–973 CrossRef CAS.
- Y. J. Zhang, D. Wang, R. P. Lee, S. M. Henning and D. Heber, J. Agric. Food Chem., 2009, 57, 7395–7400 CrossRef CAS.
- Y. J. Zhang, D. Krueger, R. Durst, R. Lee, D. Wang, N. Seeram and D. Heber, J. Agric. Food Chem., 2009, 57, 3961–3961 CrossRef CAS.
- V. L. Singleton and J. A. Rossi, Am. J. Enol. Vit., 1965, 16, 144–158 Search PubMed.
- I. F. F. Benzie and J. J. Strain, Anal. Biochem., 1996, 239, 70–76 CrossRef CAS.
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, Free Radical Biol. Med., 1999, 26, 1231–1237 CrossRef CAS.
- D. J. Huang, B. X. Ou, M. Hampsch-Woodill, J. A. Flanagan and R. L. Prior, J. Agric. Food Chem., 2002, 50, 4437–4444 CrossRef CAS.
- A. J. Stewart, W. Mullen and A. Crozier, Mol. Nutr. Food Res., 2005, 49, 52–60 CrossRef CAS.
- T. Tanaka, G. Nonaka and I. Nishioka, Chem. Pharm. Bull. (Tokyo), 1986, 34, 650–655 CAS.
- M. I. Gil, F. A. Tomás-Barberán, B. Hess-Pierce, D. M. Holcroft and A. A. Kader, J. Agric. Food Chem., 2000, 48, 4581–4589 CrossRef CAS.
- T. Tanaka, G. I. Nonaka and I. Nishioka, Chem. Pharm. Bull. (Tokyo), 1990, 38, 2424–2428.
- F. Hernandez, P. Melgarejo, F. A. Tomás-Barberán and F. Artés, Eur. Food Res. Technol., 1999, 210, 39–42 CrossRef CAS; G. A. Miguel, C. Fontes, D. Antunes, A. Neves and D. Martins, J. Biomed. Biotechnol., 2004, 338–342 Search PubMed.
- T. L. Hager, L. R. Howard, R. Liyanage, J. O. Lay and R. L. Prior, J. Agric. Food Chem., 2008, 56, 661–669 CrossRef CAS.
- S. A. Vekiari, H. M. Gordon, P. Garcia-Macias and H. Labrinea, Food Chem., 2008, 110, 1007–1011 CrossRef CAS.
- G. Borges, A. Degeneve, W. Mullen and A. Crozier, J. Agric. Food Chem., 2010, 58, 3901–3909 CrossRef CAS.
- K. R. Martin, C. G. Krueger, G. Rodriquez, M. Dreher and J. D. Reed, J. Sci. Food Agric., 2009, 89, 157–162 CrossRef CAS.
- J. Oszmianski and J. C. Sapis, J. Food Sci., 1988, 53, 1241–1242 CrossRef CAS.
- A. P. Kulkarni, S. M. Aradhya and S. Divakar, Food Chem., 2004, 87, 551–557 CrossRef CAS.
- M. A. Ross, J. Chromatogr., B: Biomed. Sci. Appl., 1994, 657, 197–200 CrossRef CAS.
- M. Serafini, J. Sci. Food Agric., 2006, 86, 1989–1991 CrossRef CAS.
- A. Crozier, I. B. Jaganath and M. N. Clifford, Nat. Prod. Rep., 2009, 26, 1001–1043 RSC.
| This journal is © The Royal Society of Chemistry 2010 |
