Comparative healing property of kombucha tea and black tea against indomethacin-induced gastric ulceration in mice: possible mechanism of action
Debashish
Banerjee
a,
Sham A.
Hassarajani
b,
Biswanath
Maity
a,
Geetha
Narayan
b,
Sandip K.
Bandyopadhyay
a and
Subrata
Chattopadhyay
*b
aIPGME&R, 244B, Acharya Jagadish Chandra Bose Road, Kolkata, 700 020, India
bBio-Organic Division, Bhabha Atomic Research Centre, Mumbai, 400 085, India. E-mail: schatt@barc.gov.in; Fax: +91-22-25505151; Tel: +91-22-25593703
First published on 3rd November 2010
Abstract
The healing activity of black tea (BT) and BT fermented with Candida parapsilosis and kombucha culture, designated as CT and KT respectively against the indomethacin-induced stomach ulceration has been studied in a mouse model. The KT sample (KT4) produced by fermenting BT for four days, showed the best DPPH radical scavenging capacity and phenolics contents. Hence the ulcer-healing activity of KT4 was compared with those of CT4 and BT. All the tea extracts (15 mg kg−1) could effectively heal the gastric ulceration as revealed from the histopathological and biochemical studies, with relative efficacy as KT4 > CT4 ∼ BT. The healing capacities of the tea extracts could be attributed to their antioxidant activity as well as the ability to protect the mucin content of the gastric tissues. In addition, the ability of KT4 to reduce gastric acid secretion might also contribute to its ulcer-healing activity. The tea preparation KT4 (15 mg kg−1) was as effective as the positive control, omeprazole (3 mg kg−1) in ulcer healing.
Introduction
The gastro-toxicity of non-steroidal anti-inflammatory drugs (NSAIDs), often leading to gastric ulceration and delayed healing, remains a crucial problem, despite recent pharmaceutical advances.1,2 The currently available synthetic anti-ulcer drugs are expensive, show side effects, and cannot prevent ulcer recurrence.2 Exploration of plants/herbs, especially the edible varieties might provide suitable alternative anti-ulcer formulations. Many taxa of medicinal plants have been assessed worldwide for their antiulcerogenic effects.3 For decades, doctors have recommended dietary adjustments aimed at preventing or treating symptoms of gastritis and ulceration, as diet may moderate the risk for gastritis or peptic ulcer.Camellia sinenesis is widely grown in the tropical humid climate of South East Asia, and decoction of its leaves (tea) is the most popular non-alcoholic beverage worldwide. Different tea preparations such as green tea, black tea, oolang tea etc. are most commonly used. The cytoprotective action of the green tea-catechins against ethanol- or restraint plus water-immersion stress-induced acute gastric mucosal injury, and acetic acid-induced chronic gastric ulcers in rats has been reported.4 In addition, epigallocatechin gallate, a constituent of green tea has been suggested to control H. pylori-related chronic inflammations or regress cancer precursor lesions, while a pectin-type acidic polysaccharide from green tea is reported to posses anti-adhesive effects against H. pylori.5 It is believed that polyphenols or polyphenol derivatives from green tea may be useful either in prevention or treatment of H. pylori-associated gastric diseases. Although black tea (BT) accounts for 80% of the total tea consumption, studies on the pharmacological properties of BT are scarce. The prophylactic action of the tea seed-derived triterpene saponins against ethanol-induced gastric mucosal lesions,6,7 and of BT extract against various ulcerogens8,9 have been reported in rat models.
Kombucha or Kargassok tea (KT) is a fermented black tea preparation that is widely consumed in parts of the erstwhile Soviet Union and Central Asia, and has become popular even in Europe and the USA. KT is made by steeping a flat, pancake-like culture, referred to as the Kombucha mushroom, which is actually a symbiotic culture of various yeasts and bacterial species including: Saccharomycodes ludwigii, Schizosaccharomyces pombe, Candida parapsilosis, Acetobacter ketogenum, Bacterium and Torula spp, etc. in brewed black tea and sugar or sucrose for about a week. It is purported to improve general health, aid longevity, boost the immune system, possess anti-oxidant, anti-ageing and chemopreventive properties, and provide relief/cure for chronic conditions such as rheumatism/arthritis, hypertension/arteriosclerosis, stomach/intestinal/liver disorders and even cancer.10 More recently, its anti-stress and hepato-protective,11 antioxidant and immunopotentiating12 as well as anti-diabetic13 properties have been reported. However, the wide-ranging claims about the health benefits of KT are primarily based on personal observations and testimonials rather than supportive scientific evidence. The primary aim of the present study was to evaluate the healing property of KT and BT against indomethacin-induced acute gastric ulceration of mice and compare the activity with that of the drug, omeprazole (Omez). Factors such as oxidative stress and acid secretion contribute to stomach ulceration. Hence the anti-oxidative property and gastric acid inhibitory capacity of the test samples were also evaluated to rationalize their ulcer healing action. To the best of our knowledge, this is the first report on the stomach ulcer healing property of KT and BT.
Results
Standardization of KT preparation and analysis of its composition
In this investigation, we prepared two types of fermented teas by fermenting BT separately with kombucha culture, and C. parapsilosis for 2, 4 and 7 days. These are designated as KT and CT, followed by a number, signifying the days of incubation for their preparation. Parameters such as pH, absorbance, viscosity and radical scavenging property of the decoctions were measured. On increasing the fermentation time, a continuous drop of pH from 6.8 to 2.6 was observed with KT. The acid-producing microorganisms present in the kombucha culture are known to produce several organic acids including acetic acid, accounting for the reduction in the pH of KT.14 Besides acetic acid, the presence of glucuronic acid and lactic acid in KT4 was confirmed by TLC analysis. The pH change in the CT samples was much less (6.8 to 5.0).A drop in absorbance during fermentation was observed with KT, which was attributed to the lower availability of free theorubigin anions at the lower pHs. The theorubigin are also known to be degraded by the kombucha culture. The absorbance change amongst the CT samples was not significant. The viscosity of KT also decreased gradually with the increase in fermentation time, possibly due to the increased consumption of the added sugar. It is worth mentioning that the multiplication of the microorganisms in the kombucha culture was confined to the biofim. Hence, this did not increase the viscosity of KT. Amongst the KT samples, KT4 showed maximum total phenolics content, and accordingly best antioxidant capacity, as revealed from the DPPH scavenging assay. Hence, the ulcer healing activity was studied using KT4 only, and the results compared with those of BT and CT4 samples. It was also felt that KT7 would not be ideal for this study as its pH was too low (2.6) due to the generation of more acetic acid, which itself is a strong stomach ulcerogen. The results are summarized in Table 1.
| Parameters | BT | CT2 | CT4 | CT7 | KT2 | KT4 | KT7 |
|---|---|---|---|---|---|---|---|
| a Measured at 530 nm. b Ratio of the viscosities of the fermented tea samples and BT. c The values are mean ± SEM (n = 5). | |||||||
| pH | 6.8 | 6.4 | 5.48 | 5.01 | 5.45 | 4.23 | 2.58 |
| Optical densitya | 0.44 | 0.41 | 0.39 | 0.36 | 0.38 | 0.15 | 0.08 |
| Relative viscosityb | 1.08 | 1.20 | 1.16 | 0.98 | 0.94 | 0.89 | |
| Total phenolics (mg GAE g−1 extract)c | 24.81 ± 2.26 | 29.16 ± 2.12 | 33.68 ± 2.28 | 34.24 ± 2.71 | 38.52 ± 0.68 | 44.89 ± 2.10 | 40.5 ± 1.56 |
| DPPH radical scavenging activity (%)c | 39.77 ± 2.14 | 43.62 ± 2.44 | 49.20 ± 3.06 | 49.70 ± 2.73 | 59.64 ± 7.81 | 73.47 ± 4.15 | 61.06 ± 7.61 |
Preliminary TLC analyses of BT, CT4 and KT4 revealed the presence of additional compounds in KT4. The three major components of KT4 were isolated and characterized as theophylline, caffeine, and theobromine. Quantification of these compounds by a HPTLC densitogram revealed that fermentation of BT with the kombucha culture led to an increase in the theobromine content from 2.7% to 3.3% along with a substantial reduction in the caffeine level from 8% to 4%. The concentration of theophylline remained nearly the same (∼77.3%).
Optimization of doses of the tea samples for gastric ulcer healing
Ulceration in the mice was induced with a single dose of indomethacin (18 mg kg−1) and the doses of the tea extracts were optimized by carrying out the treatment with BT, CT4, and KT4 (10, 15 and 20 mg kg−1) for seven days. Omez (3 mg kg−1) was used as the positive control. The doses of indomethacin and Omez were decided based on the results of our previous studies.15 To evaluate the immediate effect of the test samples, similar experiments were also carried out under a one-day treatment regime. The extent of ulcer healing was assessed from the macroscopic and histological observations of the glandular portion of the gastric mucosa of the mice. The mice receiving vehicle only showed no mucosal lesions. Indomethacin administration to mice produced acute lesions in the gastric mucosa, measured in terms of macroscopic damage scores (MDS). Treatment with the test samples accelerated the healing of gastric lesions dose-dependently. Table 2 summarizes the dose-dependent ulcer-healing effect of the tea samples on continuing the treatment for 7 days. Optimal ulcer healing was obtained with 15 mg kg−1 of all the tea samples, which did not improve significantly at a higher dose (20 mg kg−1). Hence, we used this dose (15 mg kg−1) of the tea extracts for assaying all other healing and biochemical parameters, which are presented in the following.| Group | Drug dose/mg kg−1 | Macroscopic damage scores (MDS)b | MDS reduction (%)c |
|---|---|---|---|
| a Stomach ulceration in mice was induced by oral administration of indomethacin (18 mg kg−1). Different doses of the tea samples were used for these experiments. b The MDS were measured on day seven after indomethacin administration. The values are mean ± SEM, from three independent experiments, each with 5 mice per group. *p < 0.01, compared to the untreated control. c Considering a MDS value of 100 for the ulcerated, untreated mice. | |||
| Ulcerated | -- | 1.17 ± 0.03 | 0 |
| BT-treated | 10 | 0.62 ± 0.02 | 47.55 |
| BT-treated | 15 | 0.57 ± 0.03* | 51.07 |
| BT-treated | 20 | 0.55 ± 0.03 | 52.69 |
| CT4-treated | 10 | 0.53 ± 0.04 | 54.99 |
| CT4-treated | 15 | 0.45 ± 0.03* | 61.18 |
| CT4-treated | 20 | 0.43 ± 0.02 | 63.28 |
| KT4-treated | 10 | 0.52 ± 0.02 | 56.01 |
| KT4-treated | 15 | 0.36 ± 0.01* | 68.98 |
| KT4-treated | 20 | 0.35 ± 0.02 | 69.94 |
The comparative MDS reductions due to natural healing, and treatment with BT, CT4, KT4 and Omez on the 1st and 7th days of ulceration are provided in Fig. 1a and Fig. 1b respectively. Treatment with CT4, KT4 and Omez for one day reduced the MDS values by 39.2%, 59.3% and 48.1% respectively, compared to the ulcerated control group. The effect of BT was insignificant. KT4 showed a better effect than Omez. Compared to the 1st day-untreated mice (group II), the MDS of the 7th day-untreated mice (group III) was less by 34%, due to natural healing. However, the effect was more pronounced in the treated groups. Treatment with BT, CT4, KT4 and Omez for 7 days reduced the MDS by 56.1%, 61.7%, 71.5% and 64.4% respectively, compared to the group III mice. During the production of BT from green tea, a significant part of the catechins is converted to the theaflavins (TF). Our colorimetric assay14 revealed insignificant changes in the TF concentrations in BT and KT4. Hence in a separate experiment, we also studied the ulcer healing activity of TF. Treatment with TF (1 mg kg−1) for 7 days was found to provide 81.4% ulcer healing.
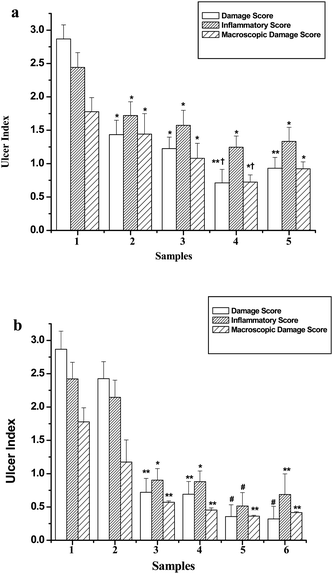 | ||
| Fig. 1 (a) Comparative healing capacities of the tea samples on the 1st day of ulceration, as revealed from the MDS, DS and IS. 1: untreated group, 2: BT group, 3: CT4 group, 4: KT4 group, 5: Omez group. Ulceration in the mice was induced by oral administration of indomethacin (18 mg kg−1). Different tea samples (15 mg kg−1) and Omez (3 mg kg−1) were used as the drugs. The assays were carried out 10 h after indomethacin administration and the values are mean ± SEM, from three independent experiments, each with 5 mice per group. *p < 0.05, **p < 0.01, compared to the untreated control (group II); †p < 0.05, compared to Omez-treatment. (b) Comparative healing capacities of the tea samples under the optimized treatment regime, as revealed from the MDS, DS and IS. 1: Untreated 1st day group, 2: untreated 7th day group, 3: BT group, 4: CT4 group, 5: KT4 group, 6: Omez group. Ulceration in the mice was induced by oral administration of indomethacin (18 mg kg−1). Different tea samples (15 mg kg−1) and Omez (3 mg kg−1) were used as the drugs. The assays were carried out 7 days after indomethacin administration and the values are mean ± SEM, from three independent experiments, each with 5 mice per group. *p < 0.05, **p < 0.01, #p < 0.001, compared to the untreated control (group III). | ||
Histological assessment of ulcer healing
The histopathological photographs of mice stomachs belonging to various groups are shown in Fig. 2a–g. Presence of crypts in the mucosal glandular layer with intact submucosa and muscular layer, and vaso congestion in serosa were noticeable in the normal mucosal epithelium (Fig. 2a). The stomach of the group II mice showed ruptures in different portions of the mucosal epithelial layer. Inflammatory infiltrates containing neutrophils were observed in the lamina propria, muscle coat and serosal layer with altered nucleus/cytoplasmic ratio. The submucosal and muscle layers were also not intact and inflammatory granules were seen in submucosa (Fig. 2b). The acute nature of ulceration was evident from the partial natural healing observed in the untreated control mice. Even on the 7th day of ulceration, multiple areas of ulceration, and hyperemia in the submucosal layer along with inflammatory exudates were noticed (Fig. 2c).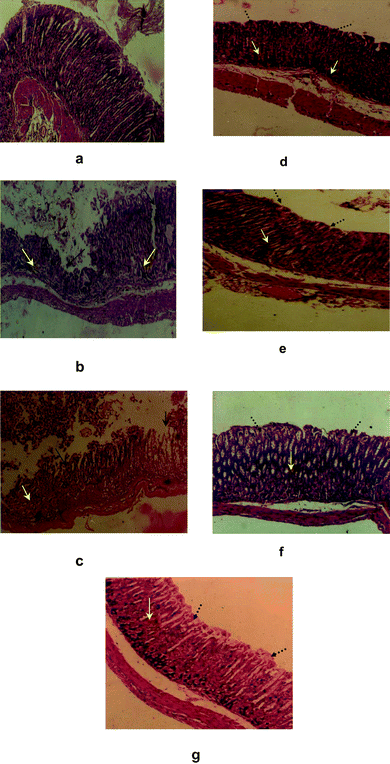 | ||
| Fig. 2 Histological assessment of acute gastric mucosal injury induced by indomethacin (18 mg kg−1) in mice and its prevention by BT, CT4, KT4 (15 mg kg−1) and Omez (3 mg kg−1). Section of mice stomachs obtained from a: normal control mice; b: untreated control mice 10 h after indomethacin administration; c: untreated control mice seven days after indomethacin administration; d–g: mice treated with BT, CT4, KT4 and Omez for seven days after indomethacin administration. Black, yellow and black dotted arrows indicate areas of mucosal damage, inflammatory cells and areas of cryptic proliferation respectively. | ||
All the tea samples showed a potent healing effect, because regenerative changes along the ulcerated margin were noticed in the 7-day treatment groups (Fig. 2d–f). Cryptic proliferation (crypt hyperchromasia) and lack of frank denudation were the prominent features of the healing. However, the effects of different test samples were different. The stomachs of the BT-treated mice showed an intact epithelial layer and mucosal glandular layer. However, the muscle layer was not intact and inflammatory exudates along with hyperemic submucosa were observed in their stomach tissues. The effect of CT4-treatment was also similar. But the presence of crypts as well as vasocongestion in muscle and serosal layers was noticed in the stomachs of the CT4-treated mice. KT4 treatment led to complete regeneration of the intact epithelial and muscle layers. The mucin containing cells were also prominent in the mucosal glandular layer, although hyperemic submucosa was still present. Omez treatment led to normal submucosa and an intact muscle layer. But the epithelial layer was not intact in this group of mice. Amongst the test samples, KT4 and Omez reduced the inflammation significantly and restored the mucosal architecture to near normalcy (Fig. 2f and Fig. 2g). The effects of BT and CT4 were also impressive, not significantly different from each other, but less than that of KT4.
For better appreciation of the above results, the histopathological slides were also quantified in terms of damage scores (DS) and inflammatory score (IS). Compared to the ulcerated mice, those treated with BT, and CT4 for one day showed a reduction of DS by 50.0% and 56.3% respectively, while KT4 and Omez reduced the DS by 75.2% and 67.5%. KT4 was more potent than Omez. Likewise, BT, CT4, KT4, and Omez also reduced the IS by 29.5%, 35.5%, 49.0%, and 45.4% respectively, compared to the corresponding untreated group.
Compared to the group II mice, natural healing during seven days decreased the DS and IS values of the group III mice marginally (15.3% and 11.4% respectively). Treatment with BT and CT4 for seven days reduced the DS by 70.3% and 71.5%, compared to the group III mice. KT4 and Omez showed comparable efficacy reducing the DS by 85.3% and 86.8% respectively. The reductions in IS by BT and CT4 (58–59%), KT4 (∼76%) and Omez (∼68%) also showed a similar trend. The results are summarized in Fig. 1a and Fig. 1b respectively.
Effect of BT, CT4 and KT4 on oxidative stress
Indomethacin administration markedly stimulated the TBARS formation in the gastric tissues by 34.5% on the 1st day, compared to the normal value. BT and CT4 reduced it by 13.5% and 18.6% respectively, compared to that of the group II mice. KT4 and Omez reduced the TBARS level almost equally (∼31%). Even after seven days of ulceration, the TBARS content in the untreated group remained significantly higher (49.5%) than the normal value. Treatment with BT, CT4, KT4 and Omez for seven days reduced it by 27.2%, 23.2%, 33.1%, and 37.1% respectively, compared to the group III mice. The effect of KT4 and Omez was similar, restoring the TBARS level to the normal value. Likewise, the protein carbonyl contents of the ulcerated mice were significantly elevated by 79% and 67.7% respectively on the 1st and 7th days of ulceration, compared to the normal values. One-day treatment with BT reduced it by 16.7%, while CT4, KT4 and Omez reduced it by ∼26–28%. Treatment with BT, CT4 and KT4 for seven days reduced it by 22.5%, 23.1% and 28.6%, compared to the corresponding untreated mice (group III). Omez showed a significantly better effect than the tea samples, reducing the parameter by 47.0%, compared to that of the untreated group. The results are presented in Tables 3a and 3b.| Parameters | Group I normal control | Group II ulcerated control | Group IV BT-treated | Group V CT4-treated | Group VI KT4-treated | Group VI I Omez-treated |
|---|---|---|---|---|---|---|
| a Stomach ulceration in mice was induced by oral administration of indomethacin (18 mg kg−1). Tea samples (15 mg kg−1) and Omez (3 mg kg−1) were used as the drugs. The assays were carried out 10 h after indomethacin administration and the values are mean ± SEM (n = 15). *p < 0.05, **p < 0.01, compared to normal mice; †p < 0.05, compared to untreated control (group II). | ||||||
| TBARS (nmoles mg−1 protein) | 1.16 ± 0.15 | 1.56 ± 0.15* | 1.35 ± 0.10 | 1.27 ± 0.14 | 1.08 ± 0.073† | 1.08 ± 0.04† |
| Protein carbonyls (nmoles mg−1 protein) | 1.24 ± 0.14 | 2.22 ± 0.30** | 1.85 ± 0.11 | 1.64 ± 0.20† | 1.65 ± 0.10† | 1.59 ± 0.07† |
| Mucin (μg g−1 tissue) | 362.00 ± 17.84 | 230.70 ± 15.77* | 268.67 ± 30.43 | 279.00 ± 16.37† | 264.33 ± 7.62 | 276.71 ± 8.82† |
| Parameters | Group I normal control | Group III ulcerated control | Group VIII BT-treated | Group IX CT4-treated | Group X KT4-treated | Group XI Omez-treated |
|---|---|---|---|---|---|---|
| a Stomach ulceration in mice was induced by oral administration of indomethacin (20 mg kg−1). Tea samples (15 mg kg−1) and Omez (3 mg kg−1) were used as the drugs. The assays were carried out on day seven after indomethacin administration and the values are mean ± SEM (n = 15). *p < 0.05, **p < 0.01, compared to normal mice; †p < 0.05; ††p < 0.01, compared to untreated control (group III). | ||||||
| TBARS (nmoles mg−1 protein) | 1.01 ± 0.10 | 1.51 ± 0.12* | 1.10 ± 0.12† | 1.16 ± 0.18† | 1.01 ± 0.12† | 0.95 ± 0.05† |
| Protein carbonyls (nmoles mg−1 protein) | 1.27 ± 0.09 | 2.13 ± 0.17** | 1.65 ± 0.13† | 1.62 ± 0.10† | 1.52 ± 0.10† | 1.13 ± 0.04††,# |
| Mucin (μg g−1 tissue) | 334.00 ± 11.40 | 248.00 ± 10.21* | 280.00 ± 15.30 | 314.33 ± 14.77† | 311.67 ± 6.23† | 301.71 ± 4.41† |
The non-protein thiol (NP-TSH) level in the gastric tissues of the group II mice was similar to that of the normal control. However, the NP-TSH level decreased by 14.2% on the 7th day of ulceration, compared to the normal value. Treatment with BT and KT4 restored it to normalcy.
Effect of BT, CT4, and KT4 on gastric mucin
Our results (Tables 3a and 3b) showed that indomethacin administration decreased the gastric mucin in mice significantly (36.3%) on the 1st day, compared to that in normal mice. Treatment with BT, CT4, KT4 and Omez for one day augmented the mucin content by 16.5%, 20.9%, 14.6%, and 19.9% respectively, compared to the group II mice. Even after seven days, the mucin content in the untreated mice was only 74.3% of the normal value. Except BT, the other test samples improved the mucin content significantly. The order of efficacy of the test samples was CT4 ∼ KT4 > Omez > BT.Anti-secretory effect of BT, CT4, KT4 and Omez
Pylorus ligation for 6 h resulted in the accumulation of gastric secretion and an increase in the total acid output of the gastric juice in the untreated mice. The tea extracts and Omez reduced the volume of gastric juice and total acid output (Table 4). CT4 and BT reduced the acid output by ∼24% while KT4 and Omez reduced the acid concentration by 28.6% and 30.8% respectively, compared to the untreated mice. Likewise, compared to the untreated mice, KT4 and Omez reduced the volume of secreted gastric juice by 25.3% and 19.0% respectively, while the effect of CT4 and BT was much less (10–14%).| Treatments | Gastric juice (ml/100 g body wt.) | Total gastric acid (μEq/100g body wt.) |
|---|---|---|
| a Immediately after pylorus ligature, the test samples (15 mg kg−1) and Omez (3 mg kg−1) were injected intraduodenally. The mice were killed 6 h after pylorus ligation, the volume of the gastric juice was measured and the total acid content was determined by titrating with 0.1 N NaOH. The values are mean ± SEM (n = 15). *p < 0.05 compared to the untreated mice. | ||
| Vehicle | 0.95 ± 0.09 | 4.55 ± 0.04 |
| BT | 0.85 ± 0.08 | 3.45 ± 0.05* |
| CT4 | 0.82 ± 0.05 | 3.46 ± 0.05* |
| KT4 | 0.71 ± 0.08* | 3.25 ± 0.02* |
| Omez | 0.77 ± 0.09* | 3.15 ± 0.04* |
Discussion
Oxygen free radicals are known to play a role in the induction and pathogenesis of gastrointestinal injury, mediated by various agents including indomethacin.16,17 Extensive research has proved that antioxidants might be effective not only in protecting gastric mucosal injury, but also inhibiting progression of gastric ulcer. Release of preformed mucus plays an important role in promoting epithelial recovery after acute injury.18 Besides providing significant buffering capacity for the neutralization of luminal acid, the mucoid cap can offer protection against the endogenous aggressors like acid, pepsin, and oxidants produced in the gastric lumen, as well as exogenous damaging agents, such as NSAIDs. The NSAID-produced mucosal hemorrhagic ulcer may be due to a decrease of gastric mucus production.18 Thus, drugs that arrest ulcer progression by antioxidant action, and also increase the synthesis and secretion of gastric mucus would accelerate gastric ulcer healing.The powerful antioxidant property of BT encouraged us to investigate its possible protective effect against indomethacin-induced gastric lesions in mice. In addition, we also included KT for the present investigation because of its proclaimed health benefit against various diseases. In recent years there has been a mounting interest in exploring the possibility of using BT as a supplement among patients. The Food and Agricultural Organisation (FAO) of the United Nations has stressed the need for research on the health benefits of BT in its totality, and not on certain isolated fractions/constituents. Hence, we also used BT as a whole for the studies.
The chemical constituents of KT depend on the exact microbiological composition, used for the fermentation as well as the fermentation time. However, these factors are often ignored, leading to substantial confusion regarding its physiological effect. Hence, we followed a scientific approach for the preparation of KT. For this, we identified the microorganisms in the kombucha culture and used it to prepare KT.19 The KT preparation was characterized in terms of several physical and chemical parameters, and evaluated for its ulcer-healing property. Consistent with the findings of a recent report, all the KT samples were found to be acidic.14 During the fermentation process, the yeast invertase hydrolyses sucrose into glucose and fructose, and produces ethanol via glycolysis with preference for fructose as the substrate. Subsequently, the acetic acid bacteria convert glucose and ethanol to gluconic and acetic acids respectively. Other organic acids including lactic acid are also produced by the acetic acid bacteria.20 These acids possess various health promoting attributes, and also make KT acidic. Because KT4 showed the best antioxidant capacity amongst the KT samples, it was chosen for studying the ulcer-healing property and its efficacy was compared with those of CT4 and BT.
Our macroscopic and histopathological results revealed that indomethacin administration induced marked, but acute damage to the gastric mucosa of mice. All the tea samples reduced the ulcerative damage and inflammation, the efficacy of KT4 being similar to that of Omez. The effects of BT and CT4 were also impressive, but not significantly different from each other, and less than that of KT4. The accelerated healing by the test samples was also evident within 4 h of their administration. However, the effect was more pronounced on continuing the treatment for seven days.
Tissue damage is always associated with excess generation of free radicals, leading to excessive lipid peroxidation (LPO) and loss or impairment of protein synthesis.21 These might aggravate tissue damage during stomach ulceration. Hence we assessed LPO (in terms of thiobarbituric acid reactive species [TBARS]) and protein oxidation (in terms of protein carbonyl formation) in the normal, ulcerated and treated groups of mice on day one and seven of the studies. Our results (Tables 3a and 3b) revealed that ulceration in mice was accompanied by a severe oxidative stress, resulting in the oxidation of lipids and proteins of the gastric tissues. These results are consistent with the earlier reports on the indomethacin-induced gastropathy.22,23 Due to their excellent radical scavenging capacity, the tea samples, especially KT4 provided a marked suppression of the oxidative damages and brought most of these parameters to near normalcy. This might decrease the ulcer progression and promote healing of gastric lesions induced by acute intake of indomethacin.
Depletion of the gastric mucin level also contributes to the NSAID-mediated gastropathy. Maintenance of mucus production may provide partial, but significant protection against reactive oxygen metabolites. In this study, the decreased mucin secretion in the indomethacin-administered mice indicated reduced ability of the mucosal membrane to protect the mucosa from physical damage and back diffusion of hydrogen ions. Treatment with BT, CT4, KT4, and Omez arrested the gastric mucin depletion significantly. Restoration of the gastric mucin to near normalcy would protect the ulcer crater against irritant stomach secretions (HCl and pepsine) and accelerate ulcer healing.
Mucin depletion may result because of oxidative rupture of the disulfide bridges that join the mucus subunits and maintain the structural integrity of the mucus.24 The sulfhydryl compounds (NP-TSH) prevent the mucosal rupture by antioxidant action. The decrease in endogenous thiol (glutathione) in ethanol induced gastric injury and its role in mucosal protection has been demonstrated earlier.23 Hence, we assayed the gastric NP-TSH levels of ulcerated as well as BT and KT4-treated mice. Our results of reduction of gastric NP-TSH in the 7th day-ulcerated mice and its restoration to normal level by BT and KT4 clearly demonstrated that the antioxidative property of BT and KT4 contributes to protection of the gastric mucosa. The ulceration-induced lipid peroxidation might increase glutathione consumption, reducing the NP-TSH level. The regeneration of the sulfhydryl compounds by the tea samples would help in recycling endogenous antioxidant vitamins, and prevent lipid peroxidation.
Suppressors of acid secretion such as proton pump inhibitors (like Omez) and histamine second receptor antagonists have been the mainstay for promotion of ulcer healing.2 In view of this, we also assessed the healing potential of the tea samples using the pylorus ligation ulcer model. In this study, the test samples, KT4, and Omez could significantly reduce the volume of gastric juice and total acid output. However, KT4 was administered to the duodenum, and the pH of the test solutions was ∼4.5. Therefore, its effects were secondary resulting from increased secretin generation, neural pathways and/or other mechanisms. Following pyloric ligation, migration of neutrophils into the mucosa has been observed in experimental animals.25 This suggests that besides gastric hypersecretion, oxidative stress is involved in this model of gastric ulceration also. It is therefore possible that the beneficial effect of the tea samples with the pyloric ligated mice may be partly due to their antioxidative property. The suppression of gastric acid by the tea samples also followed the trend of their antioxidant activity.
The tea decoction is a complex mixture of products comprising of a group of biopolymers, theaflavins and the water-soluble thearubigins with undefined chemical structures.26 Hence, we did not attempt to analyze the tea decoctions completely. Our HPTLC analysis revealed a gradual reduction in the caffeine level with simultaneous increase in theobromine concentration during the fermentation of BT with the kombucha culture. This might be attributed to N-demethylation of caffeine in the process. Similar oxidative chemical transformation of caffeine was reported earlier.27
Overall, TF was found to account for most of the healing activity of BT in this study. TF also might be the major contributor in the healing action of KT4 because its concentrations in KT4 and BT were practically the same. Presently the exact reason of the better efficacy of KT4 over BT remains elusive and multiple factors including gastric acid suppression may be responsible for this. Due to its higher phenolics content, KT4 is a better free radical scavenger that might account for its superior ulcer healing property compared to BT. Beyond a certain concentration, the three major components of the tea samples (theophylline, caffeine, and theobromine) are known to have adverse effects on gastrointestinal tracks.28 Hence these are unlikely to contribute to the healing activity of the tea samples. In contrast, the significantly reduced caffeine concentration in KT4 may be beneficial for ulcer healing, because caffeine is suggested to aggravate an existing ulcer by stimulating acid secretion. Many organic acid proton donors are known to decrease intestinal secretion.29 Some of these such as lactic acid helps digestive action and reduces acid secretion, while butyric acid strengthens the gut walls.30 Thus, some of the organic acids, produced by the fermentation of sugar by the kombucha culture may also contribute to the ulcer healing. In addition, the low pH of KT4 may be beneficial in controlling bacterial infection that will also reduce inflammation.
Conclusions
Overall, our results clearly revealed the healing ability of BT against indomethacin-induced stomach ulceration. The activity is augmented by its fermentation by the kombucha culture, but not significantly by C. parapsilosis alone. The healing action of the BT, CT4 and KT4 was due to their antioxidant action that helped in preventing rupture of the protecting gastric mucin. The increased phenolics content and some of the constituent small organic acids in KT4 might account for its superior ulcer healing effect than that of BT. BT is conventionally regarded as harmful for the stomach, while the effect of KT on the stomach has been questioned earlier.31 From this perspective, the present finding is very interesting and dispels doubt about the gastronomical harmful effect of BT and KT. Apparently, the gastrotoxicity (if any) of KT might be due to the unhygienic mode of its preparation, leading to contamination by Apergillus, Bacillus, or even by leaching of lead from the ceramic container used for its preparation. These results, taken together established the potential of KT4 as a potential anti-ulcerogenic formulation.Experimental section
Chemicals and reagents
Leaves of C. sinenesis (Brooke Bond, Red label) were procured from the local market. Alcian blue, indomethacin, bovine serum albumin (BSA), haematoxylene, alum, eosin, butylated hydroxytoluene (BHT), guanidine hydrochloride, trifluoroacetic acid (TFA), and sucrose were procured from Sigma, St. Louis, MO. Other reagents used were 2-thiobarbituric acid (TBA), ethanol, butanol and ethyl acetate (all from E. Merck, Mumbai, India), trichloroacetic acid (TCA, Thomas Baker, Mumbai, India), hydrogen peroxide (35%, Lancaster, Morecambe, U.K.), 2,4-dinitrophenyl hydrazine (DNPH), disodium hydrogen phosphate and sodium dihydrogen phosphate (BDH, Mumbai, India).Starter cultures
The kombucha culture was procured from a home brewer and maintained in the laboratory. It was grown at 28 ± 2 °C in a sterilized medium containing tea, sucrose and distilled water. The baby culture was used for further propagation, or for fresh batches of fermentation. The isolated bacteria from the culture were identified as Acetobacter aceti and A. pasteurianus, from their biochemical properties, and by comparison with different Acetobacter strains. The yeasts were identified as Saccharomyces cerevisiae, Zygosaccharomyces bailii and Brettanomyces bruxellensis according to conventional phenotype characterization and the Yeasts Identification Programme.19 This was also confirmed by scanning electron micrographs (data not shown). The Candida parapsilosis culture was maintained on Sabouraud's dextrose agar. The culture was identified by colonial morphology, formation of pseudohyphae and periodical biochemical tests.32Preparation of fermented teas
Tea leaves (0.5% w/v) were added to distilled water boiled for 15 min, and allowed to steep for 20 min. The decoction was passed through a nylon sieve and autoclaved. Concentrated sucrose solution was added to it, to achieve a final sucrose concentration of 10%. The tea sample was either left unfermented (designated as BT) or allowed to ferment in the presence of C. parapsilosis culture or kombucha culture for 2, 4 and 7 days. At the end of the designated periods, the tea samples were collected, and their volumes and pHs were measured. Each of the extracts were lyophilized, sterilized by millipore filtration and stored at −20 °C. The fermented black tea decoctions were designated as CT2, CT4, CT7 (for C. parapsilosis) or KT2, KT4, KT7 (for kombucha culture) respectively, depending on the culture and time used for the fermentation.Determination of phenolic contents
Following a known method,33 the amounts of total phenolics in the tea extracts were determined. Gallic acid monohydrate was used as the standard, and the total polyphenolic content is expressed as mg gallic acid equivalent (GAE) g−1 of the extract.Analysis of theaflavin (TF) and thearubigins (TR)
The theaflavin and thearubigins contents in BT and KT4 were estimated by a known method, with a brief modification.14 The tea extracts (25 ml) was extracted with isobutyl methyl ketone (IBMK, 25 ml), the IBMK extract (1 ml) was mixed with aqueous 45% ethanol (9 ml) and absorbance (A) measured at 380 nm. Ten ml of IBMK phase was extracted with 10 ml of 2.5% disodium hydrogen phosphate. After extraction and phase separation, 1 ml of IBMK phase was mixed with 9 ml of 45% ethanol and absorbance was measured at 380 nm (B). 10 ml of aqueous phase from the first step was extracted with 10 ml of n-butanol. After phase separation, 1 ml of n-butanol layer was mixed with 9 ml of 45% ethanol and absorbance was measured at 380 nm (C). Concentration of theaflavin and thearubigins was calculated from the absorbance values as given below.| TF (%) = 4.313 × B |
| TR (%) = 13.643 × (A + C − B) |
Characterization of the chemical constituent of KT4
The KT4 extract (50 ml) was successively extracted with chloroform, ethyl acetate and butanol (each 50 ml × 3 times). The individual extracts were concentrated in vacuo to obtain 0.514 g, 0.302 g and 0.221 g of the residues respectively. Normal phase preparative TLC (silica gel G, ethyl acetate: methanol: water = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as the solvent), followed by a preparative TLC (RP-18 silica gel plate, same solvent) of the chloroform extract afforded caffeine, which was characterized from its physical and spectral characteristics. The caffeine concentrations in tea samples were also quantified by HPTLC densitograms.
1 as the solvent), followed by a preparative TLC (RP-18 silica gel plate, same solvent) of the chloroform extract afforded caffeine, which was characterized from its physical and spectral characteristics. The caffeine concentrations in tea samples were also quantified by HPTLC densitograms.
Caffeine: mp: 237 °C; UV (MeOH) λmax: 275 nm (log ε 3.99); IR (KBr): 1700 cm−1; 1H NMR (200 MHz, CDCl3): δ 3.15 (s, 3H, 3-CH3), 3.33 (s, 3H, 7-CH3), 3.86 (s, 3H, 1-CH3), 7.1 (s, 1H).
Preliminary TLC (silica gel G, ethyl acetate: methanol: water = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) investigation of the ethyl acetate and 1-butanol extracts showed a common fluorescent spot at Rf 0.9. The compound was isolated by reverse phase preparative TLC under the above mentioned conditions from the ethyl acetate extract where its concentration was substantial. It was characterized as theobromine.
1) investigation of the ethyl acetate and 1-butanol extracts showed a common fluorescent spot at Rf 0.9. The compound was isolated by reverse phase preparative TLC under the above mentioned conditions from the ethyl acetate extract where its concentration was substantial. It was characterized as theobromine.
Theobromine: mp: 355 °C; UV (MeOH) λmax: 272 nm (log ε 4.01); IR (KBr): 1694 cm−1; 1H NMR (200 MHz, CDCl3): δ 1.55 (s, 6H, 2 × CH3), 3.33 (s, 1H, NH), 3.97 (s, 1H).
Following a similar protocol, theophylline was also isolated from the 1-butanol extract, and characterized as above.
Theophylline: mp: 270 °C; UV (MeOH) λmax: 272 nm (log ε 4.00); IR (KBr): 1680 cm−1; 1H NMR (200 MHz, CDCl3): δ 3.49 (s, 3H, 3-CH3), 3.66 (s, 3H, 1-CH3), 7.25 (s, 1H, 7-H), 12.20 (s, 1H).
Preparation of the test samples
The test samples were prepared from BT, CT4, KT4 and Omez as aqueous suspensions in 2% gum acacia as the vehicle, and administered to the mice orally. Any possibility of microbial contamination in the samples was excluded prior to their administration to the mice.Protocol for ulceration and healing studies
Male Swiss albino mice, bred at the Dr B. C. Roy Post Graduate Institute of Basic Medical Sciences, Kolkata, India were procured after obtaining clearance from the Animal Ethics Committee of the centre. All the experiments were conducted with strict adherence to the ethical guidelines laid down by European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes. In addition, the ethical guidelines, laid by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), constituted by the Animal Welfare Division, Government of India on the use of animals in scientific research was followed. The mice (6–8 weeks old, 25–30 g) were reared on a balanced laboratory diet as per National Institute of Nutrition, Hyderabad, India and given tap water ad libitum. They were kept at 20 ± 2 °C, 65–70% humidity, and 12 h day/12 h night cycles. The experiments were performed by two investigators blinded to the group and treatment of animals, which were identified by typical notches in the ear and limbs [performed at a pre-weaning stage to minimize the pain to the animals], and then randomized. Ulceration in the mice was induced by administering indomethacin (18 mg kg−1, p. o., single dose) dissolved in distilled water and suspended in the vehicle, gum acacia (2%). The animals were deprived of food, but had free access to tap water, 24 h before ulcer induction.For the standardization of doses, the respective test samples (10, 15 and 20 mg kg−1, p. o.) were given to the mice once daily for 7 days, starting the first dose 6 h after the indomethacin administration. In the subsequent six days, the test samples were given at 9 AM on each day. Five mice were taken in each group and each experiment was repeated three times. The mice were sacrificed on the 1st and 7th day, 4 h after administering the last dose of the test samples. The extent of healing was assessed from the macroscopic damage scores (MDS) of the untreated and treated ulcerated mice.
Assessment of ulcer healing from MDS
The mice were sacrificed after an overdose with thiopental. The stomach from the normal and treated groups were removed rapidly, opened along the greater curvature, and thoroughly rinsed with normal saline. The ulcerated gastric mucosal areas were visualized using a transparent sheet and a dissecting microscope. The MDS was assessed34 by grading the gastric injury on a 0–4 scale, based on the severity of hyperemia and hemorrhagic erosions: 0 – almost normal mucosa, 0.5 – hyperemia, 1 – one or two lesions, 2 – severe lesions, 3 – very severe lesions, 4 – mucosa full of lesions (lesions – hemorrhagic erosions, hyperemia – vascular congestions).Studies on the histopathological and biochemical parameters
Based on our MDS results, we assessed the histopathological, and biochemical parameters under the optimized doses of the individual test samples [BT, CT4, KT4 (each 15 mg kg−1, p. o.) and Omez (3 mg kg−1, p. o.)]. The mice were equally divided in eleven groups as follows:Group I – normal mice; Group II – ulcerated mice and sacrificed after 10 h; Group III – ulcerated mice, and sacrificed after 7 days; Group IV–VII – ulcerated mice, treated with BT, CT4, KT4 and Omez respectively, and sacrificed 4 h after administration of test samples on the 1st day; Group VIII–XI – ulcerated mice, treated with BT, CT4, KT4 and Omez respectively, and sacrificed 4 h after administration of test samples on the 7th day. Group I–III control groups of mice were given the vehicle (0.2 ml) during the period of study.
Histological studies
The ulcerated portions of the stomach were sectioned after fixing in 10% formol saline solution. After 24 h of fixation followed by embedding in a paraffin block, it was cut into sections of 5 micron onto a glass slide, stained with haematoxylene-eosin and the histology examined under a light microscope. One centimetre length of each histological section was divided into three fields. The damage score (DS) was assessed by scoring each field on a 0–4 scale as described previously:34 0 – normal mucosa, 1 – epithelial cell damage, 2 – glandular disruption, vasocongestion or edema in the upper mucosa, 3 – mucosal disruption, vasocongestion or edema in the mid-lower mucosa, and 4 – extensive mucosal disruption involving the full thickness of the mucosa. The overall mean value of the damage scores (DS) for each of the fields was taken as the histological ulcer index for that section.Likewise, the inflammatory scores (IS)35 were assigned after reviewing all slides to assess the range of inflammation as follows: 0 – normal mucosa, 1 – minimal inflammatory cells, 2 – moderate number of inflammatory cells, and 3 – large number of inflammatory cells.
Histological sections were coded to eliminate an observer bias. Data for the histological analyses are presented as the mean ± SEM from the review of a minimum of three sections per animal and five animals per group.
Quantification of lipid and protein damages
The glandular stomach tissues were pooled from five animals and their wet weights were noted. The tissues were pooled from five animals, rinsed with appropriate buffer, homogenized with a glass-teflon homogenizing tube in a 50 mM phosphate buffer, pH 7.4. The supernatant, obtained after centrifugation at 1200 × g was used for the biochemical studies.The lipid peroxidation products were estimated36 with minor modifications. Briefly, 1 ml of each of the tissue homogenates and ice-cold 20% TCA solution containing 0.01% BHT was incubated for 15 min. The samples were centrifuged at 1200 × g for 15 min, and the supernatant centrifuged again at 1200 × g for 30 min to obtain the mitochondrial pellets. These were washed with a buffer (150 mM KCl and 20 mM phosphate buffer) and finally suspended in a phosphate buffer (50 mM, pH 7.4). The mitochondrial membrane fraction (1 ml) was treated with TCA/TBA/HCl (2 ml, 15% TCA, 0.375% TBA, 0.25N HCl) containing 0.01% BHT, heated on a boiling water bath for 15 min, cooled and centrifuged at 3000 × g for 5 min, the red chromophore in the supernatant was extracted with 1-butanol (2 ml). The amount of TBARS was calculated from its absorbance at 535 nm (ε = 1.56 × 105 M−1cm−1).
The protein carbonyl contents were measured following a reported method37 with minor modifications. Briefly, the tissue homogenate was incubated for 60 min with 10 mM DNPH in 2M HCl in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 with intermittent shaking. After incubating the mixture with ice-cold 20% TCA solution for 15 min, followed by centrifugation at 1200 × g for 10 min, the pellet obtained was washed three times with ethanol:ethyl-acetate (1
4 with intermittent shaking. After incubating the mixture with ice-cold 20% TCA solution for 15 min, followed by centrifugation at 1200 × g for 10 min, the pellet obtained was washed three times with ethanol:ethyl-acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 ml). The washed pellet was redissolved in 1 ml of guanidine reagent (6M guanidine in 20 mM potassium phosphate buffer, pH 2.3), centrifuged and the carbonyl content of the supernatant was assayed from the absorbance at 362 nm (ε = 22,000 M−1 cm−1).
1, 1 ml). The washed pellet was redissolved in 1 ml of guanidine reagent (6M guanidine in 20 mM potassium phosphate buffer, pH 2.3), centrifuged and the carbonyl content of the supernatant was assayed from the absorbance at 362 nm (ε = 22,000 M−1 cm−1).
Non-protein thiol (NP-TSH) assay
Following a reported method,38 the gastric mucosal NP-TSH was measured. Briefly, the glandular stomach homogenates were prepared in 0.2 M Tris-HCl buffer, pH 8.2 containing 20 mM EDTA and centrifuged at 1200 × g for 15 min. An aliquot of the homogenate (1 ml) was treated with ice-cold 20% TCA (1 ml), centrifuged at 3000 × g for 5 min, and the supernatant (1 ml) was added to Tris-HCl buffer (2 ml, 0.8 M, pH 9) containing 20 mM EDTA, and mixed with DTNB (0.1 ml, 10 mM). The NP-TSH content was calculated from the absorbance of the chromogen at 412 nm (ε = 13.6 × 104 M−1 cm−1).Mucin assay
Following a reported method,39 the free mucin content in the gastric tissues was estimated by measuring the amount of alcian blue bound to mucus. Briefly, the glandular stomach tissues were incubated with a 1% buffered sucrose solution of alcian blue in (0.1%) sodium acetate at 37 °C for 60 min. After incubation, the tissues were washed with sucrose and centrifuged. The supernatant was extracted with MgCl2, and the amount of alcian blue was estimated spectrophotometrically at 610 nm. The quantity (μg) of alcian blue g−1 wet glandular tissue was calculated.Determination of gastric juice secretion
Mice were randomly divided into predesignated groups of five animals each, which were fasted for 24 h with free access to water. Briefly, a midline incision was made under ether anaethesia following which a portion of the abdomen was opened below the xiphoid process. The pylorus portion of the stomach was lifted and ligated. During this process, care was taken to avoid the traction to the pylorus or damage to its blood supply. The stomach was closed by interrupted sutures.40Immediately after pylorus ligature, the tea samples (each 15 mg kg−1) or the positive control, Omez (3 mg kg−1) were injected intraduodenally. The animals were killed 6 h after pylorus ligation by cervical dislocation under ether anaesthesia. For collection of the gastric juice, the abdomen was opened and another ligature placed around the oesophagus close to the diaphragm. The stomach was removed, inspected internally, and its content drained into a graduated centrifuge tube to determine the total amount of gastric-juice acid (ml/100 g). Following washing the mucosal side of the stomach with distilled water (2 ml), centrifugation at 3000 × g for 15 min was carried out. The total acid content (μEq) in the supernatant volume was determined by titration with 0.1 N NaOH.
Statistical analysis
The data are presented as mean ± SEM. Parametric data which includes all the biochemical parameters were analyzed using a paired ‘t’ test for the paired data or one way analysis of variance (ANOVA) followed by a Dunnet multiple comparisons post test. Nonparametric data (histology scoring) were analyzed using Kruskal-Wallis test (nonparametric ANOVA) followed by a Dunn's multiple comparisons post test. A probability value of p < 0.05 was considered significant.References
- F. Halter, A. Schamassman, B. M. Peskar and A. S. Tarnawski, Cyclooxygenase-2 implications on maintenance of gastric mucosal integrity and ulcer healing: controversies and perspectives, Gut, 2001, 49, 443–453 CrossRef CAS.
- J. L. Wallace, Recent advances in gastric ulcer therapeutics, Curr. Opin. Pharmacol., 2005, 5, 573–577 CrossRef CAS.
- E. Yesilada, and I. A. Gurbuz, Compilation of the studies on the antiulcerogenic effects of medicinal plants. ( 2003) In: S. Singh, V. K. Singh, J. N. Govil (ed.), Recent Progress in Medicinal Plants, vol. II: Phytochemistry and Pharmacology. SCI Tech Publishing LLC, Houston, USA, pp. 111–174 Search PubMed.
- K. Hamaishi, R. Kojima and M. Ito, Anti-ulcer effect of tea catechin in rats, Biol. Pharm. Bull., 2006, 29, 2206–2213 CrossRef CAS.
- S. Y. Lee, Y. W. Shin and K. B. Hahm, Phytoceuticals: mighty but ignored weapons against Helicobacter pylori infection, J. Dig. Dis., 2008, 9, 129–139 Search PubMed.
- M. Yoshikawa, T. Morikawa, N. Li, A. Nagatomo, X. Li and H. Matsuda, Bioactive saponins and glycosides. XXIII. Triterpene saponins with gastroprotective effect from the seeds of Camellia sinensis – theasaponins E3, E4, E5, E6, and E7, Chem. Pharm. Bull. (Tokyo), 2005, 53, 1559–1564 CrossRef CAS.
- T. Morikawa, N. Li, A. Nagatomo, H. Matsuda, X. Li and M. Yoshikawa, Triterpene saponins with gastroprotective effects from tea seed (the seeds of Camellia sinensis), J. Nat. Prod., 2006, 69, 185–190 CrossRef CAS.
- S. Maity, J. R. Vedasiromoni, L. Chaudhuri and D. K. Ganguly, Role of reduced glutathione and nitric oxide in the black tea extract-mediated protection against ulcerogen-induced changes in motility and gastric emptying in rats, Jpn. J. Pharmacol., 2001, 85, 358–364 CrossRef CAS.
- S. Maity, J. R. Vedasiromoni and D. K. Ganguly, Anti-ulcer effect of the hot water extract of black tea (Camellia sinensis), J. Ethnopharmacol., 1995, 46, 167–174 CrossRef CAS.
- H. Tietze, Kombucha the Miracle Fungus, Harald Tietze Publ., India, 2000 Search PubMed.
- T. Pauline, P. Dipti, B. Anju, S. Kavimani, S. K. Sharma, A. K. Kain, S. K. Sarada, M. Sairam, G. Ilavazhagan, K. Devendra and W. Selvamurthy, Studies on toxicity, anti-stress and hepato-protective properties of Kombucha tea, Biomed Environ. Sci., 2001, 14, 207–213 Search PubMed.
- M. Sairam, B. Anju, T. Pauline, D. Prasad, A. K. Jain, S. S. Mongia, S. K. Sharma, B. Singh, R. Singh, G. Ilavazhagan, D. Kumar and W. Selvamurthy, Effect of kombucha tea on chromate(VI)-induced oxidative stress in albino rats, J. Ethnopharmacol., 2000, 71, 235–240 CrossRef CAS.
- U. S. Hiremath, M. P. Vaidehi and B. J. Mushtari, Effect of fermented tea on the sugar levels of NIDDM subjects, The Indian Practitioner, 2002, 55, 423–425 Search PubMed.
- R. Jayabalan, S. Marimuthu and K. Swaminathan, Changes in content of organic acids and tea polyphenols during kombucha tea fermentation, Food Chem., 2007, 102, 392–398 CrossRef CAS.
- D. Banerjee, B. Maity, A. K. Bauri, S. K. Bandyopadhyay and S. Chattopadhyay, Gastroprotective property of Myristica malabarica against indomethacin-induced stomach ulceration: A mechanistic exploration, J. Pharm. Pharmacol., 2007, 59, 1555–1565 CrossRef.
- K. Biswas, U. Bandyopadhyay, I. Chattopadhyay, A. Varadaraj, E. Ali and R. K. Banerjee, A novel antioxidant and antiapoptotic role of Omez to block gastric ulcer through scavenging of hydroxyl radical, J. Biol. Chem., 2003, 278, 10993–11001 CrossRef CAS.
- H. Utsumi, K. Yasukawa, T. Soeda, K.-i. Yamada, R. Shigemi, T. Yao and M. Tsuneyoshi, Noninvasive mapping of reactive oxygen species by in vivo electron spin resonance spectroscopy in indomethacin-induced gastric ulcers in rats, J. Pharmacol. Exp. Ther., 2006, 317, 228–235 CAS.
- K. D. Rainsford, The effects of aspirin and other nonsteroid antiinflammatory analgesic drugs on gastrointestinal mucus glycoprotein biosynthesis in vivo: relationship to ulcerogenic actions, Biochem. Pharmacol., 1978, 27, 877–885 CrossRef CAS.
- C. H. Liu, W. H. Hsu, F. L. Lee and C. C. Liao, The isolation and identification of microbes from a fermented tea beverage, haipao and their interactions during haipao fermentation, Food Microbiol., 1996, 13, 407–415 CrossRef.
- C. Dufresne and E. Farnworth, Tea, Kombucha, and health: a review, Food Res. Intnl., 2000, 33, 409–421 Search PubMed.
- S. Szabo and D. Hollander, Pathway of gastrointestinal protection and repair: mechanism of action of sucralfate, Am. J. Med., 1989, 86, 23–31 CrossRef CAS.
- J. R. Avila, C. A. de la Lastra, M. J. Martin, V. Motilva, I. Luque, D. Delgado, J. Esteban and J. Herrerias, Role of endogenous sulfhydryls and neutrophil infiltration in the pathogenesis of gastric mucosal injury induced by piroxicam in rats, Inflamm. Res., 1996, 45, 83–88 CrossRef CAS.
- V. Bertrand, F. Guessous, A. L. Le Roy, B. Viossat, H. Fessi, A. El Abbouyi, J. P. Giroud and M. Roch-Arveiller, Copper-indomethacinate associated with zwitterionic phospholipids prevents enteropathy in rats: effect on inducible NO synthase, Dig. Dis. Sci., 1999, 44, 991–999 CrossRef CAS.
- J. J. Bernier and C. Florent, Les défences de l'estomac, Recherche, 1986, 117, 614–621 Search PubMed.
- L. Rastogi, G. K. Patnaik and M. Dikshit, Free radicals and antioxidant status following pylorus ligation induced gastric mucosal injury in rats, Pharmacol. Res., 1998, 38, 125–132 CrossRef CAS.
- D. A. Balentine, S. A. Wiseman and L. C. M. Bouwens, The chemistry of tea flavonoids, Crit. Rev. Food Sci. Nutr., 1997, 37, 693–704 CrossRef CAS.
- R. H. Stadler, J. Richoz, R. J. Turesky, D. H. Welti and L. B. Fay, Oxidation of caffeine and related methylxanthines in ascorbate and polyphenol-driven Fenton-type oxidations, Free Radic. Res., 1996, 24, 225–10 CrossRef CAS.
- J. O. Ibu, Ac. Iyama, C. T. Ijije, D. Ishmael, M. Ibeshim and S. Nwokediuko, The effect of Cola acuminata and Cola nitida on gastric acid secretion, Scand. J. Gastroenterol., 1986, 21(Supp. 124), 39–45 CrossRef.
- G. S. Forsyth, R. A. Kapitany and D. L. Hamilton, Organic acid proton donors decrease intestinal secretion caused by enterotoxins, Am. J. Physiol, 1981 Sep, 241(3), G227–34 Search PubMed.
- Günther Frank; Kombucha: Healthy Beverage and Natural Remedy from the Far East, Ennsthaler, 1991 Search PubMed.
- R. Srinivasan, S. Smolinske and D. Greenbaum, Probable gastrointestinal toxicity of kombucha tea, J. Gen. Int. Med., 1997, 12, 643–645 Search PubMed.
- J. A. Barnett, R. W. Payne and D. Yarrow, Yeasts: Characteristics and Identification, third ed. Cambridge University Press, Cambridge, 1983, pp. 159–160 Search PubMed.
- V. L. Singleton and J. A. Rossi Jr., Colorimetry of total phenolics with phosophomolybdic-phosphotungstic acid reagents, Am. J. Enol. Viticul., 1965, 16, 144–158 Search PubMed.
- D. Dokmeci, M. Akpolat, N. Aydogu, L. Doganay and F. N. Turan, L-Carntine inhibits ethanol-induced gastric mucosal injury in rats, Pharmacol Rep., 2005, 57, 481–488 Search PubMed.
- W. L. Beck and R. Xavier, Mechanism of NSAID induced gastrointestinal injury defined using mutant mice, Gastroenterol., 2000, 119, 699–705 CrossRef.
- H. Esterbauer, J. Gebicki, H. Puhl and G. Jurgens, The role of lipid peroxidation and antioxidants in oxidative modification of LDL, Free Radic. Biol. Med., 1992, 13, 341–390 CrossRef CAS.
- P. J. Shah, M. S. Gandhi, M. B. Shah, S. S. Goswami and D. Santani, Study of Mimusops elengi bark in experimental gastric ulcers, J. Ethnopharmacol., 2003, 89, 305–311 CrossRef.
- J. Sedlak and R. H. Lindsay, Estimation of total protein-bound, and non-protein sulfhydryl groups in tissue with Ellman's reagent, Anal. Biochem., 1968, 25, 192–205 CrossRef CAS.
- M. Tariq and A. L. Moutaery, Menadione protects gastric mucosa against ethanol-induced ulcers, Exp. Toxicol. Pathol., 2005, 56, 393–399 CrossRef CAS.
- D. A. Brodie, The mechanism of gastric hyperacidity produced by pyloric ligation in the rat, Am. J. Dig. Dis. Sci., 1966, 111, 231–241 Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
