Trace elements in recent groundwater of an artesian flow system and comparison with snow: enrichments, depletions, and chemical evolution of the water†
William
Shotyk
*a,
Michael
Krachler
a,
Werner
Aeschbach-Hertig
b,
Stephen
Hillier
c and
Jiancheng (James)
Zheng
d
aInstitute of Earth Sciences, University of Heidelberg, INF 236, D-69120, Heidelberg, Germany. E-mail: William.Shotyk@geow.uni-heidelberg.de; Fax: +49 (6221) 54 5228; Tel: +49 (6221) 54 4803
bInstitute of Environmental Physics, University of Heidelberg, INF 229, D-69120, Heidelberg, Germany
cMacaulay Institute, Craigiebuckler, Aberdeen, AB15 8QH, Scotland
dGSC Northern Canada, Geological Survey of Canada, 601 Booth Street, Ottawa, Ontario K1A 0E8, Canada
First published on 14th September 2009
Abstract
Snow samples collected from hand-dug pits at two sites in Simcoe County, Ontario, Canada were analysed for major and trace elements using the clean lab methods established for polar ice. Potentially toxic, chalcophile elements are highly enriched in snow, relative to their natural abundance in crustal rocks, with enrichment factor (EF) values (calculated using Sc) in the range 107 to 1081 for Ag, As, Bi, Cd, Cu, Mo, Pb, Sb, Te, and Zn. Relative to M/Sc ratios in snow, water samples collected at two artesian flows in this area are significantly depleted in Ag, Al, Be, Bi, Cd, Cr, Cu, Ni, Pb, Sb, Tl, V, and Zn at both sites, and in Co, Th and Tl at one of the sites. The removal from the waters of these elements is presumably due to such processes as physical retention (filtration) of metal-bearing atmospheric aerosols by organic and mineral soil components as well as adsorption and surface complexation of ionic species onto organic, metal oxyhydroxide and clay mineral surfaces. In the case of Pb, the removal processes are so effective that apparently “natural” ratios of Pb to Sc are found in the groundwaters. Tritium measurements show that the groundwater at one of the sites is modern (ie not more than 30 years old) meaning that the inputs of Pb and other trace elements to the groundwaters may originally have been much higher than they are today; the M/Sc ratios measured in the groundwaters today, therefore, represent a conservative estimate of the extent of metal removal along the flow path.
Lithogenic elements significantly enriched in the groundwaters at both sites include Ba, Ca, Li, Mg, Mn, Na, Rb, S, Si, Sr, and Ti. The abundance of these elements can largely be explained in terms of weathering of the dominant silicate (plagioclase, potassium feldspar, amphibole and biotite) and carbonate minerals (calcite, dolomite and ankerite) in the soils and sediments of the watershed. Arsenic, Mo, Te, and especially U are also highly enriched in the groundwaters, due to chemical weathering: these could easily be explained if there are small amounts of sulfides (As, Mo, Te) and apatite (U) in the soils of the source area. Elements neither significantly enriched nor depleted at both sites include Fe, Ga, Ge, and P.
Environmental impactUsing the clean lab methods developed for arctic ice, snow samples collected from hand-dug pits at two sites in Simcoe County, Ontario, Canada were found to be highly enriched in potentially toxic, chalcophile elements such as Ag, As, Bi, Cd, Cu, Mo, Pb, Sb, Te, and Zn. Relative to snow, water samples collected at two artesian flows in this area are significantly depleted in Ag, Al, Be, Bi, Cd, Cr, Cu, Ni, Pb, Sb, Tl, V, and Zn. The differences between the enrichments in snow versus groundwater is probably a testimony to the remarkable natural filtration ability of the forest soils of the recharge area. Tritium measurements show that the groundwater at one of the sites is modern (ie not more than 30 years old) meaning that metal removal along the flow path is not only efficient, but also rapid. |
Introduction
The importance of human impacts on the environmental geochemical cycles of potentially toxic trace metals such as Pb has stimulated interest in the use of arctic ice as archives of global change.1–4 Measuring trace metals reliably at the concentrations found in polar snow and ice requires not only tremendous analytical sensitivity, but extreme care must be taken to avoid sample contamination.5 However, with the latest advances in clean lab methods and inductively coupled plasma sector field mass spectrometry (ICP-SMS), it has become possible to measure not only the concentrations of Pb and most other elements of contemporary environmental relevance,6,7 but also Pb isotope ratios,8–10 in arctic snow and ice.An understanding of the significance of metals such as Pb in the environment requires not only quantitative information about the chronology and intensity of their deposition from the atmosphere to the biosphere, but also their subsequent release to the hydrosphere.11 Biological availability of metals in the environment depends on bioaccessibility, and this is largely dictated by their abundance and chemical speciation in natural waters. With atmospheric contamination by trace metals recognised as a global phenomenon,12 there is increasing interest in trying to determine the extent to which metals contaminating our soils will eventually leak into lakes and streams,13–17 as well as groundwaters.18,19 As the quantity of available drinking water supplies comes under growing pressures, the quality of these resources will come under even greater scrutiny.20 Accurate descriptions of the quality of water resources can only be obtained using accurate analytical data. Because the concentrations of many trace metals in freshwaters are extremely low, once again clean lab methods and procedures are of paramount importance.21,22
With the growing concern for the quality of drinking water worldwide, it is important to be able to characterize the chemical composition of surface waters and groundwaters, in their natural condition, prior to any intervention by man, and to understand the processes regulating them. Once this has been accomplished, this chemical information can serve as a reference level, against which any impacts in future may be measured. An opportunity for such a study has recently arisen in Tiny Township, Ontario, Canada where a landfill is being constructed by the County of Simcoe, known locally as Site 41.23This region of southern Ontario is an area of abundant, naturally flowing artesian springs, and the plan is to construct the landfill in an area of groundwater discharge. Using the clean lab methods and protocols successfully developed for polar snow and ice,6,7,9,10,24,25 we recently determined the natural abundances of Sb and Pb in these groundwaters:26,27 these values provided a reference level which helped to document the contamination of bottled waters because of Sb leaching from PET28,29 and Pb leaching from glass.27
Here, in addition to Sb and Pb, other potentially toxic trace elements are considered, namely Ag, As, Bi, Cd, Co, Cr, Cu, Mo, Ni, Te, Tl, V, and Zn. To help understand natural inputs to the groundwaters from chemical weathering of minerals in the soils and sediments, lithogenic elements (Al, Ba, Be, Ca, Fe, Ga, Ge, Li, Mg, Mn, Na, P, Rb, S, Sc, Si, Sr, Th, Ti, and U) were included for comparison. The main goals of the study were to determine the extent to which potentially toxic trace elements are enriched in contemporary snow due to anthropogenic inputs, and to quantify the extent to which these elements are subsequently enriched or depleted, as the meteoric fluids migrate through the soils and sediments of the watershed.
Materials and methods
Water samples were collected from two natural artesian flows in Simcoe County, Ontario, Canada, near Elmvale (44° 35′ 00″ N, 79° 51′ 57.0″ W): the Old Johnson Farm is ca. 1.5 km N of Elmvale, in Springwater Township, and the Parnell Farm ca. 4.5 km N of Elmvale, in Tiny Township (Fig. 1a); the Parnell Farm is adjacent to Site 41. This region contains an abundance of groundwater under artesian conditions, and these represent an important source of water to the Wye River, ultimately flowing into Georgian Bay of Lake Huron, one of the Great Lakes. Eleven samples were collected at the Johnson Farm and twelve samples collected at the Parnell Farm, on 5.10.07, using the clean lab methods and procedures described earlier.26 The data obtained from the current set of measurements are consistent with the values obtained when water samples were collected from these and other flows in the area, during 2004, 2005, and 2006. To date, however, only a summary of the data for Sb26 and Pb27 has been published. | ||
| Fig. 1 a) Location of Springwater Township, Ontario, Canada as well as Luther Bog and Sifton Bog. b) Approximate location of sampling sites. The light blue hatched area corresponds to the Elmvale Clay Plain which is an area of groundwater discharge. The stippled red areas are part of the Simcoe Uplands and represent an area of groundwater recharge. The solid triangle (▲) shows the approximate location of the Parnell Flow, and the diamond (◆) the location of the Johnson Farm. | ||
Part of the St. Lawrence Platform, this region of southern Ontario is underlain by Ordovician limestones and dolostones.30 The dominant physiographic zones are the Simcoe Lowlands and the Simcoe Uplands.31 Once part of glacial Lake Algonquin, the surficial deposits of the Lowlands (2816 km2) consist of a clay plain made up of glacial drift which covers the bedrock to depths of more than 100 m. The Elmvale Clay Plain (Fig. 1b) is a typical feature of the lowlands.31 This is an area of groundwater discharge: the water in the sand aquifer below the clays is under pressure, giving rise to upward gradients and many natural flows and springs.
In contrast, the Uplands (1024 km2) represent the recharge area (Fig. 1b). The uplands are characterised by a rolling topography, up to 70 m higher than the clay plain below, with a series of broad curved ridges separated by steep-sided, flat-floored valleys. The uplands are encircled by numerous shorelines, indicating that they were islands in glacial Lake Algonquin.31 The till in the uplands consists mainly of materials derived from Precambrian bedrock which characterizes the Canadian Shield found further to the north. Containing boulders, gravel and sand, these materials are highly permeable, and streams are rare. Here the soils are not only well drained, but they contain less carbonate and are moderately acidic.
A general description of the chemical characteristics of the waters from these aquifers was provided by the County of Simcoe. Water samples from the aquifer at Site 41 which had been collected between 1999 and 2002 from boreholes at the site, yielded the following average values: pH 8.0, 182 mg/l carbonate alkalinity, major element cations and anions (mg/l): Ca 47.6, Mg 17.6, Sr 5.0, Na 6.4, K 3.6, SO42− 17.9, Cl− 1.2, DOC 1.1.32
The climate of the region can be summarised using the data available for the cities of Barrie and Midland (Fig. 1b). During 1971–2000, Midland had an average annual temperature of 6.8 °C and 1067.1 mm of precipitation (321.9 mm as snow). For Barrie, the corresponding values are 6.7 °C and 938.5 mm of precipitation (238.4 mm as snow).
Sample collection
Wearing appropriate clean lab clothing and employing polyethylene gloves, samples were collected directly into acid-cleaned, 100 ml low density polyethylene (LDPE) bottles to which high purity HNO3 (100 µl) had already been added. This acid is produced in-house and purified twice by sub-boiling distillation. Addition of 100 µl of this acid to 100 ml of water from the Johnson Farm reduced the pH to 1.7 which is sufficient to stabilise the trace metals until the samples could be measured.26 The contribution of trace metals to the samples from this acid is negligible for each element considered here.6 To minimise the risks of contamination, none of the water samples were filtered.Both of the flows run continuously. At Parnell, the water flows continuously through a steel pipe and no pumps or valves are employed in the water delivery system. In contrast, the flow at the Johnson farm runs continuously, but was sampled from an outdoor faucet. The original faucet was brass and a time series for Pb yielded elevated Pb/Sc ratios regardless of the duration of flushing.27 The small but detectable contribution of Pb from the brass faucet was eliminated simply by replacing it with one made of stainless steel in May of 2007; a second time series for Pb, after installing the stainless steel faucet, confirmed that the problem of contamination from the valve had been eliminated (unpublished data).
Water samples were each packed into three ziplock plastic bags and kept refrigerated until they could be transported to the laboratory in Germany for analyses. For transport, they were placed into an insulated plastic box containing freezer packs and shipped airfreight; the samples were still cool when they arrived at the lab.
To provide an estimate of contemporary atmospheric inputs of trace metals to the groundwaters, snow was sampled as follows: two snow pits were dug by hand using a Ti shovel, at two sites on the Old Johnson Farm (E and W of the Wye River), on 27.2.2007 and 28.2.2007 and at one site on the Parnell Farm on 28.2.2007. In each pit, samples were collected from three depths (ca. 0–10, 10–20, and 20–30 cm) and each depth treated as an individual sample. For comparison with the data obtained from these snow pits, data from two additional snow pits are included: snow was collected on 18.3.2005 from the Luther Bog north of Guelph, Ontario, and on 14.3.2005 from the Sifton Bog in the City of London, Ontario. All snow samples were packed in insulated boxes using dry ice, and shipped airfreight; the samples were still frozen when they arrived at the lab in Germany.
In the lab, snow samples which had been packed into ziplock polyethylene bags were melted in acid-cleaned Teflon beakers in a Class 10, metal-free, laminar flow clean air cabinet. The samples were acidified with 1% HNO3 (100 µl added per 10 ml of water) which had been distilled twice in a high purity quartz still. The acidified solutions were transferred to acid-cleaned polypropylene tubes until they were analyzed.
Determination of trace metals in groundwaters using ICP-SMS
All cleaning procedures and sample manipulations were carried out in a clean lab using clean benches of at least U.S. class 100 with the operator wearing PE gloves. The 100 ml LDPE bottles and screw caps used for the collection of waters were initially rinsed five times with high-purity water (18.2 MΩ cm) supplied from a MilliQ-Element system (Millipore, MA, USA). Thereafter, the bottles were filled with 10% nitric acid for at least 3 weeks. This acid had been prepared in-house and was distilled twice by sub-boiling, using a commercial instrument made of high purity quartz (MLS, Leutkirch, Germany). Similarly, the screw caps were submerged into 10% HNO3 and left in the clean bench for 3 weeks, before both the bottles and the caps were again rinsed with high-purity water and filled with 1% HNO3 for another week. Subsequently, the bottles and caps were rinsed again five times with high-purity water and dried in the clean bench overnight, before adding 100 µl high purity HNO3 to the bottles and sealing the bottles with the screw cap. For practical reasons and to reduce the risk of contamination during sampling, the acid was added to each bottle in the lab. Bottles containing acid were then packed individually in plastic bags, and sealed for transport to the field.Selected major and trace elements (Ag, Al, As, Ba, Be, Bi, Ca, Cd, Co, Cr, Cu, Fe, Ga, Ge, Li, Mg, Mn, Mo, Na, Ni, P, Pb, Rb, S, Sb, Sc, Si, Sr, Te, Th, Ti, Tl, U, V, Zn) were determined in the waters using inductively coupled plasma-sector field mass spectrometry (ICP-SMS) applying ultra clean techniques as previously adapted for the determination of trace elements in polar ice.6,7 To this end, a high efficiency, desolvating sample introduction system (APEX Q; ESI, Omaha, NE, USA) including a low flow PFA nebulizer (ESI) operated in the self-aspirating mode was employed. The ICP-SMS was operated in a Class 1000 clean room, while the microvolume autosampler (ASX-100, Cetec, Omaha, NE, USA) and the Apex were hosted in a class 100 clean bench. Details about instrument settings, acquisition and evaluation parameters are given elsewhere.6,7
For quality control purposes, SLRS-4, a certified, standard reference material (river water) was analyzed with every sample batch. The measured values are in good agreement with the certified and information values for this reference material.6,7
Concentrations of tritium in the waters
Tritium concentrations measured in a water sample from the Johnson Farm in 2006 yielded 0 tritium units (TU) but two samples collected in 2007 showed 1.83 ± 0.95 and 0.56 ± 0.87, respectively. Thus, there may be a small amount of “post bomb” water in this flow, but most of the water is older than ca. 1950. In contrast, two samples from the Parnell Flow collected in 2006 yielded 9.52 ± 0.88 and 9.97 ± 0.9 TU, respectively: this water, therefore, is modern; certainly younger than 50 years, and probably less than 30 years, although dating with tritium alone is not possible. The tritium measurements summarized here are consistent with the data obtained at three other artesian flows in the region (unpublished data).Mineral identification
At Site 41, adjacent to the artesian flow on the Parnell farm property, a bulk sediment sample had been collected approximately 2.5 m below the soil surface and provided to us for study. The sample was dried at 105 °C, wet ground and spray dried33 to produce a random powder. An X-ray powder diffraction (XRPD) pattern was recorded from 2–75° 2θ using Cobalt Kα radiation. Quantitative analysis was done by a normalised full pattern reference intensity ratio (RIR) method.34 The uncertainty in the estimated concentration of the minerals, using a 95% confidence level, is given by ±X0.35, where X = concentration in wt.%., e.g. 30 wt.% ±3.3.35Clay fractions of <2 µm were obtained by timed sedimentation, prepared as oriented mounts using the filter peel transfer technique and scanned from 2–45° 2θ in the air-dried state, after glycolation, and after heating to 300 °C for one hour. Clay minerals identified were quantified using a mineral intensity factor approach based on calculated XRPD patterns.35 For clay minerals present in amounts >10wt.%, uncertainty is estimated as better than ±5wt.% at the 95% confidence level.
Results and discussion
Abundance of elements in snow and groundwater
The measured concentrations of major and trace elements in the groundwater and snow samples are given in Table 1. With respect to the data obtained from the three snow pits taken in Springwater and Tiny Townships, the results are presented as an average of all 9 samples (Table 1). A snow pit dug at the Johnson farm in February of 2009 showed similar values to those given in Table 1. Comparable concentrations for virtually all elements are seen in the snow pits collected two years earlier in two peat bogs in southern Ontario (Table 1). Moreover, snow pit samples collected at these two peat bogs, as well as three other peatlands in southern and central Ontario (Tiny Marsh, Wye Marsh and Spruce Bog, Algonquin Park), during February and March of 2009 (data not shown) are similar to the data given for the Luther and Sifton bogs (Table 1). Thus, for the elements of interest, the snow samples collected from the Johnson and Parnell farms provide a reasonable first estimate of the contemporary atmospheric inputs to the groundwater recharge area.| Average Groundwater, Johnson (n = 11) | std. dev | Average Groundwater, Parnell (n = 12) | std. dev | Average Snow (Johnson n = 6, Parnell n = 3) | std. dev. | Average Snow, Luther Bog (n = 3) | std. dev. | Average Snow, Sifton Bog (n = 3) | std. dev. | |
|---|---|---|---|---|---|---|---|---|---|---|
| a All concentrations in ng/L (ppt) unless otherwise indicated. | ||||||||||
| Ag | 0.95 | 0.19 | 0.47 | 0.08 | 2.77 | 0.96 | 2.6 | 1.5 | 2.1 | 0.4 |
| Al (ppb) | 3.5 | 1.1 | 0.46 | 0.10 | 16 | 6 | 33 | 40 | 20 | 4 |
| As | 1591 | 73 | 244 | 38 | 71 | 22 | 68 | 72 | 66 | 14 |
| Ba (ppb) | 100 | 3 | 110 | 18 | 0.88 | 0.45 | 1.3 | 1.6 | 1.7 | 0.6 |
| Be | 0.55 | 0.06 | 0.56 | 0.11 | 1.4 | 0.5 | 2.9 | 3.5 | 2.3 | 0.7 |
| Bi | 0.13 | 0.10 | 0.83 | 0.17 | 7.6 | 3.0 | 9.7 | 7.9 | 9.7 | 4.0 |
| Ca (ppm) | 27 | 1 | 51 | 8 | 0.45 | 0.26 | 0.73 | 0.75 | 1.4 | 0.6 |
| Cd | 4.9 | 0.9 | 2.3 | 0.5 | 35 | 27 | 10 | 8 | 17 | 7 |
| Co | 8.6 | 0.9 | 16.6 | 2.7 | 18 | 9 | 21.5 | 20.8 | 31.6 | 17.1 |
| Cr | 7.7 | 3.9 | 4.6 | 2.2 | 116 | 39 | 142 | 141 | 142 | 53 |
| Cu | 64 | 77 | 17 | 9 | 852 | 584 | 455 | 482 | 696 | 96 |
| Fe (ppb) | 12 | 1 | 159 | 26 | 15 | 6 | 41 | 51 | 29 | 5 |
| Ga | 8.0 | 0.6 | 4.2 | 0.8 | 8.4 | 2.2 | 19 | 22 | 14 | 5 |
| Ge | 4.0 | 0.3 | 5.9 | 1.0 | 3.8 | 1.1 | 8.5 | 9.8 | 5.9 | 1.9 |
| Li | 3443 | 121 | 3537 | 574 | 47 | 24 | 45 | 35 | 52 | 13 |
| Mg (ppm) | 19 | 1 | 16 | 3 | 0.09 | 0.04 | 0.19 | 0.17 | 0.23 | 0.11 |
| Mn (ppb) | 7.9 | 0.4 | 7.4 | 1.2 | 1.8 | 1.0 | 3.0 | 3.3 | 3.9 | 1.1 |
| Mo | 673 | 108 | 453 | 122 | 75 | 26 | 102 | 72 | 124 | 33 |
| Na (ppm) | 9.4 | 0.5 | 3.1 | 0.5 | 0.70 | 0.43 | 0.12 | 0.07 | 0.89 | 0.30 |
| Ni | 48 | 12 | 26 | 5 | 336 | 261 | 130 | 101 | 159 | 62 |
| P (ppb) | 10 | 0.6 | 4.0 | 1.0 | 5.8 | 3.3 | 9.5 | 11.0 | 10.4 | 1.9 |
| Pb | 5.9 | 3.6 | 3.4 | 1.9 | 672 | 264 | 747 | 726 | 798 | 396 |
| Rb | 299 | 9 | 930 | 156 | 56 | 21 | 63 | 58 | 76 | 11 |
| S (ppm) | 8.6 | 0.5 | 15.4 | 2.7 | 0.58 | 0.36 | 0.32 | 0.24 | 0.56 | 0.20 |
| Sb | 1.4 | 0.2 | 2.2 | 0.4 | 31 | 18 | 50 | 55 | 66 | 29 |
| Sc | 1.3 | 0.5 | 0.8 | 0.1 | 2.3 | 0.9 | 6.8 | 7.4 | 4.7 | 1.7 |
| Si (ppm) | 17 | 1 | 8.9 | 1.6 | 0.03 | 0.02 | 0.10 | 0.14 | 0.04 | 0.01 |
| Sr (ppb) | 1207 | 41 | 122 | 20 | 1.4 | 0.7 | 1.1 | 1.0 | 2.2 | 0.9 |
| Te | 12 | 2 | 2.6 | 0.8 | 1.8 | 0.7 | 0.80 | 0.29 | 0.92 | 0.33 |
| Th | 2.0 | 0.9 | 0.07 | 0.02 | 1.8 | 0.7 | 5.5 | 6.5 | 3.1 | 0.9 |
| Ti (ppb) | 6.7 | 0.8 | 14 | 3 | 0.17 | 0.07 | 0.26 | 0.27 | 0.42 | 0.18 |
| Tl | 0.67 | 0.29 | 0.43 | 0.06 | 2.5 | 1.4 | 2.5 | 2.6 | 3.5 | 1.6 |
| U | 843 | 28 | 1299 | 221 | 4.0 | 2.3 | 9.4 | 12.2 | 7.3 | 2.3 |
| V | 25 | 3 | 9.1 | 1.7 | 172 | 105 | 233 | 288 | 314 | 135 |
| Zn (ppb) | 0.37 | 0.10 | 0.55 | 0.14 | 6.6 | 2.6 | 4.7 | 0.5 | 5.8 | 1.3 |
For most of the trace elements of environmental interest, concentrations in snow collected at all sites were much higher than in groundwater, namely Ag, Bi, Cd, Co, Cu, Cu, Ni, Pb, Sb, Tl, V and Zn (Table 1). In the case of Pb, for example, the concentrations in snow are at least 100x greater than in the groundwaters. For a few trace elements (As, Mo, Te and U), however, concentrations in groundwater are much greater than in snow (Table 1). As expected, lithogenic elements, namely Ba, Ca, Li, Mg, Na, Rb, Si, Sr, and Ti, are more abundant in groundwater compared to snow, due to chemical weathering. In contrast to these groups of elements, Fe, Ga and Ge revealed comparable concentrations in snow and groundwater.
Enrichments and depletions of elements in groundwater, compared to snow
It is worthwhile to quantify the magnitude of the changes between snow and groundwater, using a common denominator, to better understand the chemical evolution of the fluids. Scandium is commonly used as a reference element for quantifying the extent of trace element enrichment in atmospheric aerosols because there are effectively no industrial uses of Sc and therefore no anthropogenic emissions. In addition, Sc behaves conservatively during chemical weathering in soil profiles, and has no preference for a specific mineral phase.36 Scandium has already been determined in these groundwaters, and used as a reference element for Sb and Pb.26,27 Relative to the average M/Sc ratios in all snow samples collected from Simcoe County (Table 1), water samples collected at the two artesian flows in this area are significantly depleted in Ag, Al, Bi, Cd, Cr, Cu, Ni, Pb, Sb, Tl, V, and Zn at both sites (Fig. 2a,b).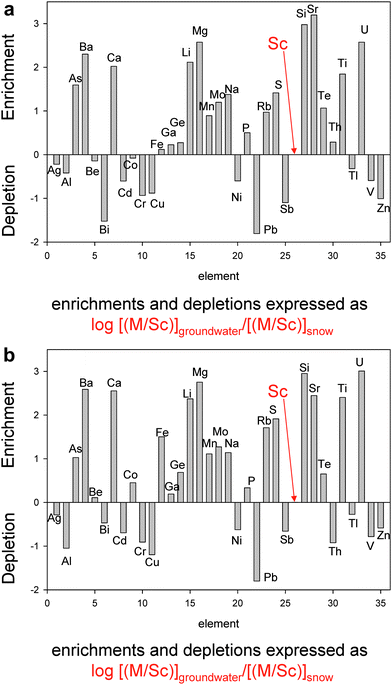 | ||
| Fig. 2 a) Enrichments and depletions of elements in groundwater, relative to snow, in the samples from Johnson Farm. b) Parnell Farm. | ||
Lithogenic elements significantly enriched in the groundwater at both sites includes Ba, Ca, Li, Mg, Mn, Na, Rb, S, Si, Sr, and Ti (Fig. 2a,b). The abundance of these elements can largely be explained in terms of weathering of the dominant silicate and carbonate minerals in the soils and sediments of the discharge area (see below). Arsenic, Mo, Te, and U are also highly enriched in the groundwaters (Fig. 2a,b), with U being especially affected. Three or four elements show slight enrichments at both sites, namely Fe, Ga, Ge, and P.
Beryllium and Co are slightly depleted in the waters from the Johnson flow, but slightly enriched at the Parnell flow: because the changes are not consistent, they are not considered further. Similarly, Th is significantly depleted at Parnell, but slightly enriched at Johnson, and is not considered further.
Enrichments of trace elements in snow due to human activities
The groundwaters are supplied with water from rain and snow in the recharge area, and to some extent the quality of rain and snow may affect the quality of the groundwater. To help understand the extent to which human activities have affected the quality of atmospheric water supplied to the aquifer, it is worthwhile to try to quantify the absolute magnitude of trace element enrichments in snow. The abundance of trace elements in the Upper Continental Crust has been used successfully as a first step to quantify trace element enrichments in continental peat bogs37–40 as well as polar snow and ice.10,25,41 Using the UCC to provide a baseline, and employing Sc as reference element, it becomes clear that Ag, As, Bi, Cd, Cu, Mo, Ni, Pb, Sb, Te, Tl, V and Zn are all significantly enriched in the snow samples (Table 2). Studies have shown significant natural enrichments of Ag and Cd in atmospheric aerosols, relative to crustal rocks,37,39,41 such that the enrichment factors reported here for the snow pits most likely overestimate the extent of anthropogenic enrichments of Ag and Cd. However, for the other elements the crustal abundance has been shown using continental bogs and arctic ice to provide a reasonable reference point for atmospheric aerosols. Specifically, the M/Sc ratios of Mo, Ni, Tl and V in aerosols from the mid-Holocene, obtained from continental bogs37–40 and polar ice,10,25 are virtually identical to crustal values. In the case of Pb and Sb, the background M/Sc ratios obtained from continental bogs37 and polar ice10,25 are within a factor of 2 of the crustal values. Thus, the profound (10 to 1000 x) enrichments of Ag, As, Bi, Cd, Cu, Mo, Ni, Pb, Sb, Te, Tl, V and Zn in contemporary snow are assumed to mainly be a reflection of human activities in northeastern North America.| Average Snow (Johnson n = 6, Parnell n = 3) | M/Sc, snow | UCC (ppm, Wedepohl 1995) | M/Sc UCC (Wedepohl, 1995) | M EF snow/UCC | M EF groundwater Johnson/UCC | M EF groundwater Parnell/UCC | |
|---|---|---|---|---|---|---|---|
| a All concentrations in ng/L (ppt) unless otherwise indicated. | |||||||
| Ag | 2.8 | 1.2 | 0.055 | 0.01 | 152 | 92 | 79 |
| Al (ppb) | 16 | 6915 | 77440 | 11063 | 0.6 | 0.2 | 0.1 |
| As | 71 | 31 | 2 | 0.29 | 107 | 4219 | 1138 |
| Ba (ppb) | 0.88 | 377 | 668 | 95 | 4.0 | 793 | 1537 |
| Be | 1.4 | 0.6 | 3.1 | 0.44 | 1.3 | 0.9 | 1.7 |
| Bi | 7.6 | 3.3 | 0.123 | 0.02 | 185 | 5.6 | 63 |
| Ca (ppm) | 0.45 | 191262 | 29450 | 4207 | 45 | 4778 | 16290 |
| Cd | 35 | 15 | 0.102 | 0.01 | 1028 | 256 | 207 |
| Co | 18 | 7.8 | 11.6 | 1.7 | 4.7 | 3.9 | 13 |
| Cr | 116 | 50 | 35 | 5.0 | 10 | 1.2 | 1.2 |
| Cu | 852 | 366 | 14.3 | 2.0 | 179 | 24 | 11 |
| Fe (ppb) | 15 | 6648 | 30890 | 4413 | 1.5 | 2.0 | 48 |
| Ga | 8.4 | 3.6 | 14 | 2.0 | 1.8 | 3.0 | 2.8 |
| Ge | 3.8 | 1.6 | 1.4 | 0.20 | 8.1 | 15 | 39 |
| Li | 47 | 20 | 22 | 3.1 | 6.4 | 830 | 1501 |
| Mg (ppm) | 0.09 | 38351 | 13510 | 1930 | 20 | 7470 | 11228 |
| Mn (ppb) | 1.8 | 764 | 527 | 75 | 10 | 79 | 131 |
| Mo | 75 | 32 | 1.4 | 0.20 | 161 | 2551 | 3017 |
| Na (ppm) | 0.70 | 298370 | 25670 | 3667 | 81 | 1941 | 1124 |
| Ni | 336 | 144 | 18.6 | 2.7 | 54 | 14 | 13 |
| P (ppb) | 5.8 | 2478 | 665 | 95 | 26 | 83 | 57 |
| Pb | 672 | 288 | 17 | 2.4 | 119 | 1.9 | 1.9 |
| Rb | 56 | 24 | 110 | 16 | 1.5 | 14 | 79 |
| S (ppm) | 0.58 | 249780 | 953 | 136 | 1835 | 47721 | 150486 |
| Sb | 31 | 13 | 0.31 | 0.04 | 299 | 24 | 66 |
| Sc | 2.3 | 1.0 | 7 | 1.0 | 1.0 | 1.0 | 1.0 |
| Si (ppm) | 0.03 | 13242 | 303480 | 43354 | 0.3 | 290 | 274 |
| Sr (ppb) | 1.4 | 580 | 316 | 45 | 13 | 20264 | 3592 |
| Te | 1.8 | 0.8 | 0.005 | 0.001 | 1082 | 12625 | 4848 |
| Th | 1.8 | 0.8 | 10.3 | 1.5 | 0.5 | 1.0 | 0.1 |
| Ti (ppb) | 0.17 | 72 | 3117 | 445 | 0.2 | 11 | 41 |
| Tl | 2.5 | 1.1 | 0.75 | 0.11 | 9.9 | 4.8 | 5.3 |
| U | 4.0 | 1.7 | 2.5 | 0.36 | 4.8 | 1788 | 4850 |
| V | 172 | 74 | 53 | 7.6 | 9.7 | 2.5 | 1.6 |
| Zn (ppb) | 6.6 | 2823 | 52 | 7.4 | 380 | 37 | 99 |
Although As, Mo, and Te are significantly enriched in snow (Table 2), the M/Sc ratios for each of these elements in groundwater is far greater than in snow. There are no industrial activities in the groundwater recharge area, so the additional enrichment of As, Mo and Te in the groundwaters, relative to atmospheric inputs, along with the profound enrichment of U, reveals a chemically reactive natural source of these elements.
Enrichment factors for trace elements in snow and groundwater
The absolute value of the enrichment factor, using Sc as reference element, and calculated relative to the M/Sc ratios in the UCC, is shown in Fig. 3. Although As, Mo, Te and U are clearly enriched in snow, the EF for these elements in groundwater is far greater (Fig. 3). In contrast to the elements enriched in groundwater, relative to snow, the following elements show significantly lower enrichments in groundwater: Ag, Bi, Cd, Cr, Cu, Ni, Pb, Sb, Tl, V, and Zn (Fig. 3). We note that Cr and Pb are particularly well removed from groundwater such that the M/Sc ratios approach the proportions typical of crustal rocks, ie they appear to reflect “natural” values. | ||
| Fig. 3 Enrichment Factor, relative to Upper Continental Crust,50 of trace metals in snow and groundwater. As, Mo, Te and U are more enriched in groundwater compared to snow, but the opposite is true for the other elements. | ||
Processes supplying major and trace elements to groundwater
As shown in the cartoon (Fig. 4), meteoric waters must percolate through approximately 100 m of coarse-grained glacial debris before they reach the shallow aquifers which are so common in the Elmvale area. The glacial debris is derived from the Canadian Shield, having been transported during the last ice age. We assume that these materials were ultimately derived from granites and their metamorphic equivalents, and therefore consist mainly of quartz, plagioclase and potassium feldspar, biotite and hornblende. In the acidic, organic-rich forest soils currently found in the recharge area and developed from these parent materials, chemical weathering will be dominated by the reaction of plagioclase feldspar, biotite, hornblende, and potassium feldspar.42 In the weathering zone of the recharge area, therefore, reactions between these minerals and meteoric fluids will contribute Na, K, Mg, Ca and Si to the solutions, in addition to the trace elements which can substitute for them, namely Li, Be, Rb, Sr, and Ba (Table 3). In the soil solutions, therefore, all of these elements will become enriched, relative to precipitation, and the meteoric waters will acquire these solutes as they percolate through the column of glacial material. | ||
| Fig. 4 Cartoon of geological cross section. | ||
| Name | Formula | Abundance | Solutes |
|---|---|---|---|
| a 1. Abundance of Dolomite + Ankerite = 4.7%; 2. Quartz is stable during chemical weathering of acidic forest soils, and is expected to make insignificant contributions to the chemical evolution of the soil solution. Virtually all of the Si in solution is expected to arise from the weathering of aluminosilicates.; 3. Although Al generally behaves conservatively during weathering, all aluminosilicates are expected to provide small quantities of Al to solution.; 4. Most of the Ca, Mg, and Sr is due to carbonate weathering in the sediments.; 5. Clay fraction (<2 µm) of the sediments dominated by illite (56%), vermiculite (36%), and chlorite (8%).; 6. Enrichment of As, Mo, and Te in the groundwaters, relative to snow, indicates that small amounts of metal sulfides are present in the glacial debris of the source area.; 7. Enrichment of U in the groundwaters, relative to snow, indicates that small amounts of apatite is present in the glacial debris of the source area. | |||
| Quartz | SiO2 | 14.6% | — |
| Plagioclase Feldspar (Albite) | NaAlSi3O8 | 21.1% | Si, Na, Ca, Sr, Ba (Pb) |
| Potassium Feldspar (Microcline) | KAlSi3O8 | 10.0% | Si, K, Rb, Li, Ba (Pb) |
| Hornblende | Na0.9K0.4Ca1.6Mg2.9Fe1.4Ti0.5Al2.4Si6O24 | 6.5% | Na, K, Ca, Mg, Si, Fe, Ti (Cr, V) |
| Hydrobiotite | Mg2.3Fe3+0.6K0.3Ca0.1Si2.8Al1.2O10(OH)1.8F0.2·3(H2O) | 8.3% | K, Mg, Ca, Fe, Si |
| Illite | K0.6(H3O)0.4Al1.3Mg0.3Fe2+0.1Si3.5O10(OH)2·(H2O) | 5.8% | K, Mg, Fe, Si |
| Chlorite | (Mg,Fe)3(Si,Al)4O10(OH)2·(Mg,Fe)3(OH)6 | 1.4% | Mg, Fe, Si |
| Calcite | CaCO3 | 22.1% | Ca, Mg, Sr |
| Dolomite | CaMg(CO3)2 | — | Ca, Mg, Sr |
| Ankerite | CaMg0.27Fe0.73(CO3)2 | — | Ca, Mg, Fe, Sr |
In the Elmvale Clay Plain which is made up of glacial lake sediments (Fig. 4), the dominant primary minerals in bulk samples include not only the silicate minerals noted above, but also calcite, dolomite/ankerite, and a number of phyllosilicates including hydrobiotite, illite, and chlorite (Table 3). Once the fluids have migrated down through the glacial debris of the Simcoe Uplands, chemical weathering of the carbonate minerals will elevate the pH to 8.0 (the measured pH of the groundwaters), generate considerable carbonate alkalinity, and contribute considerably more Ca, Mg, Sr, Ba and Mn to solution (Table 3).
Processes removing trace elements from groundwater
As shown in the cartoon (Fig. 4), meteoric waters must percolate through the forest soils of the recharge area, and a column of glacial till approximately 100 m thick, prior to reaching the aquifer. During this migration, there is considerable potential for trace metals to be removed from rain and melting snow, as metal-bearing aerosol particles are physically removed by “filtration” provided by above- and below-ground plant matter, soil humus, and mineral particles. In addition to the obvious physical removal processes, there are many chemically reactive surfaces in the soil zone which can remove ionic species from solution by adsorption and surface complexation, namely the humic acids created during the decomposition of plant and animal matter, as well as the metal (Al, Fe, Mn) oxyhydroxides and phyllosilicate (clay) minerals created during chemical weathering.In addition to the potential for trace element removal in the soils and glacial debris from which they are derived, the clay fraction (<2 µm) of the glacial lake sediments (Simcoe Lowlands) includes illite (56%), vermiculite (36%) and chlorite (8%). In contrast to the feldspars which dominate the soil profiles but have cation exchange capacities (CEC) on the order of only 1–2 meq/100 g, the clay minerals have far higher CECs, with typical values for illite and chlorite in the range 10 to 40 meq/100 g, and vermiculite on the order of 100 to 150 or more. The abundance of these clay minerals with their much large surface areas and high CECs, combined with the elevated pH values (pH 8.0) of the groundwater provides optimal circumstances for additional removal of trace metals such as Ag, Bi, Cd, Cr, Cu, Ni, Pb, Sb, Tl, V, and Zn. The processes which have led to the removal of trace elements from the groundwaters can only be speculated using the existing data. More detailed studies of the soil profiles and the glacial tills are needed to begin to understand the mechanisms of trace element removal along the groundwater flowpath. Regardless of the mechanisms of trace metal removal, the extremely low concentrations of many trace metals, relative to their abundance in contemporary snow, suggests that the removal process is very effective.
Reproducibility of the trace metal analyses of the groundwaters
The two artesian flows sampled at the Johnson and Parnell farms show some significant differences (Table 1). At the flow on the Johnson farm, for example, the concentrations of As, Cu, Sr and V are much greater, but the Fe and Rb concentrations much lower, than at Parnell. In fact, the data from 2005 and 2006 (unpublished) shows very similar and remarkably reproducible differences between the two sites. Arsenic concentrations in the groundwaters from the Johnson farm in 2005, for example, were 1.70 ± 0.47 µg/l (n = 14, three sampling campaigns) compared to Parnell which was 0.22 ± 0.10 µg/l (n = 6, one sampling campaign). Water samples collected from the Johnson farm on 13.2.09 and from the Parnell flow on 12.2.09 yielded 1.77 ± 0.02 (n = 3) and 0.31 ± 0.05 (n = 6), respectively. Also in 2005, the Ag, Cr, Cu, Mo, Sr and V concentrations in the waters from the Johnson flow were approximately double those of the Parnell flow, but Rb concentrations one-half, and Fe concentrations were one-quarter those at Parnell; similar differences were found in 2007 (Table 1). Thus, the concentration differences seen between the Johnson and Parnell flows for the sampling campaigns shown in Table 1 are also seen for the same elements in the samples collected previously and subsequently (unpublished data).Groundwater metal concentrations in perspective
The trace element concentrations in the groundwaters described here are generally rather low, with many elements significantly below 10 ng/L, namely Cd, Co, Cr, Ga, Ge, Pb, Sb, and Sc (Table 1). For some elements, however, extremely low concentrations are found in the groundwaters, e.g. Ag, Be, Bi and Tl are each below 1 ng/L. An extensive list of trace elements was recently determined in 132 brands of bottled waters from 28 countries, using the same analytical methods and procedures;43 compared to the median concentrations of trace metals in bottled waters collected worldwide and reported in that study, the groundwaters from Springwater and Tiny Townships contain significantly lower concentrations of Ag, Be, Cd, Co, Cu, Ge, Tl and V. The groundwaters analyzed here also contain far lower concentrations of Sb than bottled waters packaged in PET plastic, because of leaching from the containers,28,29 and far lower concentrations of Pb than bottled waters in glass, again because of leaching.27 The groundwaters of Springwater and Tiny Townships, therefore, provide a useful reference level against which other waters may be compared. The water samples from the Parnell flow in particular, adjacent to the planned landfill known as Site 41, should provide useful “baseline” values against which any data obtained in future may be compared.Analytical implications for studies of trace elements in natural waters
The concentrations of many trace elements reported here for these groundwaters are comparable to the lowest concentrations found in ancient ice layers from the most remote regions of the arctic. Specifically, most of the elements reported in Table 1 have also been measured in an ice core collected on Devon Island in Nunavut, Canada. The average concentrations of many of the trace metals reported here for the groundwaters are comparable to the concentrations found in the cleanest layers of ancient arctic ice. The median Pb concentrations in the groundwaters (Table 1), for example, are not significantly different from the Pb concentrations in arctic ice samples dating from the mid-Holocene, approximately 4000 to 8000 years ago, when rates of atmospheric metal deposition were at their lowest;10 the same is true of Co, Cr and V (unpublished data). Thus, the need for extreme analytical methods and precautions which are currently being employed to study trace metals in ice cores must also be employed in studying the same elements in groundwaters, to ensure the quality of the analytical data. Whether our interest concerns the predominant sources, transformations, and fate of trace metals, or to quantify exposure to humans or ecosystems, accurate data is required for trace metals in natural waters.As we noted in a previous study, the Pb concentrations found in five samples of groundwater emanating from some of the artesian flows during 2004 and 2005 were <1 ng/L,27 which are among the lowest values ever reported for Pb in water at the surface of the earth, and comparable to ancient ice from Antarctica.2 The lowest Pb concentration found to date (700 pg/L) is approximately a factor of 50 lower than the average for deep groundwaters from Switzerland,18 a factor of 5 lower than the values reported for Pb in pristine groundwaters of the USA,19 and comparable to the lowest Pb concentrations ever measured in natural waters.44 Trace metal concentrations in carbonate groundwaters have been likened to seawater45 because the concentrations of elements such as Ag, Be, Bi, Cd and Pb are so low that even today they still present enormous analytical challenges.46
Taken together these findings suggest that reliable measurements of Pb and other trace metals in uncontaminated groundwaters requires the comprehensive procedures, protocols and analytical methods developed for polar snow and ice. In particular the use of pre-cleaned sample bottles and vials, high-purity acid for cleaning and acidifying the water samples as well as systematic blank values and quality control measures have to be considered. In addition, the analytical protocols should provide detection limits well below the ng/L range to allow reliable quantification of trace elements in pristine groundwaters. The performance characteristics described here can only be achieved following strict clean laboratory procedures and by making use of the most sensitive instrumental techniques such as ICP-SMS.
To filter, or not to filter?
Although the common practice is to filter waters through a 0.45 µm membrane filter prior to measurement for trace metals,47 the concentrations of Co, Cr, Ni and Pb in these groundwaters are so low that filtration represents a significant contamination risk. For example, the blank values for Pb leaching from most kinds of membrane filters, even when determined using high purity water in a Class 100 clean bench, are commonly one to two orders of magnitude greater than the average concentration of Pb in the groundwaters. Specifically, the filter blanks obtained using high purity deionized water, in a metal-free Class 100 clean air cabinet, employing three samples of each of four brands of 10 mm syringe filters, were 110 ± 10, 30 ± 30, 10 ± 10 and 4 ± 3 ng/L, respectively.48 Even the filter which yielded the lowest blank values (4 ± 3 ng/L) is unacceptable, given the extremely low concentrations of Pb found in these waters (Table 1). Thus, at this stage, a comparison of filtered and unfiltered groundwaters of this quality is not possible.Natural purification of groundwater
Except for As, Mo, Te, and U which become enriched in the groundwater as a result of chemical weathering in the watershed, most trace metals, in particular Ag, Bi, Cd, Cr, Cu, Ni, Pb, Sb, Tl, V, and Zn, are strongly removed. The tritium concentrations in the groundwaters from the Parnell flow show that the waters are modern, ie certainly younger than 50 years, and probably younger than 30 years. Although the snow samples collected today are strongly contaminated with most of the potentially toxic trace elements (Ag, As, Bi, Cd, Cu, Mo, Ni, Pb, Sb, Te, Tl, V and Zn), in past decades the concentrations and extent of enrichments of these elements was even higher49 Therefore, the waters emanating from the springs today probably started out with even greater element concentrations, and greater enrichments, than the snow samples suggest today. Given the young age of the water and the extent of trace element removal, the natural processes responsible for the purification, although poorly understood, are certainly efficient, and deserving of further study.Acknowledgements
We are grateful to Stefan Rheinberger for expert technical support in the lab and to Tommy Nørenberg (Institute of Chemistry and Physics, Odense University, Denmark) for excellent support in the field. Discussions in Heidelberg with Andriy Cheburkin, Christian Scholz, and Margot Isenbeck-Schröter, and in Canada with Michael Powell, were helpful and sincerely appreciated.References
- M. Murozumi, T. J. Chow and C. C. Patterson, Chemical concentrations of pollutant lead aerosols, terrestrial dusts and sea salts in Greenland and Antarctic snow strata, Geochim. Cosmochim. Acta, 1969, 33, 1247–1294 CrossRef CAS.
- A. Ng and C. C. Patterson, Natural concentration of lead in ancient Arctic and Antarctic ice, Geochim. Cosmochim. Acta, 1981, 45, 2109–2121 CrossRef CAS.
- C. F. Boutron, J.-P. Candelone and S. Hong, Past and recent changes in the large-scale tropospheric cycles of lead and other heavy metals as documented in Antarctic and Greenland snow and ice: a review, Geochim. Cosmochim. Acta, 1994, 58, 3217–3225 CAS.
- C. F. Boutron, J.-P. Candelone and S. Hong, Greenland snow and ice cores: unique archives of the large scale pollution of the troposphere of the Northern Hemisphere for lead and other heavy metals, Sci. Total Environ., 1995, 160/161, 233–241 CrossRef.
- C. F. Boutron, J.-P. Candelone and U. Görlach, Ultra-trace analysis of heavy metals in ice and snow from the Antarctic and Greenland, Analusis Mag., 1992, 20, M24 CAS.
- M. Krachler, J. Zheng, D. A. Fisher and W. Shotyk, Analytical procedures for improved trace element detection limits in polar ice from Arctic Canada using ICP-SMS, Anal. Chim. Acta, 2005, 530, 291–298 CrossRef CAS.
- M. Krachler, J. Zheng, D. A. Fisher and W. Shotyk, Novel calibration procedure for improving trace element determinations in ice and water samples using ICP-SMS, J. Anal. At. Spectrom., 2004, 19, 1017–1019 RSC.
- M. Krachler, J. Zheng, D. A. Fisher and W. Shotyk, Direct determination lead isotopes (206Pb, 207Pb, 208Pb) in Arctic ice samples at pg/g levels using ICP-SMS coupled to a high efficiency sample introduction system, Anal. Chem., 2004, 76, 5510–5517 CrossRef CAS.
- W. Shotyk, J. Zheng, M. Krachler, C. Zdanowicz, R. Koerner and D. Fisher, Predominance of industrial Pb in recent snow from Devon Island, Arctic Canada, Geophys. Res. Lett., 2005, 32, L21814, DOI:10.1029/2005GL023860.
- J. Zheng, W. Shotyk, M. Krachler and D. Fisher, 15,800 years of atmospheric lead deposition on Devon Ice Cap, Nunavut, Canada: Natural and anthropogenic enrichments, isotopic composition, and predominant sources, Global Biogeochem. Cycles, 2007, 21, GB2027, DOI:10.1029/2006GB002897 2007.
- W. Shotyk and G. Le Roux. ( 2005) Biogeochemistry and Cycling of Lead. in “Biogeochemical Cycles of the Elements”, Vol. 43 of Metal Ions in Biological Systems, A. Sigel, H. Sigel, and R. K. O. Sigel, eds., M. Dekker, New York, pp. 240–275 Search PubMed.
- J. O. Nriagu, Heavy metal pollution poisoning the biosphere?, Environment, 1990, 32, 7–11 Search PubMed 28–33.
- H. Dörr and K. O. Münnich, Downward movement of soil organic matter and its influence on trace-element transport (210Pb, 137Cs) in the soil, Radiocarbon, 1989, 31, 655–663.
- E. K. Miller and A. J. Friedland, Lead migration in forest soils: response to changing atmospheric inputs, Environ. Sci. Technol., 1994, 28, 662–669 CAS.
- E. X. Wang, F. H. Bormann and G. Benoit, Evidence of complete retention of atmospheric lead in the soils of northern hardwood forested ecosystems, Environ. Sci. Technol., 1995, 29, 735–739 CAS.
- S. I. Vinogradoff, M. C. Graham, G. J. P. Thornton, S. M. Dunn, J. R. Bacon and J. G. Farmer, Investigation of the concentration and isotopic composition of inputs and outputs of Pb in waters at an upland catchment in NE Scotland, J. Environ. Monit., 2005, 7, 431–444 RSC.
- M. C. Graham, S. I. Vinogradoff, A. J. Chipchase, S. M. Dunn, J. R. Bacon and J. G. Farmer, Using size fractionation and Pb isotopes to study Pb transport in the waters of an organic-rich upland catchment, Environ. Sci. Technol., 2006, 40, 1250–1256 CrossRef CAS.
- B. Nowack, H. Xue and L. Sigg, Influence of natural and anthropogenic ligands on metal transport during infiltration of river water to groundwater, Environ. Sci. Technol., 1997, 31, 866–872 CrossRef CAS.
- S. A. Sanudo-Wilhelmy, F. K. Rossi, H. Bokuniewicz and R. J. Paulsen, Trace metal levels in uncontaminated groundwater of a coastal watershed: importance of colloidal forms, Environ. Sci. Technol., 2002, 36, 1435–1441 CrossRef CAS.
- R. M. Hirsch, P. A. Hamilton and T. L. Miller, U.S. Geological Survey perspective on water-quality monitoring and assessment, J. Environ. Monit., 2006, 8, 512–518 RSC.
- J. O. Nriagu, G. Lawson, H. K. T. Wong and J. M. Azcue, A protocol for minimizing contamination in the analysis of trace metals in Great Lake Waters, J. Great Lakes Res., 1993, 19, 175–182 CAS.
- G. Benoit, K. S. Hunter and T. F. Rozan, Sources of trace metal contamination artifacts during collection, handling, and analysis of freshwaters, Anal. Chem., 1997, 69, 1006–1011 CrossRef CAS.
- Ontario Municipal Board ( 1989) Reasons for Decision and Decision, Joint Board, Consolidated Hearings Act, 1981. CH-87-03, 11. November, 1989. Ontario Municipal Board, Suite 1201, 2300 Yonge Street, Toronto, Ontario, Canada Search PubMed.
- M. Krachler, J. Zheng, C. Zdanowicz, R. Koerner, D. Fisher and W. Shotyk, Increasing enrichments of antimony in the Arctic atmosphere, J. Environ. Monit., 2005, 7, 1169–1176 RSC.
- M. Krachler, J. Zheng, D. Fisher and W. Shotyk, Natural background levels of atmospheric antimony, variation with climate change and modern enrichments revealed by age-dated snow and ice samples from Devon Island, Arctic, Canada, Global Biogeochem. Cycles, 2008, 22, GB1015, DOI:10.1029/2007GB002998.
- W. Shotyk, M. Krachler, B. Chen and J. Zheng, Natural abundance of Sb and Sc in pristine groundwater, Springwater Township, Ontario, Canada, and implications for tracing contamination from landfill leachates, J. Environ. Monit., 2005, 7, 1238–1244 RSC.
- W. Shotyk and M. Krachler, Lead in bottled waters: comparison with pristine groundwaters and contamination from glass, Environ. Sci. Technol., 2007, 41, 3508–3513 CrossRef CAS.
- W. Shotyk, M. Krachler and B. Chen, Contamination of Canadian and European bottled waters with antimony leaching from PET containers, J. Environ. Monit., 2006, 8, 288–292 RSC.
- W. Shotyk and M. Krachler, Contamination of bottled waters with antimony leaching from PET increases with storage, Environ. Sci. Technol., 2007, 41, 1560–1563 CrossRef CAS.
- R. J. W. Douglas. ( 1976). Geology and Economic Minerals of Canada. Department of Energy, Mines, and Resources, Ottawa Search PubMed.
- L. J. Chapman and D. F. Putnam. ( 1966). The Physiography of Southern Ontario. 2nd ed. University of Toronto Press, Toronto Search PubMed.
- County of Simcoe ( 2003) Landfill Site 41, Jagger Hims Report, Supplemental Hydrogeological and Geotechnical Investigation. County of Simcoe, Midhurst, Ontario, Canada Search PubMed.
- S. Hillier. ( 2002) Spray drying for X-ray powder diffraction specimen preparation. IUCR Commission on Powder Diffraction Newsletter No. 27. pp 7–9, June 2002 Search PubMed.
- Oladipo Omotoso, Douglas K. McCarty, Stephen Hillier and Reinhard Kleeberg, Some successful approaches to quantitative mineral analysis as revealed by the 3rd Reynolds Cup contest, Clays Clay Miner., 2006, 54(6), 748 CrossRef CAS.
- S. Hiller, Quantitative analysis of clay and other minerals in sandstones by X-ray powder diffraction (XRPD). International Association of Sedimentologists, Spec Pub, 2003, 34, 213–251 Search PubMed.
- W. Shotyk, D. Weiss, J. D. Kramers, R. Frei, A. K. Cheburkin, M. Gloor and S. Reese, Geochemistry of the peat bog at Etang de la Gruère, Jura Mountains, Switzerland, and its record of atmospheric Pb and lithogenic trace elements (Sc, Ti, Y, Zr, Hf and REE) since 12,370 14C yr BP, Geochim. Cosmochim. Acta, 2001, 65(14), 2337–2360 CrossRef CAS.
- W. Shotyk, M. Krachler, A. Martinez-Cortizas, A. K. Cheburkin and H. Emons, A peat bog record of natural, pre-anthropogenic enrichments of trace elements in atmospheric aerosols since 12,370 14C yr BP, and their variation with Holocene climate change, Earth Planet. Sci. Lett., 2002, 199, 21–37 CrossRef CAS.
- M. Krachler, C. Mohl, H. Emons and W. Shotyk, Atmospheric deposition of V, Cr, and Ni since 12,370 14C yr BP recorded by a Swiss peat bog profile, Environ. Sci. Technol., 2003, 37, 2658–2667 CrossRef CAS.
- W. Shotyk and M. Krachler, Atmospheric deposition of Ag and Tl since 12,370 14C yr BP recorded by a Swiss peat bog profile, and comparison with Pb and Cd, J. Environ. Monit., 2004, 6, 427–433 RSC.
- M. Krachler and W. Shotyk, Natural and anthropogenic enrichments of molybdenum, thorium, and uranium in a complete peat bog profile, Jura Mountains, Switzerland, J. Environ. Monit., 2004, 6, 418–426 RSC.
- M. Krachler, J. Zheng, D. Fisher and W. Shotyk, Atmospheric inputs of Ag and Tl to the Arctic: comparison of a high resolution record from a snow pit (AD 1994 to 2004) with firn (AD 1840 to AD 1996) and an ice core (previous 16,000 years), Sci. Total Environ., 2008, 399, 78–89 CrossRef CAS.
- S. S. Goldich, A study in rock-weathering, The Journal of Geology, 1938, 46, 17–58 Search PubMed.
- M. Krachler and W. Shotyk, Trace and ultratrace metals in bottled waters: survey of sources worldwide and comparison with refillable metal bottles, Science of the Total Environment, 2008, 407, 1089–1096.
- M. P. Field and R. M. Sherrell, Direct determination of ultra-trace levels of metals in fresh water using desolvating micronebulization and HR-ICP-MS: application to Lake Superior waters, J. Anal. At. Spectrom., 2003, 18, 254–259 RSC.
- V. E. Hodge, K. J. Stetzenbach and K. H. Johannesson, Similarities in the chemical composition of carbonate groundwaters and seawater, Environ. Sci. Technol., 1998, 32, 2481–2486 CrossRef CAS.
- K. J. Stetzenbach, M. Amano, D. K. Kreamer and V. E. Hodge, Testing the limits of ICP-MS: determination of trace elements in ground water at the part-per-trillion level, Ground Water, 1994, 32, 976–985 CAS.
- A. J. Horowitz, K. R. Lum, J. R. Garbarino, G. E. M. Hall, C. Lemieux and C. R. Demas, Problems associated with using filtration to define dissolved trace element concentrations in natural water samples, Environ. Sci. Technol., 1996, 30, 954–963 CrossRef CAS.
- N. Rausch, L. Ukonmaanaho, T. Nieminen, M. Krachler, G. Le Roux and W. Shotyk, Evaluation of samplers and filter materials for the establishment of trace metal concentration profiles in peat bog porewaters using inductively coupled plasma-mass spectrometry, Anal. Chim. Acta, 2006, 558, 201–210 CrossRef CAS.
- D. S. Jeffries and W. R. Snyder, Atmospheric deposition of heavy metals in Central Ontario, Water, Air, Soil Pollut., 1981, 15, 127–152 CrossRef CAS.
- K. H. Wedepohl, The composition of the continental crust, Geochim. Cosmochim. Acta, 1995, 59, 1217–1232 CrossRef CAS.
Footnote |
| † Part of a themed issue dealing with water and water related issues. |
| This journal is © The Royal Society of Chemistry 2010 |
