Hypocholesterolemic activity of onion is mediated by enhancing excretion of fecal sterols in hamsters
Lei
Guan
a,
Hau Yin
Chung
*ab,
Yalun
Su
c,
Rui
Jiao
b,
Cheng
Peng
b and
Zhen Yu
Chen
*b
aDepartment of Biology, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China
bFood and Nutritional Sciences Programme, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China. E-mail: zhenyuchen@cuhk.edu.hk; anthonychung@cuhk.edu.hk; Fax: (+852) 26035745
cInstitute of Materia Medica, Chinese Academy of Medica Sciences and Peking Union Medical College, Beijing, China
First published on 13th September 2010
Abstract
Onion has been shown to favorably modify the lipoprotein profile. However, research on its underlying mechanism is lacking. The present study investigated the interaction of dietary onion powder with the protein expression of key receptors and enzymes involved in cholesterol metabolism. Thirty-six male hamsters were randomly divided into three groups and fed a high-cholesterol control diet or the two experimental diets supplemented with 1% onion powder (OP-1) or 5% onion powder (OP-5), for a period of 8 weeks. It was found that onion dose-dependently decreased plasma total cholesterol (TC) level. The change in plasma lipoprotein profile was accompanied by a greater excretion of both fecal neutral and acidic sterols. Western blot analysis revealed that onion up-regulated sterol regulatory element binding protein 2 (SREBP-2), liver X receptor alpha (LXRα) and cholesterol-7α-hydroxylase (CYP7A1) with no effect on 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) and LDL receptor (LDL-R). It was concluded that the hypocholesterolemic activity of onion powder was mediated by enhancement of fecal sterol excretion and up-regulation of LXRα and CYP7A1.
Introduction
Onion has been used as an ingredient in food by many cultures. It has been shown that onion possesses various biological activities including being antibiotic, antidiabetic, antioxidant, antiatherogenic, and anticancer.1 Traditionally, onion is also used to treat fever, dropsy, chronic bronchitis, colic, and scurvy. The chemistry of onion has been a subject of the extensive investigations. The active ingredients responsible for these activities in onion are claimed to be quercetin, methiin, propiin, isoalliin, alkyl thiosulfinate, disulfides and polysulfides.2,3Plasma total cholesterol (TC) and low-density lipoprotein cholesterol (LDL) correlate directly with risk of coronary heart disease (CHD), whereas the high-density lipoprotein cholesterol (HDL) correlates inversely with the risk. Epidemiological studies have shown a correlation between diets rich in onion and a reduced risk of mortality from coronary heart disease CHD.4,5 In healthy subjects receiving 100 g butter fat, onion juice demonstrated a significant protective action against fat-induced increases in serum TC and plasma fibrinogen with a concomitant decrease in coagulation time and fibrinolytic activity.6 Results from animal trials support this notion. Onion powder decreased blood glucose, serum TC and reduced the oxidative stress in STZ-induced diabetic rats.7 In SD rats fed a high-fat high-sucrose diet, onion was effective in lowering both plasma and hepatic TC and triacylglycerols (TG).8 When raw onion was added into diet of healthy pigs, a moderate reduction in plasma lipids was also observed.9
Plasma TC is mainly maintained by sterol regulatory element-binding protein 2 (SREBP-2) and liver X receptor-alpha (LXRα) in a coordinated manner.10 SREBP-2 governs the transcription of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) and LDL receptor (LDL-R). HMGR is a rate-limiting enzyme in cholesterogenesis, while LDLR is responsible for the removal of LDL from the circulation. LXRα activates transcription of a gene encoding cholesterol-7α-hydroxylase (CYP7A1), which is a rate-limiting enzyme in conversion of cholesterol to bile acids and responsible for elimination of excessive cholesterol in the liver.10 Despite extensive research on onion, little is known of how consumption of onion interacts with these genes and proteins involved in cholesterol metabolism in vivo. The present study was therefore undertaken to characterize the interaction of dietary onion with SREBP-2, LXRα, HMGR, LDL-R, and CYP7A1 in attempt to explore the underlying cholesterol-lowering mechanism.
Experimental
Diet
Red onion (Allium cepa Linn.) was obtained from a local market in Xinjiang, China. Onion was soaked in three volumes of boiling water. The extract was then filtered and spray-dried, leading to production of the white onion powder (OP).Three diets were prepared in the present study with the control diet being mixed with the following ingredients in proportion (g kg−1 diet): cornstarch, 508; casein, 242; lard, 50, sucrose, 119; mineral mix, 40; vitamin mix, 20; DL-methionine, 1; cholesterol, 1. The two experimental diets were prepared by adding 1% onion powder (OP-1) and 5% onion powder (OP-5) by weight into the control diet, respectively. The powdered diets were mixed with a gelatin solution (20 g L−1) in a ratio of 200 g diet per litre of solution (Table 1). Once the gelatin had set, the diets were cut into pieces of approximately 10 g cubes and stored frozen at −20 °C.
| Main nutrients | Control | OP-1 | OP-5 |
|---|---|---|---|
| Corn starch | 508 | 508 | 508 |
| Casein | 242 | 242 | 242 |
| Lard | 50 | 50 | 50 |
| Sucrose | 119 | 119 | 119 |
| Mineral mixture | 40 | 40 | 40 |
| Vitamin mixture | 20 | 20 | 20 |
| DL-Methionine | 1 | 1 | 1 |
| Cholesterol | 1 | 1 | 1 |
| Onion powder | 0 | 10 | 50 |
| Gelatin | 20 | 20 | 20 |
Hamsters
Golden Syrian male hamsters (n = 36, 100–120 g) were housed in an animal room at 25 °C with a 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h light-dark cycle. The entire experiment was approved and conducted in accordance with the guidelines set by the Animal Experimental Ethical Committee, The Chinese University of Hong Kong. Hamsters were allowed free access to a standard cereal-based chow diet (PicoLab® Rodent Diet20-Lab Diet, Australia) and water for a 2 week acclimation period. Afterwards, all hamsters were allowed free access to the control diet for additional two weeks. They were then randomly divided into three groups (n = 12 epr group), weighed, ear-punched, and fed the control, OP-1 or OP-5 diets for a period of 8 weeks. The body weight was recorded once a week, food consumption was recorded every 2 days, and total fecal output was collected weekly. Blood was collected from the retro-orbital sinus into a heparinized capillary tube under light anesthetization, using a mixture of ketamine, xylazine and saline (v/v/v, 4
12 h light-dark cycle. The entire experiment was approved and conducted in accordance with the guidelines set by the Animal Experimental Ethical Committee, The Chinese University of Hong Kong. Hamsters were allowed free access to a standard cereal-based chow diet (PicoLab® Rodent Diet20-Lab Diet, Australia) and water for a 2 week acclimation period. Afterwards, all hamsters were allowed free access to the control diet for additional two weeks. They were then randomly divided into three groups (n = 12 epr group), weighed, ear-punched, and fed the control, OP-1 or OP-5 diets for a period of 8 weeks. The body weight was recorded once a week, food consumption was recorded every 2 days, and total fecal output was collected weekly. Blood was collected from the retro-orbital sinus into a heparinized capillary tube under light anesthetization, using a mixture of ketamine, xylazine and saline (v/v/v, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) after 16 h food deprivation at weeks 0, 4, 8 and 12. At the end of 12 weeks, all the hamsters were killed after overnight fasting. Blood was collected via the abdominal aorta. The liver, heart, kidney, testis, perirenal fat and epididymal fat were also removed, rinsed with ice-cold saline, weighed, flash frozen in liquid nitrogen and stored at −80 °C until analysis.
5) after 16 h food deprivation at weeks 0, 4, 8 and 12. At the end of 12 weeks, all the hamsters were killed after overnight fasting. Blood was collected via the abdominal aorta. The liver, heart, kidney, testis, perirenal fat and epididymal fat were also removed, rinsed with ice-cold saline, weighed, flash frozen in liquid nitrogen and stored at −80 °C until analysis.
Plasma lipid and lipoprotein determinations
Plasma TC and TG levels were determined using enzymatic kits obtained from Infinity (Waltham, MA, USA) and Stanbio Laboratories (Boerne, TX, USA), respectively. The concentration of HDL was measured after precipitation of LDL and very low-density lipoprotein (VLDL) with phosphotungstic acid and magnesium chloride, using a commercial kit (Stanbio Laboratories). Non-HDL was calculated from the difference between TC and HDL.Determination of organ cholesterol
Cholesterol in organs was determined using a method described previously.11 The liver (100 mg) and heart (300 mg) were used to determine the cholesterol level. In brief, the samples with addition of 1 mg stigmastanol, as an internal standard, were homogenized in 15 ml of chloroform–methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and 3 ml saline. The chloroform–methanol phase was removed and dried under a gentle stream of nitrogen gas. After the lipid extract was mildly saponified in 5 ml of 1 N NaOH in 90% ethanol at 90 °C for one hour, 6 ml of cyclohexane were added to extract the total cholesterol. The cyclohexane phase was evaporated to dryness under nitrogen gas, and the cholesterol was converted to its trimethylsilyl (TMS)-ether derivative. The TMS-ether derivative was dissolved in hexane for GC analysis.
1, v/v) and 3 ml saline. The chloroform–methanol phase was removed and dried under a gentle stream of nitrogen gas. After the lipid extract was mildly saponified in 5 ml of 1 N NaOH in 90% ethanol at 90 °C for one hour, 6 ml of cyclohexane were added to extract the total cholesterol. The cyclohexane phase was evaporated to dryness under nitrogen gas, and the cholesterol was converted to its trimethylsilyl (TMS)-ether derivative. The TMS-ether derivative was dissolved in hexane for GC analysis.
Determination of fecal neutral and acidic sterols
Neutral and acidic sterols in the feces were determined as we described previously with slight modifications.11 Dried fecal samples (300 mg) were mildly hydrolyzed with 1 N NaOH in 90% ethanol at 90 °C for 1 h. Then, total neutral sterols were extracted with cyclohexane, and converted into their TMS-ether derivatives. The acidic sterol-containing lower aqueous phase was similarly saponified and converted into their TMS-ether derivatives. The two TMS-ether derivatives were subjected to the GC analysis.Western blotting analysis of liver SREBP-2, LDL-R, HMGR, LXRα and CYP7A1
Liver protein was extracted according to the method described previously by Vaziri and Liang with some modification.12 In brief, the liver sample was homogenized in a homogenizing buffer containing 20 mM Tris-HCL (pH 7.5), 2 mM MgCl2, 0.2 M sucrose and Complete® protease inhibitor cocktail (Roche, Mannheim, Germany). The extract was centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 15 min at 4 °C and the supernatant was collected (total protein). The total protein was centrifuged at 126
000 g for 15 min at 4 °C and the supernatant was collected (total protein). The total protein was centrifuged at 126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 60 min at 4 °C. The pellet was re-suspended in the same homogenizing buffer. The pellet protein was separated by electrophoresis on a 7% SDS-PAGE gel and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA) using a semi-dry transfer system. Membranes were blocked in 5% nonfat milk Tris-buffered saline with Tween-20 for 1 h and overnight at 4 °C in the same solution containing 1
000 g for 60 min at 4 °C. The pellet was re-suspended in the same homogenizing buffer. The pellet protein was separated by electrophoresis on a 7% SDS-PAGE gel and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA) using a semi-dry transfer system. Membranes were blocked in 5% nonfat milk Tris-buffered saline with Tween-20 for 1 h and overnight at 4 °C in the same solution containing 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 anti-LDL-R antibody (Santa Cruz Biotechnology, Inc., California, USA), 1
600 anti-LDL-R antibody (Santa Cruz Biotechnology, Inc., California, USA), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 anti-HMGR (Upstate USA Inc., Lake Placid, NY, USA), 1
500 anti-HMGR (Upstate USA Inc., Lake Placid, NY, USA), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 anti-CYP7A1 (Santa Cruz Biotechnology, Inc., California, USA), 1
200 anti-CYP7A1 (Santa Cruz Biotechnology, Inc., California, USA), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 anti-LXRα antibody, or anti-SREBP-2 antibody (Santa Cruz Biotechnology).13 The membrane was then incubated for one hour at 4 °C in diluted (1
400 anti-LXRα antibody, or anti-SREBP-2 antibody (Santa Cruz Biotechnology).13 The membrane was then incubated for one hour at 4 °C in diluted (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000) horseradish peroxidase-linked goat anti-rabbit IgG (Santa Cruz Biotechnology, Inc. California, USA), donkey anti-rabbit IgG (Santa Cruz Biotechnology, Inc. California, USA) or goat anti-mouse IgG (Calbiochem, EMD Chemicals, Inc., San Diego, CA, USA). Then, membranes were developed with ECL enhanced chemiluminescence agent (Santa Cruz Biotechnology, Inc., California, USA) and subjected to autoradiography on SuperRX medical X-ray film (Fuji, Tokyo, Japan). Densitometry was quantified using the BioRad Quantity one® software (BioRad Laboratories, Hercules, USA). Data on abundance of LDLR, HMGR, CYP7A1, LXR and SREBP-2 were normalized with β-actin (Santa Cruz Biotechnology, Inc., California, USA).
3000) horseradish peroxidase-linked goat anti-rabbit IgG (Santa Cruz Biotechnology, Inc. California, USA), donkey anti-rabbit IgG (Santa Cruz Biotechnology, Inc. California, USA) or goat anti-mouse IgG (Calbiochem, EMD Chemicals, Inc., San Diego, CA, USA). Then, membranes were developed with ECL enhanced chemiluminescence agent (Santa Cruz Biotechnology, Inc., California, USA) and subjected to autoradiography on SuperRX medical X-ray film (Fuji, Tokyo, Japan). Densitometry was quantified using the BioRad Quantity one® software (BioRad Laboratories, Hercules, USA). Data on abundance of LDLR, HMGR, CYP7A1, LXR and SREBP-2 were normalized with β-actin (Santa Cruz Biotechnology, Inc., California, USA).
Statistics
Data are expressed as mean ± standard deviation (SD). Treatment effects were statistically analyzed among the three groups using one-way analysis of variance (ANOVA) and post hoc LSD test on SigmaStat Advisory Statistical Software (SigmaStat version 16.0, SPSS Inc., Chicago, IL, USA). P-values less than 0.05 are regarded as statistically significant.Results
Body weight, food intake, food efficiency, and relative organ weight
Onion consumption had no effect on body weight among the control, OP-1 and OP-5 groups (Table 2). Food intake was expressed as g per hamster per day, whereas the food efficiency ratio was defined as the body weight gained by each hamster that consumed every 100 g of diet. It was found that food intake and food efficiency did not differ among the three groups. No difference in relative organ weight was seen among the three groups, although hamsters fed the onion powder at both doses had smaller epididymal and peripheral pads than the control group.| Control | OP-1 | OP-5 | |
|---|---|---|---|
| Data expressed as mean ± SD; n = 12 each group. | |||
| Body weight (g) | |||
| Initial | 127.1 ± 6.6 | 124.2 ± 10.2 | 124.6 ± 12.1 |
| Final | 138.0 ± 11.8 | 131.0 ± 8.4 | 132.2 ± 10.3 |
| Food Intake (g per hamster per day) | 12.19 ± 0.79 | 11.56 ± 0.56 | 12.11 ± 0.97 |
| Food Efficiency (g per 100 g diet) | 7.32 ± 1.40 | 7.72 ± 1.27 | 8.07 ± 1.70 |
| Relative organ weight (g per 100 g body weight) | |||
| Liver | 4.06 ± 0.15 | 4.06 ± 0.29 | 4.04 ± 0.16 |
| Heart | 0.35 ± 0.03 | 0.35 ± 0.02 | 0.36 ± 0.03 |
| Kidney | 0.80 ± 0.06 | 0.80 ± 0.02 | 0.78 ± 0.09 |
| Epididymal fat | 1.52 ± 0.27 | 1.45 ± 0.22 | 1.46 ± 0.33 |
| Peripheral fat | 0.93 ± 0.15 | 0.88 ± 0.14 | 0.87 ± 0.16 |
Plasma lipoproteins and organ cholesterol level
No difference in plasma lipoprotein profiles was seen among the three groups at week 0 (Table 3). At week 4 and 8, OP-5 group had plasma TC decreased by 11.2% and 20.3%, respectively, compared with the control group. Non-HDL concentration in OP-5 groups was also significantly decreased by 20.3% and 23.4%, respectively, compared with that in the control group. Similarly, the ratio of non-HDL to HDL was reduced significantly in OP-5 group compared with that in the control hamsters (Table 3). Although plasma TC and non-HDL in OP-1 group was lower, they were not statistically different from those in the control hamsters. Initially, no difference in plasma TG was seen among the three groups. At week 4, plasma TG showed a decreasing trend in the two experimental group. However, the difference in plasma TG between OP-5 and the control group became statistically significant at week 8 (Table 3). No significant differences were found in hepatic and heart cholesterol content among the three groups (Table 3).| Control | OP-1 | OP-5 | |
|---|---|---|---|
| Non-HDL= [TC]-[HDL]. Data expressed as mean ± SD; n = 12. Means at the same raw with different superscripts (a, b, c) differ significantly at p < 0.05. | |||
| TC (mg/dl) | |||
| Initial | 207.4 ± 36.4 | 207.2 ± 48.9 | 207.3 ± 48.0 |
| 4th week | 216.4 ± 24.9a | 199.4 ± 38.5ab | 192.2 ± 20.6b |
| 8th week | 215.1 ± 39.6a | 204.7 ± 46.8ab | 171.4 ± 25.0b |
| HDL (mg/dl) | |||
| Initial | 80.6 ± 14.3 | 84.7 ± 10.2 | 83.9 ± 12.1 |
| 4th week | 102.8 ± 13.9 | 112.4 ± 20.0 | 102.5 ± 17.1 |
| 8th week | 96.48 ± 8.0a | 102.83 ± 10.1a | 84.31 ± 7.4b |
| non-HDL (mg/dl) | |||
| Initial | 126.8 ± 29.9 | 122.5 ± 41.0 | 123.5 ± 42.1 |
| 4th week | 118.0 ± 7.7 | 97.7 ± 41.1 | 94.1 ± 15.0 |
| 8th week | 107.4 ± 27.8a | 99.5 ± 26.4ab | 82.3 ± 13.4b |
| HDL/TC | |||
| Initial | 0.39 ± 0.06 | 0.42 ± 0.07 | 0.42 ± 0.07 |
| 4th week | 0.46 ± 0.04 | 0.50 ± 0.11 | 0.51 ± 0.06 |
| 8th week | 0.45 ± 0.05b | 0.49 ± 0.09ab | 0.52 ± 0.03a |
| non-HDL/HDL | |||
| Initial | 1.60 ± 0.35 | 1.43 ± 0.38 | 1.48 ± 0.47 |
| 4th week | 1.17 ± 0.32 | 0.99 ± 0.54 | 0.97 ± 0.21 |
| 8th week | 1.19 ± 0.25a | 0.97 ± 0.23ab | 0.95 ± 0.11b |
| TG (mg/dl) | |||
| Initial | 155.4 ± 39.7 | 157.6 ± 45.3 | 147.3 ± 73.2 |
| 4th week | 211.9 ± 61.7 | 172.9 ± 50.5 | 167.1 ± 43.9 |
| 8th week | 218.3 ± 92.0a | 166.3 ± 73.6b | 145.4 ± 36.0b |
| Organ cholesterol (mg/g) | |||
| Liver | 80.2 ± 10.5 | 88.5 ± 11.3 | 77.6 ± 13.3 |
| Heart | 3.7 ± 0.4 | 3.7 ± 0.3 | 3.6 ± 0.2 |
Fecal sterols
The total neutral sterols are the sum of cholesterol, coprostanol, coprostanone, dihydrocholesterol, campesterol, β-sistosterol and stigmastanol. Data on fecal analysis were similar in week 4 and 8. To simplify the presentation, only data for week 8 is shown. Supplementation of onion powder into diets caused greater fecal excretion of neutral sterols compared with the control group (Table 4). The total acid sterols were the sum of lithocholic, deoxycholic, chenodeoxycholic, cholic and ursodeoxycholic acids. Similarly, onion demonstrated a dose-dependent increasing trend in fecal excretion of acidic sterols (Table 4).| Control | OP-1 | OP-5 | |
|---|---|---|---|
| a Data expressed as mean ± SD; n = 12. Means at the same raw with different superscripts (a, b, c) differ significantly at p < 0.05. | |||
| Neutral sterols (mg/day) | |||
| Coprostanol | 0.59 ± 0.14 | 0.70 ± 0.01 | 0.86 ± 0.43 |
| Coprostanone | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 |
| Cholesterol | 0.79 ± 0.19 | 0.81 ± 0.14 | 0.84 ± 0.15 |
| Dihydrocholesterol | 0.35 ± 0.06 | 0.38 ± 0.02 | 0.40 ± 0.03 |
| Campesterol | ND | 0.06 ± 0.01 | 0.10 ± 0.04 |
| Stigmasterol | ND | 0.11 ± 0.01 | 0.15 ± 0.01 |
| β-sitosterol | ND | 0.03 ± 0.01 | 0.05 ± 0.02 |
| Total | 1.76 ± 0.38b | 2.14 ± 0.15ab | 2.45 ± 0.34a |
| Acidic sterols (mg/day) | |||
| Lithocholic acid | 1.95 ± 1.25 | 3.14 ± 0.74 | 3.65 ± 0.76 |
| Deoxycholic acid | 0.52 ± 0.11 | 0.38 ± 0.05 | 0.45 ± 0.31 |
| Chenodeoxycholic + Cholic acids | 1.14 ± 0.89 | 0.68 ± 0.22 | 0.57 ± 0.95 |
| Ursodecholic acid | 0.26 ± 0.05 | 0.28 ± 0.06 | 0.59 ± 0.08 |
| Total | 3.88 ± 0.19b | 4.49 ± 0.97ab | 5.25 ± 0.89a |
Cholesterol balance
Data on the apparent cholesterol absorption was calculated as we previously described.13 Total intake of cholesterol was compared with its excretion in neutral and acidic sterols (Table 5). Net cholesterol equivalent retained was calculated by difference between the intake and the excretion of both neutral and acidic sterols. It was found that the net cholesterol retention was the highest in the control group, followed by OP-1 and OP-5 in decreasing order. The apparent cholesterol absorption was calculated by the equation [(cholesterol intake − excretion of neural and acidic sterols)/cholesterol intake] × 100. It was shown that onion powder diet could decrease the apparent cholesterol absorption in a dose-dependent manner.| Control | OP-1 | OP-5 | |
|---|---|---|---|
| Data expressed as mean ± SD; n = 12. Means at the same raw with different superscripts (a, b, c) differ significantly at p < 0.05. # Excluding the exogenous sterols namely campesterol, stigmasterol and β-sitosterol. | |||
| Food intake (g/d/hamster) | 12.19 ± 0.79 | 12.04 ± 0.51 | 12.28 ± 0.67 |
| Neutral Sterol (mg/d/hamster)# | 1.76 ± 0.38 | 1.94 ± 0.16 | 2.15 ± 0.31 |
| Acidic Sterol (mg/d/hamster) | 3.88 ± 0.19 | 4.49 ± 0.97 | 5.25 ± 1.12 |
| Total Sterol output (mg/d/hamster) | 5.64 ± 0.56 | 6.53 ± 0.61 | 6.84 ± 0.69 |
| Cholesterol Retained (mg/d/hamster) | 6.53 ± 1.46 | 5.03 ± 0.63 | 5.17 ± 0.98 |
| Apparent cholesterol absorption (% intake) | 54.73 ± 6.84a | 46.61 ± 6.75ab | 39.74 ± 3.41b |
Hepatic SREBP-2, HMGR, LDL-R, LXRα, and CYP7A1
No differences in protein mass of HMGR, LDLR and LXRβ were seen among the three groups. However, the immunoreactive mass of liver SREBP-2, LXRα and CYP7A1 was dose-dependently increased with the increasing onion powder in diets (Fig. 1 & 2).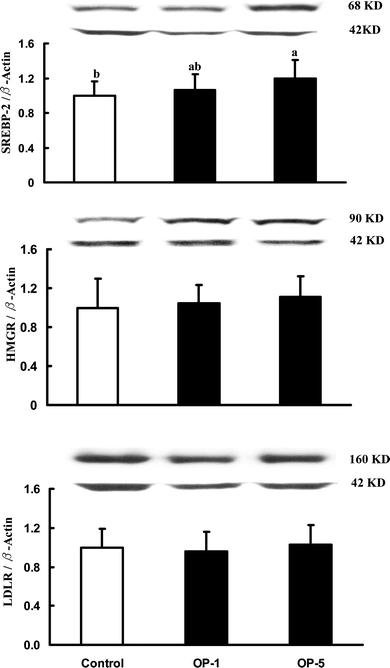 | ||
| Fig. 1 The relative immunoreactive mass of hepatic SREBP-2, HMG-CoA-R, and LDL-R in hamsters fed the control diet, and two experimental diets supplemented containing 1% onion powder (OP-1) and 5% onion powder (OP-5) for 8 weeks. Data were normalized with β-actin and values were expressed as mean ± SD, n = 12. | ||
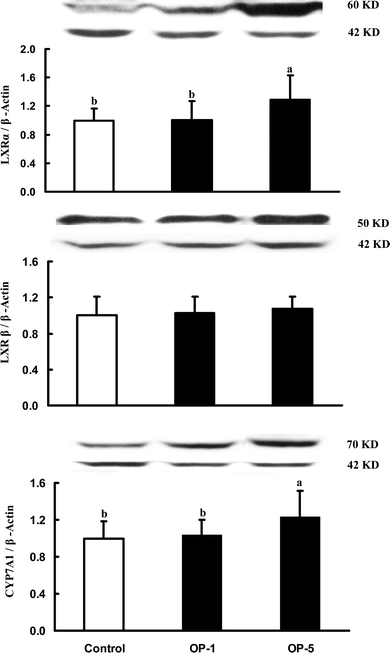 | ||
| Fig. 2 The relative immunoreactive mass of hepatic liver X receptor (LXR) and cholesterol-7α-hydroxylase (CYP7A1) in hamsters fed the control diet, and two experimental diets supplemented with 1% onion powder (OP-1) and 5% onion powder (OP-5) for 8 weeks. Data were normalized with β-actin and values were expressed as mean ± SD, n = 12. | ||
Discussion
The present study demonstrated that supplementation of onion powder in diet could favorably modify the plasma lipoprotein profile by decreasing plasma TC, non-HDL, TG and ratio of non-HDL/HDL. The beneficial effect associated with onion power appeared to be dose-dependent as the supplementation at 5% level had a greater hypocholesterolemic activity than that at 1% supplementation. In general, the present results were in agreement with those previously reported in humans and animal studies, supporting the claim that the regular consumption of onions reduces the risk of CHD.6–9There is no report to date that has investigated the underlying mechanism by which onion in diet reduces plasma cholesterol level. The present study was the first to demonstrate that onion powder in diets promoted the excretion of total fecal neutral sterols, suggesting it inhibited the cholesterol absorption and thus led to reduction in plasma TC. The fecal sterol analysis showed that the three major phytosterols, namely β-sitosterol, campesterol and stigmasterol, were present in the feces of the two onion-fed groups but they were absent in the control group. In addition, the amount of the three phytosterols in feces was in the order OP-5 > OP-1 > control, indicating that onion powder contained phytosterols. This could partially explain the hypocholesterolemic activity of onion because phytosterols compete with cholesterol for absorption. In fact, onion powder demonstrated a dose-dependent increase in the excretion of neutral sterols (Table 4).
Cholesterol is mainly eliminated via its conversion to bile acids. The present study clearly demonstrated that dietary onion was able to increase the excretion of bile acids by 16–35% (Table 4). This was partially mediated by up-regulation of LXRα and CYP7A1 proteins (Fig. 2). The observation is in agreement with that of Kumari and Augusti,2 who studied the effect of S-methyl cysteine sulfoxide, an active ingredient in onion, on the excretion of bile acids and neutral sterols, finding it increased the excretion of acidic and neutral sterols by 25 and 37%, respectively. The increase in the excretion of bile acids is likely an additional mechanism by which onion exhibited a cholesterol-lowering activity.
No study to date has examined how dietary onion interacts with the protein expressions of SREBP-2, HMGR and LDLR. Our data showed that the addition of onion into diet increased the protein mass of SREBP-2, with LDLR and HMGR being unaffected. SREBP-2 governs the expression of LDLR and HMGR. Theoretically, up-regulation of SREBP-2 should be accompanied by up-regulation of LDLR and HMGR. To explore the underlying mechanism by which dietary onion had no effect on the protein levels of LDLR and HMGR with SREBP-2 being up-regulated, the following explanation is offered. The abundance in HMGR and LDLR was already very low because hamsters in the present study were sacrificed with an empty stomach, so that cholesterol catabolism rate was nil and no effect of dietary onion on these proteins could be seen after the overnight fasting.
Conclusion
The present study was the first of its kind to investigate the interaction of dietary onion with the protein expression of hepatic SREBP-2, LDLR, HMGR, LXRα and CYP7A1. Results demonstrated that onion decreased plasma TC in a dose-dependent manner. Dietary onion-induced reduction in plasma TC was accompanied by enhanced excretion of both fecal neutral and acidic sterols, most likely by up-regulation of LXRα and CYP7A1.Abbreviations
| CHD | Coronary heart disease |
| CYP7A1 | Cholesterol 7α-hydroxylase |
| HDL | High density lipoprotein cholesterol |
| HMGR | 3-Hydroxy-3-methylglutaryl-CoA reductase |
| LDL | Low-density lipoprotein cholesterol |
| LDLR | LDL receptor |
| LXR | Liver X receptor |
| SREBP-2 | Sterol regulatory element binding protein 2 |
| TC | Total cholesterol. |
References
- K. T. Augusti, Indian J. Exp. Biol., 1996, 34, 634–640 Search PubMed.
- K. Kumari and K. T. Augusti, J. Ethnopharmacol., 2007, 109, 367–371 CrossRef CAS.
- E. Block, Garlic and Other Alliums: The Lore and the Science, Royal Society of Chemistry, Cambridge, UK, 2010 Search PubMed.
- R. K. Sogani and K. Katoch, J. Assoc. Physicians India, 1981, 29, 443–446 Search PubMed.
- M. G. Hertog, E. J. Feskens, P. C. Hollman, M. B. Katan and D. Kromhout, Lancet, 1993, 342, 1007–1011 CrossRef CAS.
- A. Bordia, H. C. Bansal, S. K. Arora and S. V. Singh, Atherosclerosis, 1975, 21(1), 15–19 CrossRef CAS.
- M. A. Bang, H. A. Kim and Y. J. Cho, Nutr. Res. Pract., 2009, 3(3), 242–246 Search PubMed.
- Y. Yamamoto and A. Yasuoka, Biosci., Biotechnol., Biochem., 2010, 74(2), 402–404 CrossRef CAS.
- E. Ostrowska, N. K. Gabler, S. J. Sterling, B. G. Tatham, R. B. Jones, D. R. Eagling, M. Jois and F. R. Sunshea, Br. J. Nutr., 2004, 91(2), 211–218 CrossRef CAS.
- Z. Y. Chen, R. Jiao and K. Y. Ma, J. Agric. Food Chem., 2008, 56, 8761–8773 CrossRef CAS.
- P. T. Chan, W. P. Fong, Y. L. Cheung, Y. Huang, W. K. K. Ho and Z. Y. Chen, J. Nutr., 1999, 129, 1094–1101 CAS.
- N. D. Vaziri and K. H. Liang, Kidney Int., 1996, 50, 887–893 CrossRef CAS.
- C. K. Lam, Z. S. Zhang, H. Yu, S. Y. Tsang, Y. Huang and Z. Y. Chen, Mol. Nutr. Food Res., 2008, 52, 950–958 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
