Effects of the fuel oil spilled by the Prestige tanker on reproduction parameters of wild mussel populations
Maren
Ortiz-Zarragoitia
,
Larraitz
Garmendia
,
María Carmen
Barbero
,
Teresa
Serrano
,
Ionan
Marigómez
and
Miren P.
Cajaraville
*
Biologia Zelularra eta Histologia Lab., Zoologia eta Biologia Zelularra Saila, Zientzia eta Teknologia Fakultatea, University of the Basque Country UPV/EHU, Sarriena z/g, 48940, Leioa, Basque Country (Spain). E-mail: mirenp.cajaraville@ehu.es; Fax: +34 946013500; Tel: +34 946012697
First published on 29th October 2010
Abstract
The aim of this work was to assess possible effects of the Prestige oil spill on reproduction parameters of mussels along the Galician and Bay of Biscay coast. Studied endpoints included sex ratio, gonad histology and vitellogenin-like proteins using the alkali-labile phosphate (ALP) method. A high prevalence of haemocytic infiltration of follicles and severe oocyte atresia was found in most localities in April 2003. Spawning gonads were observed in most impacted populations in the same sampling. In April 2004 mature small sized follicles were observed. No histopathological changes were observed in April 2005 and 2006, except a high prevalence of necrotic gametes in 6 out of 22 localities in April 2006. Female ALP levels showed high interindividual variability in April 2004, which was reduced in April 2005 and 2006. No xenoestrogenic effects were observed in male mussels. Overall, gamete alterations were detected during 2003–2004 and a recovery trend was observed afterwards.
Environmental impactSeveral works have used mussels as sentinel organism for chemical biomonitoring studies after oil spill events, but no studies have addressed the impact of oil spills on reproduction-parameters in wild mussel populations. Here we present for the first time a long-term (2003–2006) biomonitoring survey studying reproduction-related parameters, such as sex ratio, gonad histology and vitellogenin-like proteins, in 22 mussel populations from Galicia and the Bay of Biscay after the Prestige oil spill. Our results showed alterations in gamete development in mussels from the whole studied area in 2003 and 2004, but recovered later on (2005–2006). The applied reproduction-related biomarkers have been demonstrated to be a useful tool for pollution assessment and could be included in ongoing worldwide biomonitoring programmes studying mussels. |
Introduction
On November 19th, 2002, the oil tanker Prestige broke in half and sunk in international waters 133 miles from the north western coast of the Iberian Peninsula. The Prestige transported 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tones of heavy fuel oil no. 2 according to the French classification (UK classification type no. 6; Russian classification M-100). More than 60000 tones of fuel oil were spilled affecting mainly the coast of Galicia on the north west of the Iberian Peninsula. During the following months spilled oil also affected the whole northern coast of the Iberian Peninsula reaching up to Brittany.1,2 The Prestige oil spill (POS) has been considered the most important ecological disaster ever to occur in the affected area.1,3 Severe loss of intertidal invertebrate communities has been measured on POS affected beaches.4,5
000 tones of heavy fuel oil no. 2 according to the French classification (UK classification type no. 6; Russian classification M-100). More than 60000 tones of fuel oil were spilled affecting mainly the coast of Galicia on the north west of the Iberian Peninsula. During the following months spilled oil also affected the whole northern coast of the Iberian Peninsula reaching up to Brittany.1,2 The Prestige oil spill (POS) has been considered the most important ecological disaster ever to occur in the affected area.1,3 Severe loss of intertidal invertebrate communities has been measured on POS affected beaches.4,5
The area impacted by the POS is rich in bivalve production, Galicia being the most important European producer of mussels. Mussel production surpasses 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Tn per year and employs more than 8000 full-time workers in Galicia.6 Mussels have been used worldwide as sentinel species of environmental pollution in coastal areas.7,8 Mussels are excellent indicators of contamination levels in the marine environment as they are sessile and filter feed from surrounding water. Furthermore, they show a low ability to metabolize xenobiotic compounds like polycyclic aromatic hydrocarbons (PAHs) and accumulate them at high levels.9,10 Mussels are sensitive species to accidental oil spills as effects on different biomarkers have been observed after Exxon Valdez in Alaska,11Aegean Sea in Galicia,12Sea Empress in Wales,13Coral Bulker in North Portugal,14Erika in Brittany15 and the case of Prestige.16,17 Studied biological endpoints in mussels affected by an oil spill usually show a rapid recovery in 1 to 2 years after the oil spill.12,13,18 However, long lasting effects could not be discounted as shown in the case of wildlife populations affected by the Exxon Valdez oil spill.11 Bivalve populations from the Prince William Sound area were not completely recovered 17 years after the oil spill.19
000 Tn per year and employs more than 8000 full-time workers in Galicia.6 Mussels have been used worldwide as sentinel species of environmental pollution in coastal areas.7,8 Mussels are excellent indicators of contamination levels in the marine environment as they are sessile and filter feed from surrounding water. Furthermore, they show a low ability to metabolize xenobiotic compounds like polycyclic aromatic hydrocarbons (PAHs) and accumulate them at high levels.9,10 Mussels are sensitive species to accidental oil spills as effects on different biomarkers have been observed after Exxon Valdez in Alaska,11Aegean Sea in Galicia,12Sea Empress in Wales,13Coral Bulker in North Portugal,14Erika in Brittany15 and the case of Prestige.16,17 Studied biological endpoints in mussels affected by an oil spill usually show a rapid recovery in 1 to 2 years after the oil spill.12,13,18 However, long lasting effects could not be discounted as shown in the case of wildlife populations affected by the Exxon Valdez oil spill.11 Bivalve populations from the Prince William Sound area were not completely recovered 17 years after the oil spill.19
Studies dealing with reproduction effects after oil spill events are scarce and most of them focus only on population abundances. It is well known that complex oil mixtures can provoke deleterious effects on wildlife reproduction.20 Fish populations inhabiting oil and PAH polluted environments show endocrine disruption effects, such as reduced plasma hormonal levels and reduced reproduction success.21 After the Exxon Valdez oil spill, reduced estradiol levels in plasma from marine fish species such as dolly varden, yellowfin solea and pollock were measured. In dolly varden low levels of gonadotropin-I, a neurohormone responsible for steroid biosynthesis and gamete development, were also observed together with delayed gamete development.22Reduction in reproductive success was observed in pink salmon and Pacific herring from the Exxon Valdez oil spill area.23,24 Embryos obtained from both species showed low survival ratios and severe morphological deformities in the following 4 years after the oil spill.23,24
Most of the few works dealing with reproduction alterations after oil spills have been carried out in vertebrates, few of them focusing on invertebrates such as bivalve mollusks. Therefore, it is important to study the possible effects of the POS on reproduction parameters in local mussel populations. The endocrine system of bivalve mollusks is not well understood, but effects on reproduction parameters in mollusks have been described after exposure to environmental contaminants, both in the laboratory and in the field (see review by Porte et al.).25 Mussels have been shown to be sensitive to possible endocrine disruption effects provoked by PAHs.25–27 Female mussels exposed in the laboratory during gametogenesis to North Sea oil showed low levels of vitellogenin-like proteins in comparison to control females and delayed gamete development accompanied by high percentage of atretic oocytes, indicating possible anti-estrogenic effects.27 Similarly, high prevalences of atretic germ cells were observed in mussels exposed to diesel oil and PAH derivatives.28,29 Anti-estrogenic effects were also observed in field studies using soft shell clams. Female clams inhabiting a PAH contaminated harbor showed low vitellogenin-like protein levels and retarded gamete development.30,31 Although several works have demonstrated the anti-estrogenic activity of oil and PAHs,21,32 some authors have observed estrogenic effects provoked by PAHs.33,34
The water soluble fraction of the Prestigefuel oil reduced clam embryo survival35 but did not alter reproduction ability using Daphnia magna bioassays.36 Polychaetes exposed to fuel oil no. 2 during the whole life cycle showed accelerated oocyte maturation and suppression of fecundity.37 Furthermore, mussel seed collected from POS affected areas showed reduced growth in comparison to mussel seed from non-affected populations.38 Affected mussel seed populations showed higher levels of triglycerides and carbohydrates.39,40
In the present work we aimed to investigate possible reproduction effects after POS in local mussel populations, which could compromise their reproduction ability and survival. For this purpose we studied vitellogenin-like protein levels as a biomarker of endocrine disruption. In fish, vitellogenin is a bulky phospholipoglycoprotein synthesized by the liver of female fish under the regulation of estradiol. After being synthesized it is secreted to the blood and transported to the ovary, where it is endocytosed by growing oocytes. Vitellogenin is the precursor protein of vitellins present in the oocytes to feed the embryos.41 In fish, vitellogenin levels are used as a marker of xenoestrogenicity in adult males and juvenile organisms.41–44 In bivalve mollusks, vitellogenin synthesis occurs in the follicular cells of the female gonad.45,46 Regulation of vitellogenin synthesis is not well understood and hormonal control by estrogens is under discussion.47–49 Nevertheless, bivalve mollusks are known to synthesize steroid hormones50 and a relationship between estradiol levels and vitellogenesis has been suggested.48 Due to the lack of specific antibodies, measurement of vitellogenin or vitellogenin-like protein levels in bivalves has been based on indirect methods, such as the alkali-labile phosphate (ALP) method.25,51 Thus, we applied measurement of ALP levels in combination with gonad histology to study possible reproduction alterations in 22 mussel populations differently affected by the POS in Galicia and the Bay of Biscay, for a period of three years (2003–2006).
Materials and methods
Samplings and sample processing
For the purpose of the present work a biomonitoring study was performed along the North Atlantic coast of the Iberian Peninsula. Mussels (Mytilus galloprovincialis) were collected from 22 different locations (Fig. 1) selected on the basis of the severity of the impact of the oil spill and on the oil arrival conditions to the coast (fresh spilled oil versus weathered oil). Based on the first reports after the oil spill2 the impact was most severe in the coast of Galicia, where we studied 8 locations comprised of highly impacted sites (Aguiño, Caldebarcos, Camelle and Segaño) and less impacted sites (Estaca de Bares, Ons, Cíes and Oia), all affected by fresh spilled oil. We studied a further 13 localities in the Bay of Biscay affected later on by weathered oil. We also included one sampling point in the Natural Park of North Littoral of Esposende (Sao Bartolomeu do Mar, Portugal) as a possible non-impacted site. Samplings were performed in April, July and September 2003 (17 localities), February 2004 (19 localities), April, July and October 2004 and 2005, and April 2006 (22 localities). At each sampling time 30–60 adult mussels, 3.5–4.5 cm long, were collected from each locality and directly processed in the field. For histological studies 10–30 mussels from each site were carefully opened by sectioning the adductor muscle and directly fixed in 10% neutral buffered formalin. For the study of vitellogenin-like protein levels, mantle samples collected during April 2004, 2005 and 2006 were used since this is the period when mussels at this latitude show the most developed gonads.52–55 Mussel gonads (mantle tissue) from 20 animals were dissected out, chilled on ice and a small piece of tissue was removed to do a smear on a microscope slide. The sex of each animal was determined after visualization of the smear under the microscope. Afterwards the rest of the tissue was frozen individually in liquid nitrogen. Once in the laboratory they were stored at −80 °C until subsequent analysis.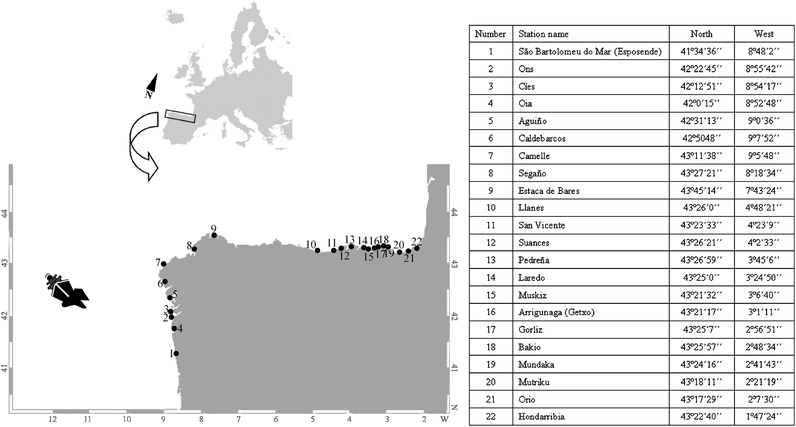 | ||
| Fig. 1 Map showing the study area, the location where the Prestige sunk and selected sampling points. In the adjacent table geographical coordinates for the location of each sampling point are shown. | ||
Sex ratio, gamete development and histopathology of the gonad
The gonad (mantle tissue) from each mussel was dissected out after fixation, placed in histological cassettes in 70° ethanol and routinely processed for paraffin embedding in a Leica ASP300 Automatic tissue processor (Nussloch, Germany). 7 μm thick sections were cut in a Leitz 1512 microtome (Vienna, Austria) and stained with hematoxylin and eosin.56 The sex of each animal was recorded and the total number of female and male mussels was calculated for each sampling time and location, including the sex data collected from mussels used for ALP measurements. With these data, the sex ratio was calculated for each sampling time and location.Gamete developmental stages were distinguished in mussel gonads following the classification of Seed.57 Then, a gonad index (GI) value was assigned to each developmental stage adapted from the description of Kim et al.:58 1 resting stage (inactive or undifferentiated); 1.5 early gametogenic stage (gametogenesis has begun but no ripe gametes visible); 3.5 advanced gametogenic stage (gametogenesis still progressing and ripe gametes and developing gametes have about equal proportions); 5 mature stage (gonad fully mature, follicles full of ova or sperm); 3.5 spawning stage (active emission of gametes, some follicles appear empty); 1.5 post-spawning stage (empty follicles and only residual gametes remain). Afterwards, a mean GI value for each location and sampling time was calculated.
In samples corresponding to April 2003, 2004, 2005 and 2006 a detailed histopathological study was carried out. Histopathological alterations such as haemocytic infiltration of follicles containing mature gametes, severe gamete atresia, necrosis and abnormal follicle development were scored for each animal and reported as a percentage of total animals in each group, for males and females separately.
Terminal transferase dUTP nick end labeling (TUNEL) assay
With the aim of identifying apoptotic gametes in the gonads of studied mussel populations, we used a commercial TUNEL assay system (DeadEndTM Colorimetric TUNEL system, Promega Corporation, Madison, WI, USA). Apoptotic cells were detected in non-stained gonad histology sections. The fragmented DNA of apoptotic cells was detected by incorporating a biotinylated nucleotide at the 3′–OH ends of the DNA fragments using the enzyme terminal deoxynucleotidyl transferase and then staining with diaminobenzidine (DAB) in a reaction catalyzed by the enzyme streptavidin horseradish peroxidase.Briefly, paraffin-embedded sections (7 μm thick) were deparaffinized and rehydrated in graded ethanol steps. Then, slides were washed in a 0.85% NaCl solution for 5 min and subsequently immersed in phosphate buffered saline (PBS) solution for 5 min. Sections were fixed in 10% neutral buffered formalin for 15 min and then washed in PBS for 2 × 5 min. Following the manufacturer's instructions, sections were covered with a 20 μg ml−1proteinase K solution and incubated for 10–30 min at room temperature in a wet chamber. Afterwards, sections were washed in PBS for 5 min, refixed in 10% neutral buffered formalin for 5 min and rinsed in PBS for 2 × 5 min. The excess of liquid was removed by tapping the slides and sections were covered for 5 min with the equilibration buffer supplied in the commercial kit. After carefully removing the excess of liquid, sections were covered with recombinant terminal deoxynucleotidyl transferase (rTdT) reaction mix and incubated at 37 °C for 60 min. Then, slides were immersed in SSC buffer for 15 min. After rinsing the slides with PBS for 2 × 5 min, endogenous peroxidase was blocked with 0.3% hydrogen peroxide solution for 5 min. Slides were washed in PBS for 2 × 5 min and incubated in a solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) of streptavidin horseradish peroxidase for 30 min. Before reaction visualization, slides were rinsed in PBS for 2 × 5 min and subsequently incubated in the DAB solution supplied in the kit for 10 min in the dark. Finally, slides were rinsed in deionized water for 2 × 5 min and mounted in Kaiser's glycerol gelatine. Apoptotic nuclei appeared stained dark brown. Negative control sections were run in parallel, where autoclaved deionized water was used instead of rTdT in the reaction mix. Positive control slides of human promyelocytic leukemia cells (HL60) previously treated with actinomycin to induce apoptosis, were also included.
500) of streptavidin horseradish peroxidase for 30 min. Before reaction visualization, slides were rinsed in PBS for 2 × 5 min and subsequently incubated in the DAB solution supplied in the kit for 10 min in the dark. Finally, slides were rinsed in deionized water for 2 × 5 min and mounted in Kaiser's glycerol gelatine. Apoptotic nuclei appeared stained dark brown. Negative control sections were run in parallel, where autoclaved deionized water was used instead of rTdT in the reaction mix. Positive control slides of human promyelocytic leukemia cells (HL60) previously treated with actinomycin to induce apoptosis, were also included.
Alkali-labile phosphate (ALP) method
Levels of vitellogenin-like proteins in mussel gonads were measured by the ALP method following the protocol described in Gagné et al..59 Briefly, the gonad from each animal was homogenized in 25 mM Hepes-NaOH buffer, pH 7.4, containing 125 mM NaCl, 1 mM dithiothreitol and 1mM EDTA using a glass-teflon pestle in an ice containing bath with a Braun Potter S homogenizer (Melsungen, Germany). The obtained homogenate was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 30 min at 2 °C in a Beckman Coulter Optima L-90 K ultracentrifuge (Palo Alto, CA, USA). The obtained supernatant was carefully removed and an aliquot of 200 μl was mixed with acetone (35% final acetone concentration) and centrifuged at 10
000 g for 30 min at 2 °C in a Beckman Coulter Optima L-90 K ultracentrifuge (Palo Alto, CA, USA). The obtained supernatant was carefully removed and an aliquot of 200 μl was mixed with acetone (35% final acetone concentration) and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 5 min for the precipitation of proteins. Another aliquot (75 μl) of the original supernatant was stored at −80 °C for protein concentration determination. The obtained pellet was dissolved in 200 μl of 1 M NaOH solution for 30 min at 60 °C in a water-shaking bath. Levels of inorganic free phosphates were determined using the phosphomolybdenum assay adapted from Stanton.60 A subsample of 75 μl was mixed with 125 μl trichloroacetic acid, 630 μl ultrapure water, 170 μl molybdenum reagent (0.02 M ammonium molybdate tetrahydrated and 5.25 M H2SO4 solution), and 50 μl Fiske-Subbarow reducer (Sigma, St. Louis, MO, USA). After incubating for 10 min, the absorbance was measured at 660 nm using a Multiskan Spektrum spectrophotometer (Thermo Labsystems, Chantilly, VA, USA). For inorganic phosphate standard curve, a series of KH2PO4 concentrations was used. ALP levels in gonads are given as μg phosphates/mg protein. Protein concentration was measured using the DC Protein Assay of Bio-Rad (Hercules, CA, USA) based on the Lowry method adapted to microtiter plates.61
000 g for 5 min for the precipitation of proteins. Another aliquot (75 μl) of the original supernatant was stored at −80 °C for protein concentration determination. The obtained pellet was dissolved in 200 μl of 1 M NaOH solution for 30 min at 60 °C in a water-shaking bath. Levels of inorganic free phosphates were determined using the phosphomolybdenum assay adapted from Stanton.60 A subsample of 75 μl was mixed with 125 μl trichloroacetic acid, 630 μl ultrapure water, 170 μl molybdenum reagent (0.02 M ammonium molybdate tetrahydrated and 5.25 M H2SO4 solution), and 50 μl Fiske-Subbarow reducer (Sigma, St. Louis, MO, USA). After incubating for 10 min, the absorbance was measured at 660 nm using a Multiskan Spektrum spectrophotometer (Thermo Labsystems, Chantilly, VA, USA). For inorganic phosphate standard curve, a series of KH2PO4 concentrations was used. ALP levels in gonads are given as μg phosphates/mg protein. Protein concentration was measured using the DC Protein Assay of Bio-Rad (Hercules, CA, USA) based on the Lowry method adapted to microtiter plates.61
Statistics
Sex ratio bias was studied using the G test of association, comparing total number of female and male mussels and normalizing for theoretical gender bias (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Other statistical analyses were performed using the SPSS 10.0 software (SPSS, Inc., Chicago, IL, USA). After testing normality (Kolmogorov–Smirnov's test) and homogeneity (Levene's test) of the data, the effect of factors sampling time and location, as well as their interaction on ALP levels was studied by two-way analysis of variance (ANOVA). Statistically significant differences in ALP levels among groups were studied using one-way ANOVA followed by the Duncan's post-hoc test. In case of GI values, non-parametric ANOVA (Kruskall–Wallis) was applied followed by Mann–Whitney's U test. The interactive effect of both sampling time and location on GI values was analyzed by Friedman's test. Correlation studies between GI and ALP levels were performed using the Spearman's correlation index. Differences in percentages of histopathological alterations among groups were studied by Chi-squared test. Statistical significance was established at p < 0.05 for all studied tests. Finally, classification studies based on hierarchical cluster analysis were performed with GI and ALP values and a principal component analysis was run to study the association between studied endpoints and to explain the variability among sampled populations.
1). Other statistical analyses were performed using the SPSS 10.0 software (SPSS, Inc., Chicago, IL, USA). After testing normality (Kolmogorov–Smirnov's test) and homogeneity (Levene's test) of the data, the effect of factors sampling time and location, as well as their interaction on ALP levels was studied by two-way analysis of variance (ANOVA). Statistically significant differences in ALP levels among groups were studied using one-way ANOVA followed by the Duncan's post-hoc test. In case of GI values, non-parametric ANOVA (Kruskall–Wallis) was applied followed by Mann–Whitney's U test. The interactive effect of both sampling time and location on GI values was analyzed by Friedman's test. Correlation studies between GI and ALP levels were performed using the Spearman's correlation index. Differences in percentages of histopathological alterations among groups were studied by Chi-squared test. Statistical significance was established at p < 0.05 for all studied tests. Finally, classification studies based on hierarchical cluster analysis were performed with GI and ALP values and a principal component analysis was run to study the association between studied endpoints and to explain the variability among sampled populations.
Results
Sex ratio
Sex ratio values for each sampling location at each sampling time are shown in Table 1. No statistically significant bias in the sex ratio of the whole studied population (G value = 0.59, p = 0.44) was detected in comparison with the theoretical sex ratio in mussel populations (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The observed sex ratio was 1
1). The observed sex ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.02 (female
1.02 (female![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) male) for the total of three years of study. When the sex ratio was analyzed separately for each sampling time, a statistically significant bias to more males was observed in September 2003 (G value = 27.98; p < 0.01), July 2004 (G value = 4.14, p < 0.05) and October 2004 (G value = 6.86, p < 0.05). On the other hand, in April 2004 significantly more females were observed (G value = 7.09, p < 0.05). Among studied locations only Cíes (G value = 7.77, p < 0.05) showed a significant bias to females (1.7
male) for the total of three years of study. When the sex ratio was analyzed separately for each sampling time, a statistically significant bias to more males was observed in September 2003 (G value = 27.98; p < 0.01), July 2004 (G value = 4.14, p < 0.05) and October 2004 (G value = 6.86, p < 0.05). On the other hand, in April 2004 significantly more females were observed (G value = 7.09, p < 0.05). Among studied locations only Cíes (G value = 7.77, p < 0.05) showed a significant bias to females (1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Finally, we found two hermaphrodite animals, one in Mutriku (April 2005) and the other in Ons (July 2005). They both showed separate male and female follicles in the mantle tissue (see Fig. 2c below).
1). Finally, we found two hermaphrodite animals, one in Mutriku (April 2005) and the other in Ons (July 2005). They both showed separate male and female follicles in the mantle tissue (see Fig. 2c below).
 | ||
| Fig. 2 Micrographs showing gonad sections stained with hematoxylin/eosin from studied mussels. (a) Male gonad section from Caldebarcos (April 2004) showing advanced gametogenic stage. (b) Female gonad section from Pedreña (April 2004) showing mature stage. (c) Hermaphroditic gonad section from Mutriku (April 2005). Separate male and female follicles are observed. (d) Female gonad section from Mundaka (April 2003) showing widespread gamete atresia. (e) Female gonad section from Muskiz (April 2003) showing severe haemocytic infiltration (asterisks) of mature follicles. (f) Female gonad section from Suances (April 2004) showing small sized follicles with mature gametes at spawning stage. (g) Female gonad section from Cíes (April 2006) showing necrotic oocytes (arrows) with dispersed cytoplasm in the form of eosinophilic spheres. (h) Gonad section showing trematode parasite infestation of gonad follicles causing castration of a mussel from S. Bartolomeu do Mar (April 2005). Scale bars in (a), (b), (d), (e), (f), (g) and (h) represent 100 μm and in (c) represents 200 μm. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M) data in studied mussel populations. — indicates no samples
M) data in studied mussel populations. — indicates no samples
| Sampling month | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2003 | 2004 | 2005 | 2006 | TOTAL | ||||||||
| April | July | September | February | April | July | October | April | July | October | April | ||
| São Bartolomeu do Mar | — | — | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
| Ons | — | — | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 1.8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Cíes | — | — | — | — | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Oia | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Aguiño | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 1.8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
| Caldebarcos | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Camelle | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Segaño | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Estaca de Bares | 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Llanes | 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| San Vicente | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Suances | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
| Pedreña | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
| Laredo | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
| Muskiz | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Arrigunaga | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
| Gorliz | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Bakio | — | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Mundaka | 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Mutriku | — | — | — | 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Orio | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
| Hondarribia | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| TOTAL | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 2.3 |
1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.02 1.02 |
Gamete development and gonad index
Microscopical observation of the gonad showed differences in gamete development patterns among stations (Fig. 3). Furthermore, Friedman's test showed that both sampling time and location had significant effects on gamete development (χ2 = 2495.48, p < 0.05). At the beginning of the present study in April 2003, most mussel populations showed mature gonads, with the exceptions of mussels from Oia, Estaca de Bares and San Vicente that showed a high number of animals in advanced gametogenesis. It is interesting to note that mussels from Aguiño and Caldebarcos, located close to the most impacted area, showed gonads mainly at spawning stage in April 2003. In July 2003, mussels from all locations were at more advanced developmental stages than in April 2003, except for mussels from Llanes, which remained in the mature stage. In most locations, some mussels were at the post-spawning stage and in Camelle and Suances the beginning of a second gametogenesis cycle could be observed. In September 2003, the gonad of most mussels was at a post-spawning stage and mussels from most locations had started the next reproductive cycle.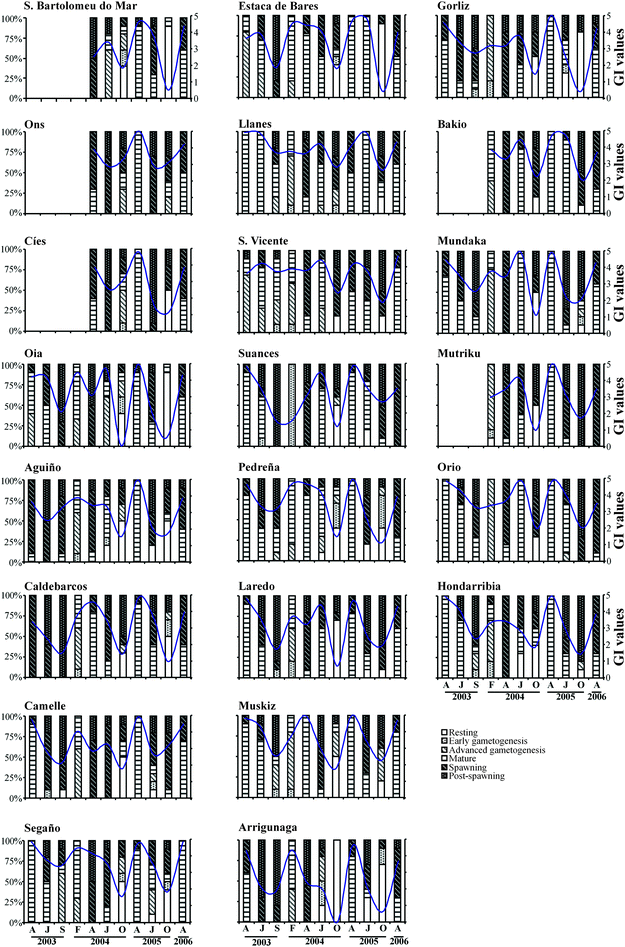 | ||
| Fig. 3 Graphs showing gamete development and gonad index from each station at each sampling time. Stacking-bar graphs show the percentage of individuals at each gametogenic stage at each sampling time. Superimposed continuous lines represent gonad index values for each sampling point at each sampling month. F: February; A: April; J: July; S: September; O: October. | ||
In February 2004 most mussel populations showed gonads in advanced gametogenesis or in mature phases, with the exception of mussels from Suances where all mussels showed early gametogenic gonads (Fig. 3). In April 2004, mussels from most locations were spawning and some had reached the post-spawning stage. However, most mussels from Caldebarcos, Estaca de Bares, Pedreña and all in Muskiz showed mature gonads (Fig. 3). In July 2004, spawning but also mature gonads were predominant in studied mussel populations, indicating the possibility of a second gametogenic cycle in some mussels of 16 out of 22 locations. Only mussels from Ons, Cíes, Caldebarcos, Camelle and Muskiz did not show this process. In October 2004, most mussel populations showed gonads in resting and post-spawning stages.
In April 2005, the predominant stage was the mature phase and in some stations mussels at spawning stage were also observed, mainly in Llanes and San Vicente (Fig. 3). In July 2005 most mussels showed spawning and post-spawning gonad stages, except mussels from Estaca de Bares and Llanes where all individuals were still at mature stage. Some localities, such as Camelle, Segaño and Gorliz showed the start of a second gametogenic cycle. In October 2005 all studied mussel populations showed gonads mainly at post-spawning and resting stages. Some locations, such as Ons, Caldebarcos, Segaño, Pedreña, Muskiz, Arrigunaga, Mundaka and Hondarribia had already started the new reproductive cycle.
In April 2006, most mussel populations showed gonads in mature and spawning stages, except those from Suances and Mutriku where all mussels were spawning (Fig. 3). Some few mussels from Aguiño, Arrigunaga and Gorliz were at post-spawning stage. Overall, most studied mussel populations showed one reproductive cycle in 2003 and 2005, and seemingly two reproductive cycles in 2004.
A hierarchical cluster analysis of GI values showed two main branches separating samplings of February, April and July (mainly gonads in mature and spawning stages) from those of September and October (gonads mainly at post-spawning or resting and early gametogenesis stages) (Fig. 4a). Furthermore, the dendrogram shows that data from samplings performed in April 2003, 2005 and 2006 are closely related but April 2004 was more related with February 2004 and July 2003, 2004 and 2005. On the other hand, no significant discrimination between most oil impacted and less impacted localities was observed (data not shown).
 | ||
| Fig. 4 Statistical classification analysis of studied endpoints. Dendrograms showing results of the hierarchical cluster analysis for GI values (a) and ALP levels (b) at each sampling time following the squared Euclidean distance between groups. Principal component analysis (PCA) of all studied endpoints for each sampling point at each sampling time is shown in (c). Circle groups mussel populations belonging to April 2005 and 2006 samplings. PC1: principal component 1. PC2: principal component 2. | ||
Gonad histopathology
The histopathological study of the gonad (Table 2) showed a high prevalence of oocyte atresia in female mussels in April 2003 (Fig. 2d), together with a high prevalence of haemocytic infiltration of gonad follicles (Fig. 2e). In April of the following year 2004 the atresia disappeared but widespread small sized mature follicles were observed in most mussel populations (Table 2). Both male and female mussels showed small follicles containing developing and full developed gametes, together with follicles of larger size with no gametes and surrounded by a high amount of reserve material (Fig. 2f). Overall, relative volume of gonad follicles was small compared with the rest of the specimens. Haemocytic infiltration of follicles was present in several stations. In April 2005, few histopathological alterations were observed. In females, atresia was scarce and low levels of infiltration of follicles by haemocytes were observed (Table 2). However, in April 2006 a high prevalence of abnormal gametes was observed in female mussels from six out of 22 studied populations (Table 2). Some abnormal gametes showed dispersed cytoplasm in the form of eosinophilic spheres (Fig. 2g). Other abnormal gametes were characterized by higher staining intensity than normal gametes. They progressively loose structure (nuclei and cytoplasm became indistinguishable) and finally they were released to the follicle lumen. Both types of abnormal gametes were studied by TUNEL assay to establish whether they appear as a result of apoptotic events. Whereas positive nuclei were evidenced in the positive control slides containing apoptotic HL60 cells, no positive nuclei were detected in studied mussel gonad sections indicating that abnormal gametes could be originated by degenerative processes such as necrosis and not by apoptotic events. In addition, in April 2006 haemocytic infiltration of gonad follicles was widespread among most studied populations, being again female mussels more affected than male mussels.| Histopathology | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haemocytic infiltrations | Small sized follicles | Oocyte atresia | Abnormal gametes | |||||||||||||
| Females | 2003 | 2004 | 2005 | 2006 | 2003 | 2004 | 2005 | 2006 | 2003 | 2004 | 2005 | 2006 | 2003 | 2004 | 2005 | 2006 |
| São Bartolomeu do Mar | — | 60 | 0 | 0 | — | 70 | 0 | 0 | — | 0 | 0 | 12 | — | 0 | 0 | 0 |
| Ons | — | 50 | 0 | 40 | — | 25 | 0 | 0 | — | 0 | 0 | 40 | — | 0 | 0 | 20 |
| Cíes | — | 20 | 0 | 30 | — | 0 | 40 | 0 | — | 0 | 0 | 0 | — | 40 | 0 | 28 |
| Oia | 60 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aguiño | 0 | 0 | 0 | 20 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 | 0 |
| Caldebarcos | 0 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 | 0 | 0 |
| Camelle | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 30 | 0 | 0 | 0 | 0 |
| Segaño | 100 | 0 | 20 | 30 | 0 | 16 | 0 | 0 | 100 | 0 | 20 | 14 | 0 | 0 | 20 | 0 |
| Estaca de Bares | 30 | 20 | 0 | 20 | 0 | 0 | 0 | 0 | 60 | 0 | 50 | 0 | 0 | 0 | 0 | 0 |
| Llanes | 15 | 10 | 25 | 16 | 0 | 44 | 25 | 0 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| San Vicente | 0 | 12 | 25 | 0 | 0 | 37 | 0 | 0 | 0 | 0 | 50 | 0 | 0 | 12 | 0 | 0 |
| Suances | 50 | 0 | 33 | 50 | 0 | 80 | 33 | 0 | 0 | 0 | 33 | 0 | 0 | 0 | 0 | 66 |
| Pedreña | 70 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 70 | 0 | 25 | 0 | 0 | 0 | 0 | 0 |
| Laredo | 50 | 100 | 25 | 28 | 0 | 75 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Muskiz | 100 | 50 | 0 | 20 | 0 | 0 | 0 | 0 | 100 | 0 | 25 | 0 | 0 | 0 | 0 | 60 |
| Arrigunaga | 0 | 100 | 65 | 100 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 25 |
| Gorliz | 0 | 0 | 0 | 20 | 0 | 50 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 25 |
| Bakio | — | 20 | 0 | 0 | — | 100 | 0 | 0 | — | 0 | 0 | 0 | — | 0 | 16 | 0 |
| Mundaka | 30 | 0 | 14 | 0 | 0 | 80 | 0 | 0 | 100 | 0 | 14 | 0 | 0 | 0 | 0 | 0 |
| Mutriku | — | 0 | 0 | 33 | — | 40 | 0 | 0 | — | 0 | 0 | 0 | — | 0 | 0 | 0 |
| Orio | 0 | 0 | 0 | 66 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 0 | 0 | 0 | 0 |
| Hondarribia | 25 | 0 | 0 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Histopathology | ||||||||
|---|---|---|---|---|---|---|---|---|
| Haemocytic infiltrations | Small sized follicles | |||||||
| Males | 2003 | 2004 | 2005 | 2006 | 2003 | 2004 | 2005 | 2006 |
| São Bartolomeu do Mar | — | 0 | 0 | 0 | — | 33 | 37 | 0 |
| Ons | — | 16 | 0 | 20 | — | 50 | 0 | 0 |
| Cíes | — | 0 | 0 | 0 | — | 20 | 0 | 0 |
| Oia | 0 | 25 | 0 | 0 | 0 | 50 | 0 | 0 |
| Aguiño | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 0 |
| Caldebarcos | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 0 |
| Camelle | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Segaño | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Estaca de Bares | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Llanes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| San Vicente | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 0 |
| Suances | 50 | 60 | 14 | 25 | 0 | 40 | 0 | 0 |
| Pedreña | 15 | 16 | 0 | 0 | 0 | 0 | 0 | 0 |
| Laredo | 0 | 0 | 0 | 33 | 0 | 0 | 0 | 0 |
| Muskiz | 12 | 20 | 0 | 0 | 0 | 0 | 0 | 0 |
| Arrigunaga | 25 | 60 | 50 | 67 | 0 | 80 | 0 | 0 |
| Gorliz | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 0 |
| Bakio | — | 0 | 0 | 0 | — | 0 | 0 | 0 |
| Mundaka | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mutriku | — | 0 | 20 | 25 | — | 0 | 0 | 0 |
| Orio | 0 | 0 | 50 | 66 | 0 | 0 | 0 | 0 |
| Hondarribia | 0 | 60 | 70 | 40 | 0 | 0 | 0 | 0 |
Parasite prevalence during the whole study period was very low in the gonads. Only 4 individuals showed trematode infestation of gonad follicles, which provoked castration of infected mussels (Fig. 2h).
Statistical analysis of histopathology data showed that sex dependent differences occurred, female mussels showing higher prevalence of histopathologies than male mussels. Females showed statistically significant higher prevalence of haemocytic infiltration of follicles than male mussels in April 2003 (χ2 = 20.73; p < 0.05) and April 2006 (χ2 = 6.13; p < 0.05). In April 2004, small sized follicle prevalence was higher in female mussels than in male mussels (χ2 = 12.48; p < 0.05). Regarding station dependent effects, we did not observe any significant differences among studied stations. Only mussels from Arrigunaga, both female and male, showed significantly higher prevalence levels for all studied pathologies. As to possible statistically significant differences between sampling years, in the case of female mussels prevalence of oocyte atresia was significantly higher in 2003 than in other years (χ2 = 38.85, p < 0.05). Similarly, prevalence of oocyte atresia was higher in 2005 (χ2 = 8.33, p < 0.05) and 2006 (χ2 = 9.42, p < 0.05) than in 2004. Finally, abnormal gamete prevalence was higher in 2006 (χ2 = 5.67; p < 0.05) than in other studied years.
ALP levels
Levels of vitellogenin-like proteins in mussel gonads measured as ALP levels are shown in Fig. 5. Female mussels showed significantly higher ALP values than male mussels in the three samplings (Fig. 5), in agreement with the female-specific synthesis of vitellogenin-like proteins. In female mussels, ALP levels in April 2004 were highly variable among stations and inside each population (Fig. 5). Accordingly, ALP levels and GI values were not significantly correlated during April 2004 sampling (Spearman's r2 = 0.339). In April 2005, differences among stations in ALP levels of female mussels were reduced in comparison with April 2004 (Fig. 5), which was related with a more homogeneous gamete development observed during April 2005, most females being at mature gonad stage (Fig. 5). ALP levels and GI values were significantly correlated (Spearman's r2 = 0.557) in female mussels in the April 2005 sampling. In April 2006, ALP values in female mussels were overall lower than in 2005 but more homogeneous within each location (Fig. 5). These lower values were related with gonads mainly at spawning stage observed in April 2006 (Fig. 3). The association between ALP and GI values was confirmed by a significant correlation (Spearman's r2 = 0.773), which was stronger than in April 2005. A hierarchical cluster analysis of ALP levels in female mussels showed that April 2005 and 2006 were closely associated between them and separated from April 2004 (Fig. 4b). As in the case of GI values, no discrimination between most oil impacted locations and less impacted locations was obtained (data not shown). | ||
| Fig. 5 Graphs showing ALP levels in the gonads of mussels in studied April months (2004, 2005 and 2006). Bars represent ALP levels (mean ± standard deviation) in gonads of female mussels. Matrices at the top of each graph represent results after ANOVA analysis of data from female mussels. Localities belonging to the same statistical group are marked with a black dot. As no significant differences were observed between male mussels, the mean value at each sampling is represented with a black line for comparison purposes. | ||
In the case of male mussels, no differences in gonad ALP levels between sampling localities or sampling years were observed (Fig. 5). No correlation between ALP levels and GI values was obtained at any sampling time for male mussels.
Principal component analysis
Principal component analysis was performed with data on GI, ALP and histopathology obtained in April 2004, 2005 and 2006. More than 70% of the variability was explained by two components (Fig. 4c). The first component was highly associated with GI (association coefficient: −0.904) and ALP levels (association coefficient: −0.754) and separated samples of 2005 and 2006. The second component showed high association with small sized follicle prevalence (association coefficient: −0.696) and haemocytic infiltration of gonad follicles (association coefficient: 0.654) and separated samples of 2004 from those of 2005 and 2006. Therefore, variability in the spatial distribution of studied samples was based on gamete development (GI and ALP levels) and observed histopathologies such as small sized follicles and haemocytic infiltration.Discussion
In the present work we investigated the effects caused by the POS on reproduction of wild mussel populations from Galicia and the Bay of Biscay, which were differently impacted by the oil spill.62 Most alterations occurred during 2003 and 2004 and disappeared later on.There were differences in gamete development from year to year but not between sexes. The present results demonstrated that in studied mussel populations gametogenesis proceeded during winter months and the active reproductive period occurred in spring months. These observations agree with reproductive cycles described before by other authors for the north western Iberian Peninsula and in the Bay of Biscay for mussel populations.52–55,63 During 2003 and 2005 there was mainly one reproductive cycle but in 2004 two reproductive peaks appeared to occur. These differences in reproductive output from year to year are common to mussel populations worldwide. Gamete development and reproduction are controlled by biotic and abiotic factors such as food availability, temperature and salinity, which are highly variable.53,63–65
Alterations in sex ratios have been observed in freshwater mussels inhabiting rivers polluted with endocrine disruptors.66 No significant bias on sex ratio was measured in the present study, and the resulting ratio close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was as previously reported53,67 except in Cíes. Only two hermaphrodite animals were detected during the whole study. This is in accordance with previous data reported by Villalba53 on the north western coast of Spain. An increase in hermaphroditism has been reported in bivalves inhabiting highly polluted environments67 and in mussels from the locality of Txatxarramendi in the Biosphere's Reserve of Urdaibai.69 The histopathological study of mussel gonads in April showed a high prevalence of atresia in female mussels in 2003. This could lead to a possible decrease in gamete quality in affected female mussels. A high prevalence of atretic gametes has been observed in mussels exposed to diesel oil and PAH derivatives,28,29,70 to North Sea oil and to a mixture of oil, alkylphenols and PAHs similar to produced water released by offshore oil-extraction platforms.27 However, in the following year 2004, no atresia was observed but a striking observation was the reduced size of follicles both in male and female mussels. These small follicles with mature gametes have been described before by Villalba53 in mussels from Galicia. This author used the term “imperfect ripeness” to describe this process, but the reason and possible effects on mussel reproductive ability are unknown.53 It is unclear whether the presence of small sized follicles in 2004 could be related to the two reproductive peaks found in the same year.
1 was as previously reported53,67 except in Cíes. Only two hermaphrodite animals were detected during the whole study. This is in accordance with previous data reported by Villalba53 on the north western coast of Spain. An increase in hermaphroditism has been reported in bivalves inhabiting highly polluted environments67 and in mussels from the locality of Txatxarramendi in the Biosphere's Reserve of Urdaibai.69 The histopathological study of mussel gonads in April showed a high prevalence of atresia in female mussels in 2003. This could lead to a possible decrease in gamete quality in affected female mussels. A high prevalence of atretic gametes has been observed in mussels exposed to diesel oil and PAH derivatives,28,29,70 to North Sea oil and to a mixture of oil, alkylphenols and PAHs similar to produced water released by offshore oil-extraction platforms.27 However, in the following year 2004, no atresia was observed but a striking observation was the reduced size of follicles both in male and female mussels. These small follicles with mature gametes have been described before by Villalba53 in mussels from Galicia. This author used the term “imperfect ripeness” to describe this process, but the reason and possible effects on mussel reproductive ability are unknown.53 It is unclear whether the presence of small sized follicles in 2004 could be related to the two reproductive peaks found in the same year.
In female mussels, ALP levels in April 2004 were highly variable among stations, while in April 2005 and 2006 the variability among groups was reduced and values were more homogeneous. In male mussels ALP levels were always low, suggesting that the fuel oil spilled by the Prestige did not cause xenoestrogenic effects. In female mussels, ALP levels and gamete development (gonad index) were positively correlated in April 2005 and 2006, as it could be expected based on the seasonal variation of vitellogenin-like proteins and oocyte growth.47,69,71 This correlation was not found in April 2004, indicating that natural environmental factors were not the only factors controlling observed differences in reproductive cycle and that a possible alteration in mussel reproduction occurred during 2004. Similarly, mussels collected in Galicia during the winter of 2003–2004 lost seasonal variability of nitric oxide synthesis in haemocytes.72 This could indicate altered endocrine regulation and reproduction as nitric oxide is a signaling molecule controlled by estradiol in mussels and participates in the immunoresponse and reproduction.73,74 Effects on gamete development and reduction in ALP levels in female mussel gonads have been described after exposure to 0.5 ppm North Sea oil for 3 weeks.27 Intermoult female crabs Carcinus maenas exposed for 28 days to sediments obtained from POS affected areas showed a trend to reduced vitellogenin levels.75 Low ALP levels have been measured in bivalve populations inhabiting PAH contaminated sites30 together with delayed gametogenesis.31 Delayed gametogenesis was also observed in clams feed on PAH contaminated algae and exposed throught sediments76 and in mussels exposed for three months to low doses of the water accommodated fraction of three crude oils.77 However, accelerated spawning occurred at high oil exposure doses,77 similar to that observed in stations severely impacted by the POS (Aguiño and Caldebarcos) in April 2003.
According to biomarker analysis in mussel digestive gland, mussel health started to recover after the POS at the end of 2004.18 The results of the present work also indicate no significant gamete alterations in 2005 and 2006, but long-lasting effects on mussel reproduction ability, embryo development and growth can not be discarded. Blue mussels exposed to North Sea oil during the whole gametogenic cycle showed early gamete atresia and reduced fertility. Obtained larvae showed delayed development and severe abnormalities.78 Similarly, scallops (Mizuhopecten yessoensis) from polluted areas in the Sea of Japan showed reduced fertilization, delayed larval development and increased larval abnormalities,68 as shown in oysters (Crassostrea virginica) and Dwarf surfclams (Mulinia lateralis) exposed to water soluble fractions of three crude oils79 and in mussel embryos exposed to different PAHs.80 Seawater samples collected in December 2002 from Galicia after POS were embryotoxic to oyster (Crassostrea gigas) and clam (Venerupis pullastra).35 A reduction in survival ability has been described in mussel seeds collected in February 2003 in areas highly impacted by the POS.39 Mussel seeds affected by POS showed lower growth than non-impacted or less impacted mussels.38 Therefore, long-lasting effects on affected populations can not be ruled out.
Conclusions
Mussel populations appeared to be affected in the whole studied area and most alterations in studied endpoints were observed after 1–2 years of POS. Thus, high prevalences of oocyte atresia and haemocytic infiltration were found in most localities in 2003 whereas small sized follicles and very variable ALP levels were observed in 2004. Gamete alterations disappeared in 2005, but in 2006 a high prevalence of necrotic gametes was found at six localities, which deserves further research. In agreement, cluster analysis and PCA clearly distinguished mussels sampled in April 2004 from those sampled in April 2005 and April 2006. PAH accumulation levels in the same mussel populations were high in Galicia and Bay of Biscay during 2003 and 2004, and returned to pre-spill levels in 2005 and 2006.62,81,82 At present, the meaning and relevance of gamete alterations found in 2003–2004 are not known. Gamete atresia accompanied by haemocytic infiltration observed in most localities in 2003 could be associated with an increased energy consumption and demand to cope with detoxification and defense metabolism.Acknowledgements
This work was supported by the Spanish Ministry of Science and Technology through “Urgent Research Actions on Coastal areas” and project PRESTEPSE (VEM2003-20082-CO6-01) and by the Basque Government through ETORTEK actions (IMPRES) and through a grant to Consolidated Research Groups (Ref: GIC07/26-IT-393-07).Notes and references
- J. Albaigés, B. Morales-Nin and F. Vilas, Mar. Pollut. Bull., 2006, 53, 205 CrossRef CAS.
- M. González, A. Uriarte, R. Pozo and M. Collins, Mar. Pollut. Bull., 2006, 53, 369 CrossRef CAS.
- A. Del Valls, Ciencias Marinas, 2003, 29, i Search PubMed.
- R. De la Huz, M. Lastra, J. Junio, C. Castellanos and J. M. Viétez, Estuarine, Coastal Shelf Sci., 2005, 65, 19 CrossRef CAS.
- J. Junoy, C. Castellanos, J. M. Viéitez, M. R. de la Huz and M. Lastra, Mar. Pollut. Bull., 2005, 50, 526 CrossRef CAS.
- G. Caballero-Miguez, M. D. Garza-Gil and M. M. Varela-Lafuente, Mar. Policy, 2009, 33, 288 CrossRef.
- E. D. Goldberg, Mar. Pollut. Bull., 1975, 6, 111 CrossRef.
- M. P. Cajaraville, M. J. Bebianno, J. Blasco, C. Porte, C. Sarasquete and A. Viarengo, Sci. Total Environ., 2000, 247, 295 CrossRef CAS.
- J. Oehlmann, U. Schulte-Oehlmann, 2002. Molluscs as bioindicators. In: Markert, B. A., Breure, A. M., Zechmeister, H. G. (ed.), Bioindicators and biomonitorings.Elsevier, New York, USA, pp. 577 Search PubMed.
- R. Smolders, L. Bervoets, V. Wepener and R. Blust, Hum. Ecol. Risk Assess., 2003, 9, 741 CrossRef CAS.
- C. H. Peterson, S. D. Rice, J. W. Short, D. Esler, J. Bodkin, B. E. Ballachey and D. B. Irons, Science, 2003, 302, 2082 CrossRef CAS.
- C. Porte, X. Biosca, M. Solé and J. Albaigés, Biomarkers, 2000, 5, 436 CrossRef CAS.
- R. J. Law, J. E. Thain, M. F. Kirby, Y. T. Allen, B. P. Lyons, C. A. Kelly, S. Haworth, E. A. Dyrynda, P. E. J. Dyrynda, J. S. Harvey, S. Page, M. D. Nicholson, D. R. P. Leonard, 1998. The impact of the Sea Empress oil spill on fish and shellfish. In: Edwards, R., Sime, H. (ed.), The Sea Empress Oil Spill: Proceedings of the International Conference held in Cardiff, 11–13 February 1998, Chartered Institute of Water and Environmental Management, London, UK, pp. 109–136 Search PubMed.
- S. M. Moreira, M. Moreira-Santos, R. Ribeiro and L. Guilhermino, Ecotoxicology, 2004, 13, 619 CrossRef CAS.
- G. Boquené, S. Chantereau, C. Clérendeau, E. Beausir, D. Ménard, B. Raffin, C. Minier, T. Burgeot, A. P. Leszkowicz and J. F. Narbonne, Aquat. Living Resour., 2004, 17, 309 Search PubMed.
- I. Marigómez, M. Soto, C. Cancio, A. Orbea, L. Garmendia and M. P. Cajaraville, Mar. Pollut. Bull., 2006, 53, 287 CrossRef CAS.
- A. Orbea, L. Garmendia, I. Marigómez and M. P. Cajaraville, Mar. Ecol.: Prog. Ser., 2006, 306, 177 CrossRef CAS.
- M. P. Cajaraville, L. Garmendia, A. Orbea, R. Werding, A. Gómez-Mendikute, U. Izagirre, M. Soto and I. Marigómez, Mar. Environ. Res., 2006, 62, S337 CrossRef CAS.
- Exxon Valdez Oil Spill Trustee Council, 2006. Exxon Valdez Oil Spill Restoration Plan. Update on injured resources and services, 2006, Anchorage, AK, USA. pp. 42 Search PubMed.
- WHO/IPCS (World Health Organization/International Progamme on Chemical Safety), Global assessment of the state-of-the-science of endocrine disruptors, 2002, WHO/IPCS/EDC/02.2, Geneva, Switzerland Search PubMed.
- P. Matthiessen, Pure Appl. Chem., 2003, 75, 2197 CrossRef CAS.
- S. Y. Sol, L. L. Johnson, B. H. Horness and T. K. Collier, Mar. Pollut. Bull., 2000, 40, 1139 CrossRef CAS.
- S. D. Rice, R. E. Thomas, R. A. Heintz, A. C. Wertheimer, M. L. Murphy, M. G. Carls, J. W. Short, A. Moles, Synthesis of long-term impacts to pink salmon following the Exxon Valdez oil spill: persistence, toxicity, sensitivity, and controversy. Final Report project 99329, 2001,pp. 77 Search PubMed.
- M. G. Carls, G. D. Marty and J. E. Hose, Can. J. Fish. Aquat. Sci., 2002, 59, 153 CrossRef.
- C. Porte, G. Janer, L. C. Lorusso, M. Ortiz-Zarragoitia, M. P. Cajaraville, C. Fossi and L. Canesi, Comp. Biochem. Physiol. Part C, 2006, 143, 303 CrossRef CAS.
- J. Lintelmann, A. Katayama, N. Kurihara, L. Shore and A. Wenzel, Pure Appl. Chem., 2003, 75, 631 CrossRef CAS.
- M. Ortiz-Zarragoitia and M. P. Cajaraville, Arch. Environ. Contam. Toxicol., 2006, 50, 361 CrossRef CAS.
- D. M. Lowe, Mar. Ecol. Res., 1988, 22, 243 Search PubMed.
- D. Lowe and R. K. Pipe, Mar. Environ. Res., 1987, 22, 243 CrossRef CAS.
- F. Gagné, C. Blaise, J. Pellerin and S. Gauthier-Clerc, Mar. Environ. Res., 2002, 53, 295 CrossRef CAS.
- S. Gauthier-Clerc, J. Pellerin, C. Blaise and F. Gagné, Comp. Biochem. Physiol., Part C, 2002, 131, 457 CrossRef CAS.
- J. M. Navas and H. Segner, Aquat. Toxicol., 2000, 51, 79 CrossRef CAS.
- J. M. Nicolas, Aquat. Toxicol., 1999, 45, 77 CrossRef CAS.
- M. M. H. Van Lipzig, N. P. E. Vermeulen, R. Gusinu, J. Legler, H. Frank, A. Seidel and J. H. N. Meerman, Environ. Toxicol. Pharmacol., 2005, 19, 41 CrossRef CAS.
- R. Beiras and L. Saco-Alvarez, Water, Air, Soil Pollut., 2006, 177, 457 CrossRef CAS.
- J. M. Navas, M. Babín, S. Casado, C. Fernández and J. V. Tarazona, Mar. Environ. Res., 2006, 62, S352 CrossRef CAS.
- S. S. Rossi and J. W. Anderson, Water, Air, Soil Pollut., 1978, 9, 155 CrossRef CAS.
- L. G. Peteiro, J. M. F. Babarro, U. Labarta and M. J. Fernández-Reiriz, ICES J. Mar. Sci., 2006, 63, 1005 CAS.
- U. Labarta, M. J. Fernández-Reiriz, J. L. Garrido, J. M. F. Babarro, J. M. Bayona and J. Albaigés, Mar. Ecol.: Prog. Ser., 2005, 302, 135 CrossRef CAS.
- L. G. Peteiro, U. Labarta and M. J. Fernández-Reiriz, Comp. Biochem. Physiol. Part C, 2007, 145, 588 CrossRef.
- A. Arukwe and A. Goksøyr, Comp. Hepatol., 2003, 2, 4 CrossRef.
- M. G. Marin and V. Matozzo, Mar. Pollut. Bull., 2004, 48, 835 CrossRef CAS.
- A. Goksøyr, J. Toxicol. Environ. Health, Part A, 2006, 69, 175 Search PubMed.
- T. H. Hutchinson, G. T. Ankley, H. Segner and C. R. Tyler, Environ. Health Persp., 2006, 114(Suppl. 1), 106.
- T. Matsumoto, A. M. Nakamura, K. Mori and T. Kayano, Zool. Sci., 2003, 20, 37 CrossRef CAS.
- M. Osada, M. Harata, M. Kishida and A. Kijama, Mol. Reprod. Dev., 2004, 67, 273 CrossRef CAS.
- C. Blaise, F. Gagné, J. Pellerin and P. D. Hansen, Environ. Toxicol., 1999, 14, 455 CrossRef CAS.
- M. Osada, T. Takamura, H. Sato and K. Mori, J. Exp. Zool., 2003, 299a, 172 CrossRef CAS.
- A. M. Puinean, P. Labadie, E. M. Hill, M. Osada, M. Kishida, R. Nakao, A. Novillo, I. P. Callard and J. M. Rotchell, Aquat. Toxicol., 2006, 79, 376 CrossRef CAS.
- G. Janer and C. Porte, Ecotoxicology, 2007, 16, 145 CrossRef CAS.
- V. Matozzo, F. Gagné, M. G. Marin, F. Ricciardi and C. Blaise, Environ. Int., 2008, 34, 531 CrossRef CAS.
- S. Ogueta, B. J. Gómez, T. Serrano, M. Soto, Proceedings of the 5th National Congress on Aquaculture (Sant Carles de la Rápita, Spain). Barcelona University Press, Barcelona, Spain, 1995, 169 Search PubMed.
- A. Villalba, Aquaculture, 1995, 130, 269 CrossRef.
- A. T. Mikhailov, M. Torrado, J. Méndez and M. J. López, Mar. Biol., 1996, 126, 77 CrossRef CAS.
- M. P. Suárez, C. Alvarez, P. Molist and F. San Juan, J. Shellfish Res., 2005, 24, 531.
- M. Gamble, I. Wilson, The hematoxylin and eosin. In: Bancroft, J. D., Gamble, M. (ed.), Theory and practice of histological techniques.Churchill Livingstone-Elsevier Science, London, UK, 2002, pp. 125–138 Search PubMed.
- R. Seed, Oecologia, 1969, 3, 277 CrossRef.
- Y. Kim, K. A. Ashton-Alcox, E. N. Powell, Gonadal analysis. NOAA Histological techniques for marine bivalve mollusks: Update. NOAA Technical Memories NOS NCCOS 27,Silver Spring, USA, 2006, pp. 1–18 Search PubMed.
- G. Gagné, C. Blaise, J. Pellerin, E. Pelletier, M. Douville, S. Gauthier-Clerc and L. Viglino, Comp. Biochem. Physiol., Part C, 2003, 134, 189 CrossRef CAS.
- M. G. Stanton, Anal. Biochem., 1968, 22, 27 CrossRef CAS.
- H. J. Fryer, G. E. Davis, M. Manthorpe and S. Varon, Anal. Biochem., 1986, 153, 262 CAS.
- L. Bartolomé, M. Deusto, N. Etxebarria, P. Navarro, A. Usobiaga and O. Zuloaga, J. Chromatogr., A, 2007, 1157, 369 CrossRef CAS.
- J. Cáceres-Martínez and A. Figueras, Aquaculture, 1998, 162, 141 CrossRef.
- R. I. E. Newell, Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (North and Mid-Atlantic) –blue mussel. US Fish and Wildlife Service of Biology and Reproduction 82 (11.102). US Army Corps of Engineers, TR EL-82-4, 1989, 25 Search PubMed.
- F. G. Figueiras, U. Labarta and M. J. Fernández-Reiriz, Hydrobiologia, 2002, 484, 121 CrossRef.
- C. Blaise, F. Gagné, M. Salazar, S. Salazar, S. Trottier and P. D. Hansen, Fresenius Environ. Bull., 2003, 12, 865 CAS.
- E. Kenchington, B. MacDonald, L. Cao, D. Tsagkarakis and E. Zouros, Genetics, 2002, 161, 1579 CAS.
- M. A. Vaschenko, I. G. Syasina, P. M. Zhadan and L. A. Medvedeva, Hydrobiologia, 1997, 352, 231 CrossRef.
- M. Ortiz-Zarragoitia and M. P. Cajaraville, Ecotoxicol. Environ. Saf., 2010, 73, 693 CrossRef CAS.
- A. Goksøyr, A. Arukwe, J. Larsson, M. P. Cajaraville, L. Hauser, B. D. Nilsen, D. Lowe, P. Matthiessen, Molecular/cellular processes and the impact on reproduction. In: Lawrence, A., Hemingway, K. (ed.), Effects of pollution on fish.Blackwell Publishing, Oxford, UK, 2003, pp. 179–220 Search PubMed.
- C. Blaise, F. Gagné, J. Pellerin, P. D. Hansen and S. Trottier, Environ. Toxicol., 2002, 17, 170 CrossRef CAS.
- A. Novas, R. Barcia and J. I. Ramos-Martínez, Aquat. Toxicol., 2007, 85, 285 CAS.
- L. Barbin, I. Boarini, P. G. Borasio, P. Barion, S. Fiorini, R. Rossi and C. Bioindi, Gen. Comp. Endocrinol., 2003, 130, 215 CrossRef CAS.
- G. B. Stefano, P. Cadet, K. Mantione, J. J. Cho, D. Jones and W. Zhu, Endocrinology, 2003, 144, 1234 CrossRef CAS.
- C. Morales-Caselles, M. L. Martín Díaz, I. Riba and T. A. DelValls, Fresenius Environ. Bull., 2009, 18, 140 CAS.
- H. Frouin, J. Pellerin, M. Fournier, E. Pelletier, P. Richard, N. Pichaud, C. Rouleau and F. Garnerot, Aquat. Toxicol., 2007, 82, 120 CrossRef CAS.
- M. P. Cajaraville, I. Marigómez and E. Angulo, Comp. Biochem. Physiol., Part C: Pharmacol., Toxicol. Endocrinol., 1992, 102, 103 CrossRef CAS.
- T. Baussant, M. Ortiz-Zarragoitia, M. P. Cajaraville, R. K. Bechmann, I. C. Taban, S. Sanni. Mar. Pollut. Bull., submitted Search PubMed.
- A. Renzoni, Mar. Pollut. Bull., 1975, 6, 125 CrossRef.
- J. Bellas, L. Saco-Álvarez, O. Nieto and R. Beiras, Mar. Pollut. Bull., 2008, 57, 493 CrossRef CAS.
- J. A. Soriano, L. Viñas, M. A. Franco, J. J. González, M. H. Nguyen, J. M. Bayona and J. Albaigés, J. Environ. Monit., 2007, 9, 1018 RSC.
- B. Fernández, M. Albentosa, L. Viñas, A. Franco, J. J. González and J. A. Campillo, Ecotoxicology, 2010, 19, 735 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
