Natural attenuation of nutrients in a mediterranean drainage canal†‡
Fotini E.
Stamati
*,
Nikolaos
Chalkias
,
Daniel
Moraetis
and
Nikolaos P.
Nikolaidis
Department of Environmental Engineering, Technical University of Crete (TUC), University Campus, 73100, Chania, Crete, Greece. E-mail: fotini.stamati@enveng.tuc.gr; nikos_env@yahoo.com; moraetis@mred.tuc.gr; nikolaos.nikolaidis@enveng.tuc.gr; Fax: +30 28210 37847; Tel: +30 28210 37831
First published on 9th December 2009
Abstract
This research is aimed at elucidating the removal mechanisms of nutrients due to natural attenuation in drainage canals in Evrotas River delta in Greece. We investigated nutrients fluxes in groundwater, sediments, and reeds (Phragmites Australis and Arundo Donax) of the drainage canal. Groundwater fluxes indicated that the rate of mineralization was 37.6 mg N/m2 day. The accumulation of toxic ammonia was prevented through the nitrification process (26.6 mg N m−2 day−1). The decrease of NO3–N flux in groundwater in the riparian zone was calculated to be 56.1 mg N m−2 day−1 (20.48 g N m−2 year−1). Phosphate was adsorbed to sediments and its load to the drainage canal was minimized. Harvesting of above ground reed biomass in mid June, when maximum standing stock of nutrients was attained for both plants, would remove 2.73 g P m−2 and 11.2 g N m−2. All the phosphorous (1.39 g P/m2 year−1) and 76.5% of the nitrate nitrogen (14.64 g N m−2 year−1) entering the drainage canal was taken up by plants. Drainage canal management is suggested as an efficient low cost–high gain agri-environmental measure, which is easy to be adapted by farmers, to reduce diffuse nutrient pollution.
Environmental impactIn this work, the efficiency of natural attenuation of nutrients (i.e. denitrification and adsorption of phosphates) and phytoremediation (P.australis and A.donax nutrient uptake and harvesting the above-ground biomass) was assessed in a Mediterranean drainage canal. Drainage canal management was suggested as an efficient low cost–high gain agri-environmental measure, which can be easily adapted by farmers, to reduce diffuse nutrient pollution. This work aimed at improving our understanding of the biogeochemical cycles of nutrients in the drainage canal ecosystem and supporting the design and implementation of watershed water quality protection technologies, based on natural attenuation mechanisms. |
1. Introduction
Nitrogen (N) and Phosphorous (P) inputs are essential for increasing agricultural production and maintaining the economic viability of farming systems worldwide. Increases in worldwide use of N fertilizers combined with average N use efficiencies of 50% have contributed to the eutrophication of surface and coastal waters. Fertilized farmland is frequently the main non-point source of nitrogen and phosphorous excess input to surface and groundwater ecosystems.6 A number of approaches have been identified to reduce nutrient (especially nitrate) losses to surface waters including controlled drainage, diverting or directing drainage discharge through natural or constructed wetlands, bioreactors (zones that surround or border the drain pipes) and in-stream denitrification.21,32,4,7,8,25,14Agricultural drainage canals have been used in poorly drained agricultural landscapes for the regulation of water retention to allow crop production and to mitigate pollution (nutrients, pesticides and herbicides) as well as for erosion prevention. Drainage canals provide habitat for both aquatic and terrestrial biota and operate as a nutrient pool due to the decomposition of organic matter (lacking otherwise in dry and intensively managed agricultural areas).
Drainage canals, usually situated in river deltas, are areas of accumulation for organic debris (sediment deposition) and growth of macrophytes, such as Phragmites australis (common reeds) and Arundo donax (giant reeds). Such areas have suitable anaerobic conditions and electron donors for denitrification.15 In addition, plants (like reeds) can also promote phosphorous adsorption onto the sand and prevent ammonia accumulation by the release of oxygen from their roots. The removal of N in riparian wetlands, zones, strips and drainage canals is mainly attributed to denitrification. Therefore, drainage canals are likely to act both as narrow buffers in filtering runoff waters and as phosphorous pools during the dormant stage. Although ditch performance has been shown to be highly variable,19 no holistic studies are available on the functioning of small field drains, with or without permanent water.14
Plant N and P uptake is often considered less important compared to mitigation of nutrients in riparian buffers due to denitrification and phosphate adsorption in sediments. Most of the nutrients taken up by vegetation are released back into the water once the vegetation dies and decomposes.31 On the other hand, a pan-European study demonstrated that annual N retention in vegetation and litter accounts for 13–99% of the total mitigation.13 Although higher N uptake and retention is found in forested buffers, periodic harvesting of herbaceous biomass contributes considerably to the retention of N. It has been shown that plants, like reeds, take in nutrients during the growing period and release them back into the aquatic environment and their roots after the foliar period.11 Consequently, cutting the reeds at the proper time results in the overall reduction of nutrients in the receiving surface water bodies.23
The chemistry of such systems is very complex with dissolved, colloidal and particulate materials biogeochemically interacting within soils, sediments, and organisms. Elucidating the function of drainage canals in the removal of chemicals will assist in the design and implementation of water quality protection technologies22 based on natural attenuation mechanisms. In Greece, 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hectares of such canals exist in the delta plains of Evrotas, Achelloos, Axios and other rivers (http://www.minagric.gr). The objective of this research was to assess the efficiency of natural attenuation of nutrients in a Mediterranean drainage canal.
000 hectares of such canals exist in the delta plains of Evrotas, Achelloos, Axios and other rivers (http://www.minagric.gr). The objective of this research was to assess the efficiency of natural attenuation of nutrients in a Mediterranean drainage canal.
2. Methodology
The drainage canal under study was located in Evrotas River delta in Greece (Skala region) and drained fields of orange groves (4500 m2). The length of the canal was 180 m and the width of the vegetated zone was approximately 1.5 m. The average density growth of both Phragmites australis and Arundo donax was 15 clones per m2. Plants covered two distinct areas of 160 and 20 m length for Phragmites australis and Arundo donax, respectively.To monitor the temporal 3-dimensional variability of hydrology and chemistry of surface and ground water in the drainage canal, eleven multi-level (3, 4 and 5 m) wells were installed (Fig. 1). Field sampling (groundwater and surface water sampling) was conducted in order to assess the fate and transport of nutrients as they move from the groundwater to the drainage canal. In addition, laboratory studies were used to assess the biogeochemical processes that control the cycles of nitrogen and phosphorous and evaluate the efficiency of the sediments to attenuate pollutants. Finally, the fluxes in nutrient (nitrogen and phosphorous) uptake by Phragmites australis and Arundo donax were measured on a monthly basis in order to determine the timing of reed harvesting that will maximize the removal of nutrients by plant uptake but also keep the N/P ratio high enough to avoid toxic algal blooms.24
 | ||
| Fig. 1 Multi-level probe design in relation to drainage canal. | ||
Field hydrologic studies
The water depth of the wells was monitored on a monthly basis. The hydraulic characteristics of the sub-surface were determined by conducting single-well pumping tests and the infiltration capacity of the fields was estimated by conducting in situ infiltration experiments. Horton's equation was used to obtain the infiltration rate (f = fc + (fco − fc) e(−kt)) where fco and fc are the initial and final infiltration rates and k is an empirical constant. The surface runoff (overland flow) to the drainage canal was estimated by converting the measured daily precipitation in the region (Elos station, at 4 m altitude) to an hourly precipitation (WDMUtil version 2.2, Basin) and then subtracting the infiltration rate. Potential water deficit was also estimated by subtracting daily potential evapotranspiration (PET) (Hamon's equation) from precipitation.Surface and ground water chemistry monitoring
The multilevel wells and the drainage canal water were sampled every 2 months (11/06, 1/07, 3/07, 5/07, 7/07, 11/07, 3/08, 5/08), with a peristaltic pump with low flow (< 1 L min−1), so as to maintain turbidity at minimum levels, and the physicochemical parameters pH, temperature, conductivity, dissolved oxygen (DO), and redox potential (Eh) were measured in situ using the following electrodes: Orion 9107 pH meter, Orion 081010 DO meter, and Orion 011050 conductivity meter. The samples were filtered through a 0.45 μm nylon filter and analyzed using a Hack 2010 spectrophotometer for nitrates (NO3–N, cadmium reduction method, 8039), nitrites (NO2–N, diazotization method, 8507), ammonia (NH3–N, salicylicate method, 10023), phosphates (PO4–P, phosVer3 method, 8048), total phenols (Folin Ciocalteu method), dissolved organic carbon (DOC, direct method Patent Pending, 10129 or by a TOC analyzer-Shimadzu 5050, after the removal of inorganic carbon by air sparging for 10 min), chemical oxygen demand (COD, reactor digestion method, 8000), and total nitrogen (TN, TNT persulfate digestion method, 10071) or Kheldalh nitrogen (TKN, by the Kjeldahl digestion technique with a Hach digestahl digestion apparatus, Nessler method, 8075). Dissolved organic nitrogen (DON) was derived by the abstraction of ammonia from the TKN.Laboratory process studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 soil to water ratio), and particle size distribution (wet sieving, 2–0.063 mm). When the soil sample was fine (less than 63 μm), Laser Diffraction Size Analysis was conducted. Bulk chemical analysis was conducted (by energy dispersive X-ray fluorescence sprectroscopy (EDS-XRF), 174 using a Bruker S2 Ranger XRF spectrometer and major elements (Si, Al, Ti, Fe, Mn, Mg, Ca, 175 Na, K, and P) were determined by using fusion beads. Soil organic C was determined by the Walkley–Black (WB) acid dichromate digestion technique (Soil Survey Laboratory Methods Manual, 2004) and Total Kjeldahl N and Total Phosphorous by the Kjeldahl digestion technique. All sediment analysis was run in triplicates.
2.5 soil to water ratio), and particle size distribution (wet sieving, 2–0.063 mm). When the soil sample was fine (less than 63 μm), Laser Diffraction Size Analysis was conducted. Bulk chemical analysis was conducted (by energy dispersive X-ray fluorescence sprectroscopy (EDS-XRF), 174 using a Bruker S2 Ranger XRF spectrometer and major elements (Si, Al, Ti, Fe, Mn, Mg, Ca, 175 Na, K, and P) were determined by using fusion beads. Soil organic C was determined by the Walkley–Black (WB) acid dichromate digestion technique (Soil Survey Laboratory Methods Manual, 2004) and Total Kjeldahl N and Total Phosphorous by the Kjeldahl digestion technique. All sediment analysis was run in triplicates.
3. Results
Hydrologic balance
The velocity of ground water was determined to be 0.062 m day−1 (travel time, 16 d m−1) and close to drainage canal-where the gradient is steeper-was 0.354 m day−1 (travel time, 3 d m−1) using measured hydraulic conductivities (0.691 m day−1 for groundwater, and 0.587 m day−1 for drainage canal recharge) and hydraulic gradients established by the piezometric heads of the water table. The steady state infiltration rate was estimated using Horton's equation to be 0.0135 cm min−1 and the constant K was 0.125 ± 0.003 cm min−1. Average potential evapotranspiration was 899 ± 54.7 mm, and precipitation was 543 ± 199 for the hydrologic years 2000–01 to 2006–07. Precipitation during 2006–2007 was 425 mm, while for 2007–2008 (until 31/05/2008) was 492 mm, indicating dry conditions. The estimated average potential water deficit for the studied region is presented in Fig. S1 of the ESI‡. The corresponding variation in the piezometric heads of groundwater is presented in Fig. S2 of the ESI‡. Surface runoff to the drainage canal was estimated to take place only during precipitation events higher than 25 mm day−1 (Table S1, ESI‡). It was estimated that the annual runoff from the drainage canal was 2521 m3 y−1 based on field measurements and mass balance modeling (for the calendar year 2007); 86% originated from groundwater and the remainder from overland flow and direct precipitation, equally.Surface and ground water chemistry monitoring
Total and seasonal averages results of the eight sampling sessions of ground and surface water are presented in Table 1 and Fig. 2 (detailed data are found in Tables S2 and S3 in the ESI‡), respectively. Groundwater of the orange grove field was anoxic with high COD, total phenols, DOC and DON and ammonia and seasonally with nitrates. The high organic load was due to the type of soil which is turf. The drainage canal had significantly lower concentrations of ammonia, COD, and DOC. Nitrate concentrations in drainage canal were less than in ground water with the exception when there was significant contribution from surface runoff. There was generally a consistent decrease of pollutants between ground water and drainage canal suggesting natural attenuation mechanisms in action. Dissolved organic N ranged, on average (sampling sessions), from 34 to 84% of total N for ground water. The average ratio of DOC to DON in ground water was relatively low ranging from 3.6 to 33.8 with a total average of 15, suggesting an abundance of organic N.| Ground water | Drainage Canal | |
|---|---|---|
| Number of sampling sessions | 7 | 5 |
| Number of total samples | 105 | 7 |
| Temperature (°C) | 19.6 (2.5) | 18.9 (2.3) |
| pH | 7.23 (0.11) | 7.31 (0.20) |
| DO/mg L−1 | 1.6 (0.6) | 6.1 (1.7) |
| Conductivity/μS cm−1 | 941 (227) | 758 (255) |
| Eh/mV | 111.0 (37.7) | 171.4 (26.0) |
| COD/mg L−1 | 18.8 (20.3) | 6.8 (5.1) |
| NO2–N/mg L−1 | 0.030 (0.089) | 0.006 (0.002) |
| NO3–N/mg L−1 | 0.842 (2.360) | 4.034 (5.479) |
| NH3–N/mg L−1 | 0.352 (0.277) | 0.035 (0.015) |
| PO4–P/mg L−1 | 0.093 (0.073) | 0.109 (0.153) |
| Total phenols/mg L−1 | 1.648 (1.084) | 1.119 (0.724) |
| DOC/mg L−1 | 10.8 (7.6) | 3.6 (2.8) |
| DON/mg L−1 | 3.024 (1.645) | 2.297 (1.772) |
| DIN/mg L−1 | 1.201 (2.433) | 4.075 (5.471) |
| DON/TDN | 0.71 (0.22) | 0.48 (0.40) |
| DOC/DON | 14.5 (21.7) | 2.9 |
| DIN/DIP molar ratio | 58.3 (121.5) | 326.1 (358.1) |
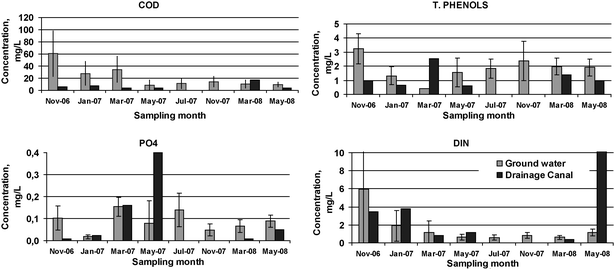 | ||
| Fig. 2 Seasonal averages from eight sampling sessions of physicochemical parameters of surface water and ground water underneath drainage canal at Skala. | ||
The molar DIN/DIP ratio for ground water was highly variable, ranging, on average (sampling sessions), from 10 to 288, with an average of 58, whereas the ratio for the drainage canal ranged from 6 to 849, suggesting P limitation to eutrophication. The drainage canal phosphate concentrations were also highly variable, ranging from 9 μg L−1 to 399 μg L−1, and exceeded the eutrophication criteria for lakes (20 μg L−1). The drainage canal was oligotrophic due to reeds’ P uptake and once the reeds were cut (December 2006) it became successively mesotrophic to eutrophic.
Sediment physicochemical characteristics
The results of the physicochemical characterization of the sediment are presented inTable S4 of the ESI‡. The pH of the sediment was slightly basic (7.64), while conductivity was not high (218 mS cm−1). Dry bulk density was estimated to be 1125 kg m−3. The texture of the sediment was silty. Total organic carbon content was 11434 mg kg−1 whereas total Kjeldahl nitrogen (TKN) was 1886 mg kg−1 and the total phosphorous is 3124 mg kg−1. Therefore, the organic matter was enriched in nitrogen and phosphorous and the C/N ratio was 6. The bulk chemical analysis (XRF results) indicated that sediments contained mostly aluminium (15.5%) and silicon (54.8%) oxides, while the high percentage of loss of ignition implied high content of organic matter.The sediment samples contained significant amounts of exchangeable nitrogen content, 4.65 ± 0.36 mg NH3-N kg−1, 17.79 ± 8.39 mg NO3-N kg−1, and 56.53 ± 7.18 mg DON kg−1. Short term PMN and PTSN was also significant, 15.21 and 73.73 mg N kg−1, respectively. Anaerobic conditions prevented nitrification during the experiment. Mineralization rate, estimated by the leaching kinetic experiment (Fig. 3), was found to be 0.21 mg N L−1 d−1, and the total capacity (adjusted for 7 d) was 10 mg N kg−1 sediment, which was consistent with the short term PMN values. The partitioning coefficient (kd), mL g−1, for EMN, and PMN, was 400 and 600 mL g−1, respectively, while for DON, it was much lower at 200 mL g−1, indicating the trend of the sediment to release DON. The sediment released 80 mg DON kg−1 sediment (Fig. 3). The aromaticity estimated in the leachate of the PMN test (ArI–DOC, 1.169 ± 0.052 L mg−1 C m, ArI DON, 280, 3.076 ± 0.431 L mg−1 N m) compared with the aromaticity observed in a range of Greek agricultural soils27 could be considered to be of low-medium class explaining the enhanced mineralization response of the sediment. The decline in NO3-N concentration, observed in the kinetic experiment, could be attributed to denitrification since dissolved oxygen was negligible and the redox potential was below 100 mV. Finally, the redox potential (Eh) of the sediment reached values lower than −50 mV in 200 h, (Fig. S3 in the ESI‡) suggesting the potential for denitrification under anaerobic conditions and available electron donors.
 | ||
| Fig. 3 Kinetic release of dissolved nitrogen forms and dissolved organic carbon. | ||
The sediment also released small quantities of phosphates (0.465 ± 0.265 mg PO4-P kg−1) as it was indicated from the leaching experiment. On the other hand, it had a large capacity to adsorb phosphorous and no plateau was reached in the sorption experiment (Fig. S4 ESI‡). The partitioning coefficient (kd) was estimated to be 300 mL g−1 and the retardation factor 1092. Equilibrium P concentration (EPC0) was estimated to be 0.08 mg L−1 consistent with the value obtained from the empirical equation of Smith et al.,.26
Phragmites Australis and Arundo Donax temporal nutrient content
Nutrient (TKN and total P) concentration of Phragmites australis (averages for plots S2 and S3) and Arundo donax (plot S1) for the three parts (upper, middle, lower) of the above-ground biomass and the below-ground biomass (roots and rhizomes) for plot S1 are presented in Fig. 4 (growing periods of 2007 and 2008). | ||
| Fig. 4 Temporal variation of shoot content in N and P of a) A.donax (S1) and b) P.australis (S2–S3) from May to September. | ||
Nutrient concentration patterns were very similar for both growing periods. Maximum N concentration was observed in March/April for Phragmites australis (S2–S3 plots, approx. 30–31, 12–18, 6–8 g kg−1 for upper, middle, and lower biomass respectively for the two growing seasons), while for Arundo donax the exact time was not distinct but seemed to be in April/May (S1 plots, approx. 26–39, 17–19, 10–17 g kg−1 for upper, middle, and lower biomass, respectively). On the other hand, maximum P concentrations for the upper and middle parts were in May for the S1 plot and March/April for the S2–S3 plots (approx. S1: 4.5–5 and 3.2–4 g kg−1, S2–S3: 4–5.6, and 3–4.8 g kg−1 for upper, and middle biomass respectively).
In subsequent months, there was a gradual decrease in P concentration of these two parts and an increase in that of lower part and below-ground biomass, while nitrogen concentration decreased for the three parts of the above ground plants biomass. This decrease of nutrient concentration could be partially attributed to dilution due to increasing biomass, since maximum biomass was usually observed after maximum nutrient concentrations were reached. In addition, there was also the possibility that P had returned to the roots as it has been reported in other studies,20 where 25–50% of phosphorous was considered to be translocated from stems and leaves to roots for fertilization of next season's growth.11
In general, during the monitoring period, nutrient concentrations were higher in the upper part and lower in the lower part of above ground biomass, apart from certain periods of low concentrations, where concentration values among the three parts were relatively identical (15/2/2008, data not shown, and 1/8/2008). The upper part (leaves) had higher N (and not P) content and a N/P molar ratio compared to the middle and lower parts indicating the N requirement of leaves for chlorophyll formation. On the other hand, during growth periods where there was a great need of P for the formation of new tissues, the N/P ratio was decreased in the above ground biomass, and then remained relatively constant.
During the growing season in 2008, the biomass was at a maximum soon after the maximum concentrations in June for P.australis (47 g per reed clone, 705 g m−2) and in late July for A.donax (204 g per reed clone, 3.1 kg m−2) (Fig. S5 in the ESI‡) in accordance with other studies which also showed maximum reed biomass in early summer.10,12 Above-ground biomass has been found to range from 97 g m−2 (pure nutrient substrate, translocation ecotype)20 to 1500 g m−2 (rich nutrient substrate, assimilation ecotype)17 in August (Table S5 in the ESI‡) for P.australis.2,3,17,18,20,34 On the other hand, maximum peak standing stock of nutrients was attained in June for both plants (A.donax: 432 mg P per shoot and 2023–2132 mg N per shoot in July, P.australis: 151 mg P per shoot, 586 mg N per shoot) (Fig. 4). Converting these contents to mg g−1 DW (dry weight), then P.australis exhibited 12.4 mg N g−1 DW and 3.2 mg P g−1 DW, while A.donax exhibited 18.4 mg N g−1 DW and 3.74 mg P g−1 DW. In the literature, nutrient contents of P.australis were observed during the summer in the range of 17.5–24.3 mg N g−1 DW and 1.3–3.14 mg P g−1 DW. Accounting for the reed density, the areal nutrient content was 8.78 g N m−2 and 2.26 g P m−2 for P.australis and 30.34 g N m−2 and 6.48 g P m−2 for A.donax, which were consistent with literature (Table S6 in the ESI‡) values that range between 17.8 and 35 g N m−2 and 0.96–3 g P m−2.1,16–18,29,35
4. Discussion
Nitrogen buffering processes
Drainage canals are areas of accumulation for organic matter (source of nutrients for microrganisms) due to erosion and growth of plants such as Phragmites australis and Arundo donax, that are important for nitrogen microbial processes (mineralization, nitrification, denitrification). In the drainage canal under study, the substrate was turf enriched in organic nitrogen. Groundwater exhibited high levels of DOC (approx. 14 mg L−1) and DON (approx. 2.5 mg L−1). Mineralization of organic nitrogen (15 mg kg−1 PMN, 0.21 mg L−1 d) was enhanced due to low aromaticity of DON which was released from the sediments. The reduction of groundwater DON flux passing through the riparian zone was an estimation of mineralized nitrogen for the study period and it was estimated to be on average 37.6 mg N m−2 day−1 (13.72 g m−2 year−1).Nitrification is an important aerobic process for the prevention of toxic ammonia accumulation. The nitrification process was occurring (even though the sediments redox state was anaerobic) due to the release of oxygen by the reed's roots. Oxygen release from the roots of macrophytes to the surrounding substrate has a positive influence on plant growth by oxidizing reduced, phytotoxic metabolites in the substrate (e.g. S2−, Fe2+, Mn2+),33 promoting phosphorous adsorption onto the sand and preventing ammonia accumulation. The reduction of groundwater ammonia flux passing through the riparian zone indicated that the amount of nitrified nitrogen during the study period was on average 26.6 mg N m−2 day−1 (9.72 g N m−2 year−1). Denitrification was the main process responsible for the buffering capacity of drainage canals against diffuse nitrate pollution.15 Denitrifers require in addition to electron donors, anaerobic and reductive conditions. Such conditions were observed in our case, since the groundwater exhibited both low dissolved oxygen (mean value 1.6 mg L−1) and redox potential (mean value 111 mV, range: −182.5 to +340.8 mV) (Table 1). Moreover, sediment redox potential under anoxic conditions was also low, at −50 mV (Fig. 4, S1). The reduction of groundwater NO3-N flux passing through the riparian zone was on average 56.1 mg N m−2 day−1 (20.48 g N m−2 year−1). This nitrogen was removed from the system before entering the surface water. These fluxes were similar to other studies found in the scientific literature.9,28
Phosphates buffering processes
Sediments showed a large capacity to adsorb phosphorous. The DIP concentration in groundwater was higher than the equilibrium concentration (EPC0 = 0.08 mg L−1) and the groundwater phosphate load was retained by the sediments. On the other hand, the levels of phosphorous in the drainage canal were seasonally below the EPC0 making the process inactive. Root oxygen release was also important for adsorption as it oxidized the soluble Fe2+ to the Fe3+ which precipitated as oxyhydroxides and bound phosphate. Carlyle et al.5 suggested that low soluble reactive phosphorous (SRP) concentrations occurred in groundwater with DO concentrations > 3 mg L−1 and low Fe2+, and, on the contrary, high SRP concentrations of > 0.05 mg L−1 were associated with low DO and high Fe2+ concentrations in areas of buried channel sediments near the river bank. In this study, PO4-P ranged from 0.009 (method detection limit) to 0.437 mg L−1, with DO from 0.45 to 5.00 mg L−1 and ORP from 140 to −215 (outlier −382). When the DO was higher than 3 mg L−1 the PO4-P ranged from 0.058 to 0.183 mg L−1. However, there was no correlation of PO4-P concentrations higher than EPC0 with DO and ORP. Thus, we could assume that the redox potential enhances denitrification and not iron (Fe3+) reduction.Management issues of reed biomass
Harvesting of above-ground biomass in June, when peak nutrient content of reeds was observed and N/P ratio of surface water was high enough (Table S3 in the ESI‡) to avoid toxic algal blooms, would remove 0.74 kg P (2.73 g P m−2) and 3.02 kg N (11.2 g N m−2). In total, 76.5% of nitrate nitrogen (14.64 g N m−2 year−1) and phosphorous (1.39 g P m−2 year−1) entering the drainage canal would be removed by plant uptake. The suggested time for harvesting was also consistent with the period suggested by the Hellenic Ornithological Society for the harvesting of reeds in the region (15th of June to the 30th of September, personal communication with the Hellenic Ornithological Society) that was determined by considering the ecological functioning of the habitat and it was also in accordance with Valkama et al.305. Conclusions
This field and laboratory study revealed that in the riparian zone of the agricultural drainage canal under study in the Evrotas river delta, natural attenuation mechanisms (denitrification and adsorption of phosphates), as well as phytoremediation (P.australis and A.donax nutrient uptake and harvesting of their above ground biomass), could remove significant quantities of N and P that would otherwise follow their path to the drainage canal and eventually to the sea. The harvesting of above-ground biomass of reeds (P.australis and A.donax) in the drainage canals of the alluvial Evrotas river plain was suggested to take place in mid June when peak standing stock of nutrients was attained for both plants P.australis and A.donax, the N/P ratio of surface water was high enough to avoid toxic algal blooms, and water depth is shallow enough so that root aeration will not be affected. Overall, drainage canal management is suggested as an efficient low cost–high gain agri-environmental measure, which is easy to be adapted by farmers, to reduce diffuse nutrient pollution. | ||
| Fig. 5 Temporal variation of standing stock of nutrients of (a) A.donax (S1) and (b) P.australis (S2–S3) from May to September. | ||
Acknowledgements
This work is a part of the European Union funded project LIFE05-EnviFriendly (http://www.EnviFriendly.tuc.gr). Mrs Stamati was financially supported by a PhD fellowship from the Bodosaki Foundation. We would like also to acknowledge Anna Androulaki, Konstantinos Annousis, George Katsimalis, Vassilios Papadoulakis, Elpida Peroulaki, Ourania Tzoraki, and Katerina Valta, for field and laboratory assistance.References
- I. L. Bayly and T. A. O'Neill, Seasonal ionic Fluctuations in a Phragmites communis community, Can. J. Bot., 1972, 50, 2103–2109 CrossRef CAS.
- G. Bjorndahl, Influence of winter harvest on stand structure and biomass production of the common reed, Phragmites australis (Cav.) Trin.ex. Steud. In Lake Takern, Southern Sweden, Biomass, 1985, 7, 303–319 CrossRef.
- P. Boszke, K. Bociąg and J. Szmeja, Population structure and regeneration of Phragmites australis (Cav.) Trin. ex Steud. in flood control ditches in the depression wetland (Żuławy Wiślane, northern Poland), Pol. J. Ecol., 2005, 53, 3–12 Search PubMed.
- M. R. Burchell, R. W. Skaggs, G. M. Chescheir, J. W. Gilliam and L. A. Arnold, Shallow subsurface drains to reduce nitrate losses from drained agricultural lands, Transactions of the ASAE, 2005, 48, 1079–1089 Search PubMed.
- G. C. Carlyle and A. R. Hill, Groundwater phosphate dynamics in a river riparian zone: effects of hydrologic flowpaths, lithology and redox chemistry, J. Hydrol., 2001, 247, 151–168 CrossRef CAS.
- European Environment Agency. 1999. Nutrients in European eco- systems. Environmental Assessment Rep. 4. European Environ. can occur. Agency, Copenhagen Search PubMed.
- R. O. Evans, W. R. Skaggs and W. J. Gilliam, Controlled versus conventional drainage effects on water quality, Journal of Irrigation and Drainage Engineering, 1995, 121, 271–276 Search PubMed.
- N. R. Fausey, K. W. King, B. J. Baker, R. L. Cooper, Controlled drainage performance on Hoytville soils. Proceedings of the Eighth International Drainage Symposium ASAE, St. Joe, MI ( 2004), p. 49085 Search PubMed.
- E. Fustec, A. Mariotti, X. Grillo and S. Sajus, Nitrate removal by denitrification in alluvial round water: role of a former channel, J. Hydrol., 1991, 123, 337–354 CrossRef CAS.
- J. García-Pintado, M. Martínez-Mena, G. G. Barberá, J. Albaladejo and V. M. Castillo, Anthropogenic nutrient sources and loads from a Mediterranean catchment into a coastal lagoon: Mar Menor, Spain, Sci. Total Environ., 2007, 373, 220–239 CrossRef CAS.
- W. Graneli and D. Solander, Influence of aquatic macrophytes on phosphorous cycling in lakes, Hydrobiologia, 1988, 170, 245–266 CAS.
- W. Graneli, S. E. B. Weisner and M. D. Sytsma, Rhizome dynamics and resource storage in Phragmites australis, Wetlands Ecology and Management, 1992, 1, 239–247.
- M. M. Hefting, J. C. Clement, P. Bienkowski, D. Dowrick, C. Guenat, A. Butturini, S. Topa, G. Pinay and J. T. A. Verhoeven, The role of vegetation and litter in the nitrogen dynamics of riparian buffer zones in Europe, Ecological Engineering, 2005, 24, 465–482 CrossRef.
- I. Herzon and J. Helenius, Agricultural drainage ditches, their biological importance and functioning, Biological Conservation, 2008, 141, 1171–1183 Search PubMed.
- K. M. Hiscock and T. Grischek, Attenuation of groundwater pollution by bank filtration, J. Hydrol., 2002, 266, 139–144 CrossRef CAS.
- I. Kufel and L. Kufel, Heavy metals and mineral nutrient budget in Phragmites Australis and Typha Angustifolia, Symposia Biologica Hungarica, 1985, 29 Search PubMed.
- H. Kuhl, P. Woitke and J. G. Kuhl, Strategies of Nitrogen Cycling of Pragmites australis at two sites differing in Nutrient Availability, Int. Revue ges. Hydrobiologia, 1997, 82, 57–66 Search PubMed.
- V. Kuusemets and K. Lohmus, Nitrogen and phosphorous accumulation and biomass production by Scirpus sylvaticus and Phragmites australis in a horizontal subsurface flow constructed wetland, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2005, 40, 1167–1175 Search PubMed.
- P. Legacherie, O. Diot, N. Domange, V. Gouy, C. Floure, C. Kao, R. Moussa, J. M. Robbez-Masson and V. Szleper, An indicator approach for describing the spatial variability of artificial stream networks with regard to herbicide pollution in cultivated watersheds, Ecological Indicators, 2006, 6, 265–279 Search PubMed.
- I. Lippert, H. Rolletschek, H. Kühl and J. G. Kohl, Internal and external nutrient cycles in stands of Phragmites australis– a model for two ecotypes, Hydrobiologia, 1999, 408–409, 343–348 CrossRef.
- C. C. Madramootoo, W. R. Johnston, J. E. Ayars, R. O. Evans and N. R. Fausey, Drainage Water Quality Issues In North America, Journal of Irrigation and Drainage Engineering, 2007, 56, 535–545 Search PubMed.
- B. A. Needeman, P. J. A. Kleinman, J. S. Strock and A. L. Allen, Improved management of agricultural drainage ditches for water quality protection: an overview, J. Soil Water Conserv, 2007, 62, 171–178 Search PubMed.
- N. P. Nikolaidis, T. Koussouris, T. E. Murria, I. Bertahas, A. Diapoulus and G. Konstantinos, Seasonal Variation of Nutrients and Heavy Metals in Phragmites australis of Lake Triclonis, Greece, Lake and Reservoir Management, 1996, 12, 364–370 Search PubMed.
- N. P. Nikolaidis, A. P. Karageorgis, V. Kapsimalis, G. Marconis, P. Drakopoulou, H. Kontoyiannis, E. Krasakopoulou, Al. Pavlidou and K. Pagou, Circulation and nutrient modeling of Thermaikos Gulf, Greece, Journal of Marine Systems, 2006, 60, 51–62 Search PubMed.
- R. W. Skaggs, M. A. Youssef, G. M. Chescheir and J. W. Gilliam, Effect of drainage intensity on nitrogen losses from drained lands, Transactions of the ASABE, 2005, 48, 2169–2177 Search PubMed.
- D. R. Smith, B. E. Haggard, E. A. Warnemuende and C. Huang, Sediment phosphorous dynamics for three tile fed drainage ditches in Northeast Indiana, Agricultural Water Management, 2005, 71, 19–32 Search PubMed.
- F. E. Stamati, N. P. Nikolaidis, D. Venieri, E. Psillakis, N. KalogerakisEffects of de-vegetation on biochemical quality and soluble organic matter of calcaric Mediterranean soils: Implications for water quality. Water Research (submitted) Search PubMed.
- M. R. Trudell, R. W. Gillham and J. A. Cherry, An in situ study of the occurrence and rate of denitrification in a shallow unconfined sand aquifer, J. Hydrol., 1986, 83, 251–268 CrossRef CAS.
- K. E. Ulrich and T. M. Burton, The effects of nitrate, phosphate and potassium fertilization on growth and nutrient uptake patterns of Phragmitas australia (Car.) Trin. ex Steudel, Aquat. Bot., 1985, 21, 53–62 CrossRef.
- E. Valkama, S. Lyytinen and J. Koricheva, The impact of reed management on wildlife: A meta-analytical review of European studies, Biological Conservation, 2008, 141, 364–374 Search PubMed.
- L. V. Verchot, E. C. Franklin and J. W. Gilliam, Nitrogen cycling in piedmont vegetated filter zones.1. Surface soil processes, Journal of Environmental Quality, 1997, 26, 327–336 CAS.
- D. Wang, Z. Chen, J. Wang, S. Xu, H. Yang, H. Chen, L. Yang and L. Hu, Summer-time denitrification and nitrous oxide exchange in the intertidal zone of the Yangtze Estuary, Estuarine Coastal and Shelf Science, 2007, 73, 43–53 CrossRef.
- S. E. B. Weisner, Factors affecting the internal oxygen supply of Phragmites australis (Cav.) Trin. ex Steudel in situ, Aquat. Bot, 1988, 31, 329–335 CrossRef.
- S. E. B. Weisner and W. Graneli, Influence of substrate conditions on the growth of Phragmites australis after a reduction in oxygen transport to below-ground parts, Aquat. Bot, 1989, 35, 71–80 CrossRef.
- S. E. B. Weisner, Effects of an organic sediment on performance of young Phragmites australis clones at different water depth treatments, Hydrobiologia, 1996, 330, 189–194 CrossRef CAS.
Footnotes |
| † Part of a themed issue dealing with water and water related issues. |
| ‡ Electronic supplementary information (ESI) available: Fig. S1–S5 and Tables S1–S6. See DOI: 10.1039/b913083g |
| This journal is © The Royal Society of Chemistry 2010 |
